To assess the long-term association between prediabetes and an increased risk of cardiovascular events in patients with coronary artery disease and percutaneous coronary intervention (PCI).
MethodsA retrospective cohort study. We searched our database to identify all PCI procedures performed in 2010. Patients with no diabetes and HbA1c measurement in the index hospitalization were enrolled and divided into two groups based on HbA1c value: 5.7–6.5% for prediabetes and <5.7% for controls. Demographic, clinical, and procedure-related variables were recorded. Study endpoints were mortality, hospital admissions, myocardial infarction (MI), and revascularization procedures.
ResultsThe study population consisted of 132 subjects (82.6% males, age: 65.26±12.46 years). No difference was found as regards distribution of demographic, clinical, and procedure-related variables. A majority (64.1%) of PCI procedures were performed for ST-segment elevation MI. Prevalence of prediabetes was 40.2%. After a mean follow-up period of 42.3±3.6 months, no differences were found in outcomes between the prediabetes and control groups in total mortality (5.4% vs 1.9%; relative risk [RR] 2.86, 95% confidence interval [95% CI] 0.27–30.44; p=0.56), non-cardiovascular mortality (2.7% vs 1.9%; RR 1.43, 95% CI 0.93–22.18; p=0.79), hospital admissions (19% vs 25%; RR 1.13, 95% CI 0.73–1.73; p=0.57), MI (3% vs 1%; RR 4.28, 95% CI 0.46–39.52; p=0.30), or target lesion revascularization (3% vs 6%; RR 0.70, 95% CI 0.18–2.61; p=0.72).
ConclusionsPrediabetes, as determined by HbA1c (5.7–6.5%), is not associated with long-term adverse cardiovascular outcomes in patients with CAD and PCI.
Determinar si la prediabetes, a largo plazo, se asocia a un mayor riesgo de eventos cardiovasculares en pacientes con cardiopatía isquémica y revascularización coronaria percutánea.
Método.Cohortes retrospectivo. De los procedimientos de revascularización realizados durante 2010 se seleccionaron aquellos sin diagnóstico de diabetes y con determinación de hemoglobina glucosilada. Se constituyeron 2 grupos: prediabetes (5,7-6,5%) y control (<5,7%). Se registraron variables demográficas, clínicas e intervencionistas. Los objetivos de estudio fueron mortalidad, ingresos hospitalarios, infarto de miocardio (IM) y procedimientos de revascularización.
ResultadosLos sujetos de estudio fueron 132 (hombres 82,6%; edad 65,26 ± 12,46). No se encontraron diferencias significativas en las variables demográficas, clínicas ni intervencionistas. La prevalencia de prediabetes fue 40,2%. El 64,1% de los casos de revascularización se debieron a IM con elevación de ST. Tras un seguimiento de 42,3 ± 3,6 meses no se encontraron diferencias entre prediabetes y control en mortalidad total: 5,4% vs 1,9% (riesgo relativo [RR]: 2,86, intervalo de confianza del 95% [IC 95%]: 0,27-30,44, p = 0,56), mortalidad no cardiovascular: 2,7% vs 1,9% (RR: 1,43, IC 95%: 0,93-22,18, p = 0,79), ingresos de cualquier causa: 19% vs 25% (RR: 1,13, IC 95%: 0,73-1,73, p = 0,57), IM: 3% vs 1% (RR: 4,28, IC 95%: 0,46-39,52; p = 0,30) ni revascularización de la lesión tratada: 3% vs 6% (RR: 0,70, IC 95%: 0,18-2,61, p = 0,72).
ConclusionesEn pacientes sometidos a revascularización coronaria la presencia de prediabetes, definida según valores de hemoglobina glucosilada, no se asocia a un incremento de eventos cardiovasculares a largo plazo.
Ischemic heart disease (IHD) is the most common cause of death worldwide, accounting for approximately seven million deaths annually, 12.8% of all deaths. In Europe, it is estimated that one out of every six men and one out of every seven women will die from IHD.1 In Spain, IHD is the leading cause of death in men and the second leading cause of death in women. As regards morbidity, in 2010 there were 129,944 hospital discharges with a diagnosis of acute myocardial infarction (AMI).2
The most effective measure for coping with this health problem is cardiovascular prevention. Fifty percent of mortality reduction attributed to IHD is due to the detection and control of cardiovascular risk factors (CVRFs). When a patient develops the disease, however, effective treatment is needed, and most coronary lesions are treatable with percutaneous coronary interventions and stent implantation. The most common long-term complication of this procedure is stent restenosis.3,4 There are several associated factors. Thus, in registries of patients implanted conventional stents (bare-metal stent (BMS)), the variables inherent in coronary anatomy, the type of lesion, and the characteristics of the procedure have been identified, including long lesions (>20mm), the length of the implanted stent, small vessels (<3mm), and bifurcation lesions.3,4
There are also other associated conditions such as female sex, diabetes mellitus (DM), high blood pressure, the body mass index, chronic kidney disease, and multivessel coronary disease. An association with DM has been consistent in the different reports. Specifically, 30–50% restenosis has been shown with BMS, and although rates have decreased with the use of drug-eluting stents, diabetic patients continue to have a higher incidence of restenosis.5
These data reflect the impact of DM in this group, which is substantial considering that there are between 170 and 194 million people with DM. Its prevalence in Spain is 13.8%, and 11.6% are at risk of developing T2DM.6,7
This last subgroup encompasses the so-called prediabetes.8 The diagnostic criteria for impaired glucose tolerance and impaired basal glucose have changed in recent decades,9,10 but in 2010 the American Diabetes Association recommended the use of glycosylated hemoglobin (HbA1c) levels ranging from 5.7% to 6.4% to define prediabetes.6 Epidemiologically, prediabetes represents a significant problem, because in 2010 there were already 65 million prediabetics in Europe.11,12 From the pathophysiological viewpoint, prediabetes is related to insulin resistance and β-cell dysfunction.13,14
Prediabetes has been associated with other CVRFs and increased total cardiovascular risk. The DECODE study15 showed prediabetes to be associated with an increased risk of coronary and cardiovascular death. The prognostic impact of HbA1c in IHD was verified in a recent meta-analysis of more than 11,000 patients (the ARIC study), and its association with mortality was much more significant in patients with no established diagnosis of DM.16 Data have also been reported for approximately 900 patients followed up for 14 years suggesting that healthy individuals with prediabetes, based on HbA1c levels, are at greater risk of developing IHD.17
It, therefore, seems plausible that patients with coronary revascularization who also have prediabetes represent a population subgroup with a higher number of cardiovascular events. The limited data available in the literature show conflicting results, both short- and long-term. Timmer et al.,18 in a retrospective, observational study of 4176 non-diabetic patients with acute coronary syndrome (ACS) with ST elevation (STE-ACS), showed an association between increasing HbA1c levels and long-term mortality after dividing the population into quartiles. Thirty-day mortality was however similar. Chan et al.19 prospectively studied a cohort of 317 diabetic patients with ACS, divided into two groups based on their HbA1c level, using a threshold of 7%. There were no differences in clinical prognosis at six months. Most patients had non-ST elevation ACS (NSTE-ACS). Lazzeri et al.20 subsequently enrolled into a study 518 non-diabetic patients with STE-ACS referred for primary angioplasty, who were divided into groups with a cut-off point of HbA1c of 6.5%. No differences were found in the short- or long-term prognosis. Cicek et al.21 conducted a prospective, single center study on 374 patients with STE-ACS; after measuring HbA1c levels, they divided the population into three groups: <5.6%, 5.7–6.4%, and >6.5%. The group in the DM range had a higher hospital mortality rate, with no differences between those with and without prior DM diagnosis. A higher cardiovascular event rate was reported in the prediabetes group as compared to the group with no prediabetes. More recently, Tian et al.22 reported a post hoc analysis of a prospective, multicenter study conducted in China on a population with STE-ACS referred for primary angioplasty. The study enrolled 608 patients, and determined prognosis at 7 and 30 days. There were three groups based on their HbA1c level: <5.6%, 5.7–6.4%, and >6.5%. No significant differences in mortality and AMI were found at 7 and 30 days.
This study was undertaken to assess whether patients with ischemic heart disease undergoing percutaneous coronary revascularization who also have prediabetes represent a population at a greater long-term risk of cardiovascular events.
MethodsThis was a retrospective cohort study. The database of the hemodynamics section of Hospital Clínico Universitario Lozano Blesa was reviewed to identify those patients who had had intracoronary stents implanted in 2010. The inclusion criteria were as follows: age over 18 years, stent implantation during 2010, and the availability of an HbA1c measurement during their hospital stay. The exclusion criteria included: a diagnosis of T2DM and HbA1c levels in the T2DM range.
Study groups were defined based on the HbA1c level: a “prediabetes” group with values ranging from 5.7% to 6.4%, and a “no prediabetes” group with values <5.7%. The outcome variables (mortality, hospital admissions, myocardial infarction, and revascularization procedures)23 were verified to allow for the association to be estimated. Data were collected from procedure reports, and laboratory values were taken from the clinical records. Outcome variables were verified through the hospital Intranet, which allows access to health data in the autonomous community.
For statistical analysis, a description was given of the demographic variables in the overall sample and in each cohort, with measures of central tendency (mean) and standard deviation for the quantitative variables, and percentages for the categorical variables. A search was subsequently made for differences in variable distribution between the two study cohorts. After verifying normality with a Kolmogorov–Smirnov test, a Student's t test was used for the quantitative variables, and a Chi-square test and a Fisher exact test were used for the categorical variables. If no normality was found, a Mann–Whitney U test was used. Measurement of the incidence of the outcome variables was then continued, after which the relative risk of prediabetes as a function of the outcome variables and the corresponding confidence interval were estimated. SPSS version 15 was used. No intervention was performed, and informed consent was therefore not required. A confidentiality agreement was signed with the hospital.
ResultsBased on the inclusion criteria and the availability of the required data, a total of 132 patients with 226 coronary stents were analyzed. After patients with inadequate laboratory data (n=40) had been excluded, prediabetes was found in 40.2% of the patient sample, which was divided into prediabetes (n=37) and no prediabetes cohorts (n=55).
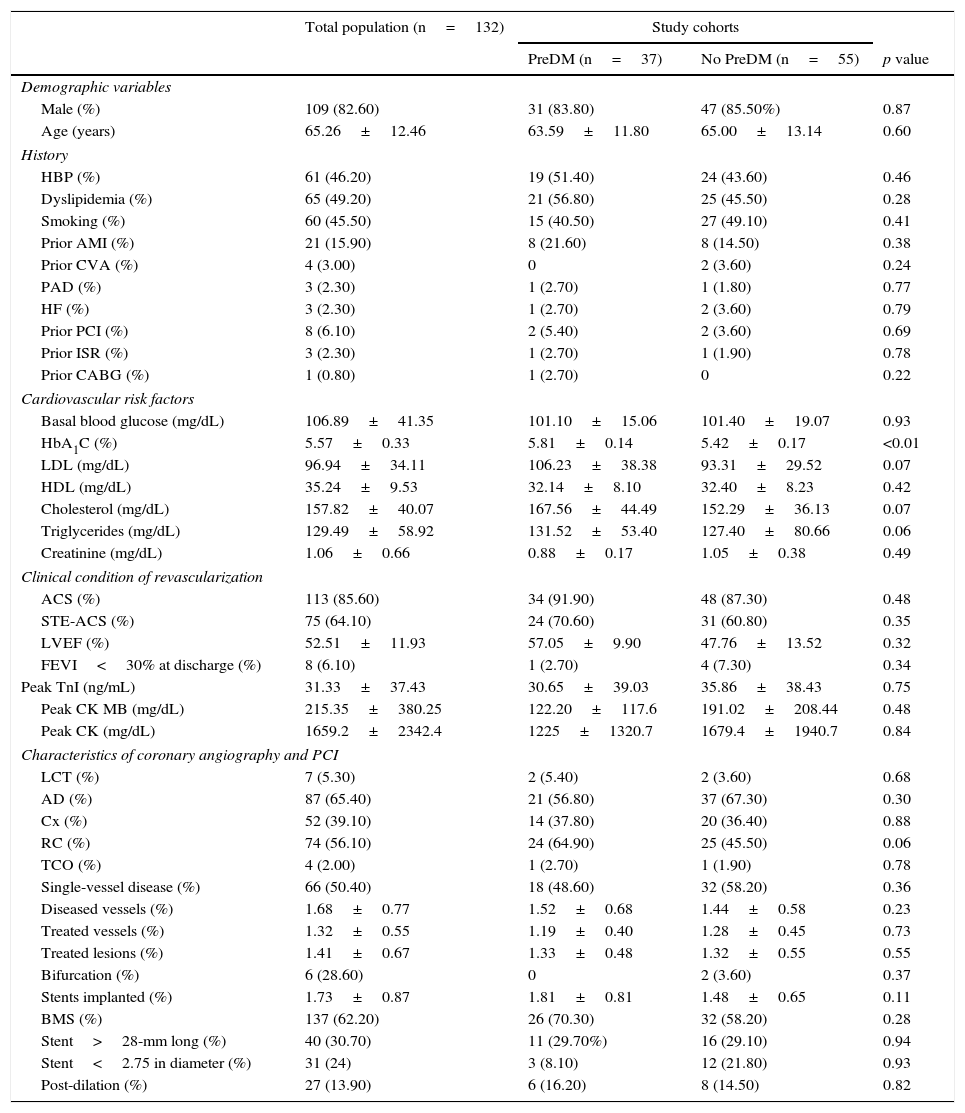
Table 1 shows the demographic characteristics and personal histories of the patient sample. No significant differences were seen between the groups in variable distribution in the study cohorts.
Study variables in the general population and by study cohort.
| Total population (n=132) | Study cohorts | |||
|---|---|---|---|---|
| PreDM (n=37) | No PreDM (n=55) | p value | ||
| Demographic variables | ||||
| Male (%) | 109 (82.60) | 31 (83.80) | 47 (85.50%) | 0.87 |
| Age (years) | 65.26±12.46 | 63.59±11.80 | 65.00±13.14 | 0.60 |
| History | ||||
| HBP (%) | 61 (46.20) | 19 (51.40) | 24 (43.60) | 0.46 |
| Dyslipidemia (%) | 65 (49.20) | 21 (56.80) | 25 (45.50) | 0.28 |
| Smoking (%) | 60 (45.50) | 15 (40.50) | 27 (49.10) | 0.41 |
| Prior AMI (%) | 21 (15.90) | 8 (21.60) | 8 (14.50) | 0.38 |
| Prior CVA (%) | 4 (3.00) | 0 | 2 (3.60) | 0.24 |
| PAD (%) | 3 (2.30) | 1 (2.70) | 1 (1.80) | 0.77 |
| HF (%) | 3 (2.30) | 1 (2.70) | 2 (3.60) | 0.79 |
| Prior PCI (%) | 8 (6.10) | 2 (5.40) | 2 (3.60) | 0.69 |
| Prior ISR (%) | 3 (2.30) | 1 (2.70) | 1 (1.90) | 0.78 |
| Prior CABG (%) | 1 (0.80) | 1 (2.70) | 0 | 0.22 |
| Cardiovascular risk factors | ||||
| Basal blood glucose (mg/dL) | 106.89±41.35 | 101.10±15.06 | 101.40±19.07 | 0.93 |
| HbA1C (%) | 5.57±0.33 | 5.81±0.14 | 5.42±0.17 | <0.01 |
| LDL (mg/dL) | 96.94±34.11 | 106.23±38.38 | 93.31±29.52 | 0.07 |
| HDL (mg/dL) | 35.24±9.53 | 32.14±8.10 | 32.40±8.23 | 0.42 |
| Cholesterol (mg/dL) | 157.82±40.07 | 167.56±44.49 | 152.29±36.13 | 0.07 |
| Triglycerides (mg/dL) | 129.49±58.92 | 131.52±53.40 | 127.40±80.66 | 0.06 |
| Creatinine (mg/dL) | 1.06±0.66 | 0.88±0.17 | 1.05±0.38 | 0.49 |
| Clinical condition of revascularization | ||||
| ACS (%) | 113 (85.60) | 34 (91.90) | 48 (87.30) | 0.48 |
| STE-ACS (%) | 75 (64.10) | 24 (70.60) | 31 (60.80) | 0.35 |
| LVEF (%) | 52.51±11.93 | 57.05±9.90 | 47.76±13.52 | 0.32 |
| FEVI<30% at discharge (%) | 8 (6.10) | 1 (2.70) | 4 (7.30) | 0.34 |
| Peak TnI (ng/mL) | 31.33±37.43 | 30.65±39.03 | 35.86±38.43 | 0.75 |
| Peak CK MB (mg/dL) | 215.35±380.25 | 122.20±117.6 | 191.02±208.44 | 0.48 |
| Peak CK (mg/dL) | 1659.2±2342.4 | 1225±1320.7 | 1679.4±1940.7 | 0.84 |
| Characteristics of coronary angiography and PCI | ||||
| LCT (%) | 7 (5.30) | 2 (5.40) | 2 (3.60) | 0.68 |
| AD (%) | 87 (65.40) | 21 (56.80) | 37 (67.30) | 0.30 |
| Cx (%) | 52 (39.10) | 14 (37.80) | 20 (36.40) | 0.88 |
| RC (%) | 74 (56.10) | 24 (64.90) | 25 (45.50) | 0.06 |
| TCO (%) | 4 (2.00) | 1 (2.70) | 1 (1.90) | 0.78 |
| Single-vessel disease (%) | 66 (50.40) | 18 (48.60) | 32 (58.20) | 0.36 |
| Diseased vessels (%) | 1.68±0.77 | 1.52±0.68 | 1.44±0.58 | 0.23 |
| Treated vessels (%) | 1.32±0.55 | 1.19±0.40 | 1.28±0.45 | 0.73 |
| Treated lesions (%) | 1.41±0.67 | 1.33±0.48 | 1.32±0.55 | 0.55 |
| Bifurcation (%) | 6 (28.60) | 0 | 2 (3.60) | 0.37 |
| Stents implanted (%) | 1.73±0.87 | 1.81±0.81 | 1.48±0.65 | 0.11 |
| BMS (%) | 137 (62.20) | 26 (70.30) | 32 (58.20) | 0.28 |
| Stent>28-mm long (%) | 40 (30.70) | 11 (29.70%) | 16 (29.10) | 0.94 |
| Stent<2.75 in diameter (%) | 31 (24) | 3 (8.10) | 12 (21.80) | 0.93 |
| Post-dilation (%) | 27 (13.90) | 6 (16.20) | 8 (14.50) | 0.82 |
CVA: cerebrovascular accident; BMS: bare-metal stent; CABG: coronary artery bypass graft; RC: right coronary artery; Cx: circumflex artery; AD: anterior descending artery; PAD: peripheral artery disease; LVEF: left ventricular ejection fraction; HbA1c: glycosylated hemoglobin; HDL: high density lipoprotein cholesterol; HBP: high blood pressure; AMI: acute myocardial infarction; HF: heart failure; PCI: percutaneous coronary intervention; ISR: in-stent restenosis; LDL: low density lipoprotein cholesterol; TCO: total chronic occlusion; PreDM: prediabetes; ACS: acute coronary syndrome; STE-ACS: ST elevation acute coronary syndrome; LCT: left coronary trunk; TnI: troponin i.
The high component of coronary revascularization in the setting of STE-ACS should be noted (64.1%), although this rarely had a significant impact on left ventricular systolic function at discharge. As these were patients treated in 2010, BMSs were implanted in a majority of cases (62.2%), and there were a limited number of long lesions (30.7%) or small vessel lesions (24%).
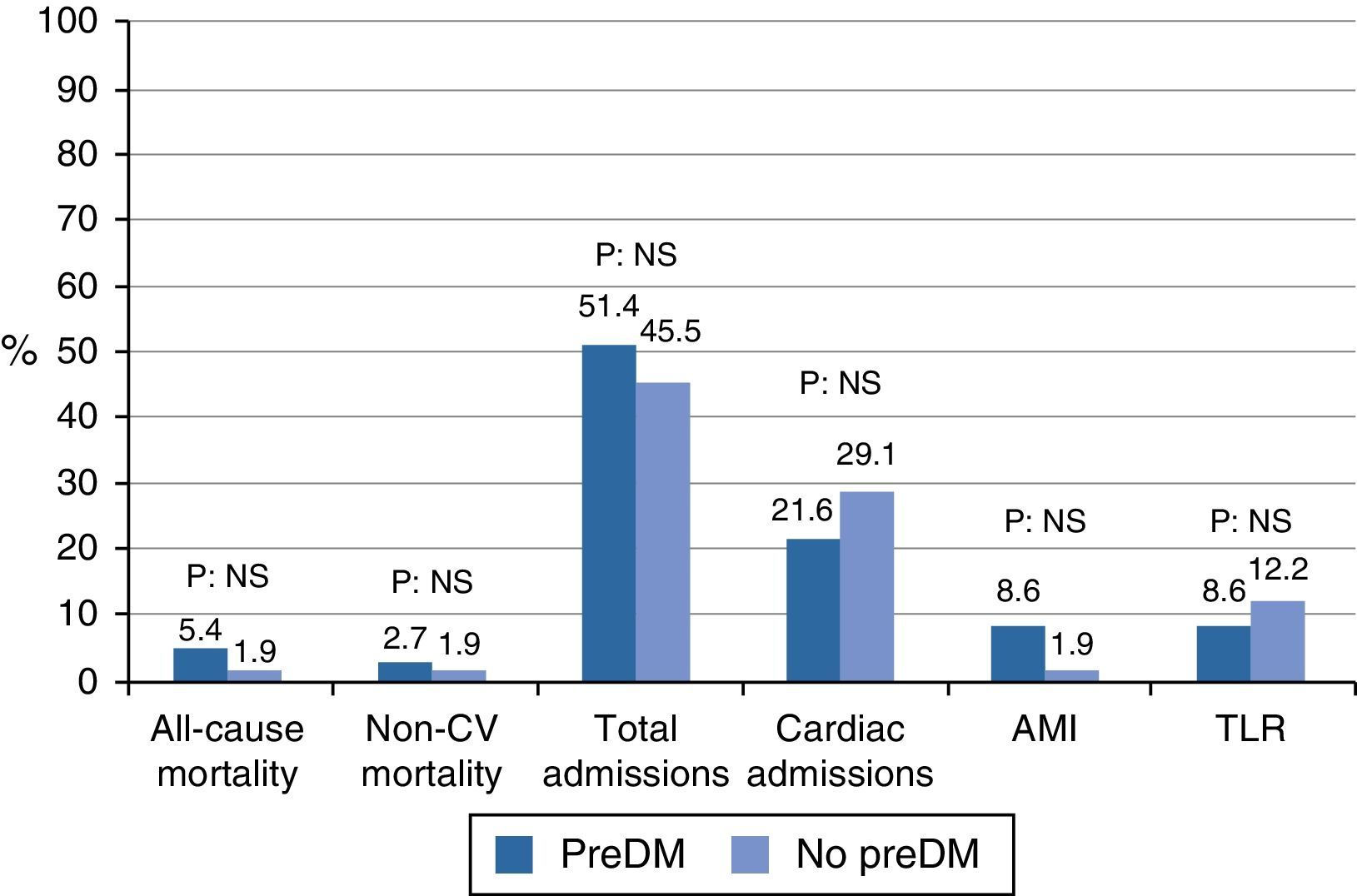
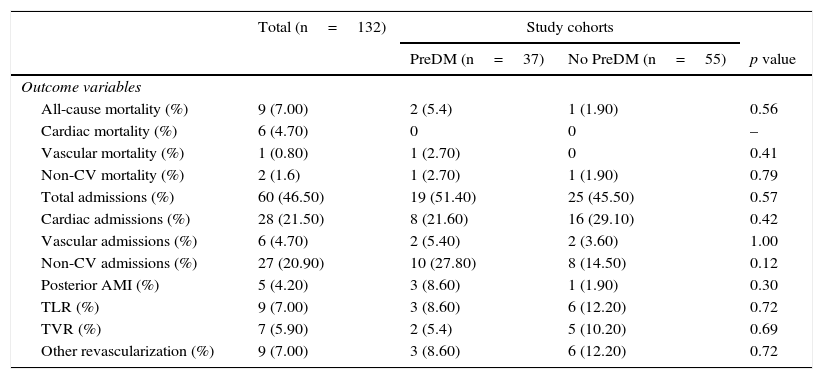
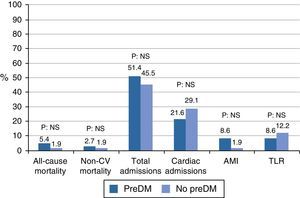
In the outcome variables (Table 2 and Fig. 1), in general, after a mean follow-up of 42.37±3.65 months, there was a low rate of events of mortality, myocardial infarction, or revascularization, with a higher occurrence of hospital admissions. Mortality was 5.4% in the prediabetes group and 1.9% in the group with no prediabetes (relative risk [RR]: 2.86, 95% confidence interval [95% CI]: 0.27–30.44, p=0.56). Cardiac mortality could not be analyzed because all the cases occurred in patients excluded from the cohort analysis. Similarly, vascular mortality could not be analyzed because no events occurred in the group with no prediabetes. There were no differences in non-cardiovascular mortality (RR: 1.43, 95% CI: 0.93–22.18, p=0.79). The low event rate in the sample explains the amplitude of CIs.
Outcome variables in the general population and by study cohort.
| Total (n=132) | Study cohorts | |||
|---|---|---|---|---|
| PreDM (n=37) | No PreDM (n=55) | p value | ||
| Outcome variables | ||||
| All-cause mortality (%) | 9 (7.00) | 2 (5.4) | 1 (1.90) | 0.56 |
| Cardiac mortality (%) | 6 (4.70) | 0 | 0 | – |
| Vascular mortality (%) | 1 (0.80) | 1 (2.70) | 0 | 0.41 |
| Non-CV mortality (%) | 2 (1.6) | 1 (2.70) | 1 (1.90) | 0.79 |
| Total admissions (%) | 60 (46.50) | 19 (51.40) | 25 (45.50) | 0.57 |
| Cardiac admissions (%) | 28 (21.50) | 8 (21.60) | 16 (29.10) | 0.42 |
| Vascular admissions (%) | 6 (4.70) | 2 (5.40) | 2 (3.60) | 1.00 |
| Non-CV admissions (%) | 27 (20.90) | 10 (27.80) | 8 (14.50) | 0.12 |
| Posterior AMI (%) | 5 (4.20) | 3 (8.60) | 1 (1.90) | 0.30 |
| TLR (%) | 9 (7.00) | 3 (8.60) | 6 (12.20) | 0.72 |
| TVR (%) | 7 (5.90) | 2 (5.4) | 5 (10.20) | 0.69 |
| Other revascularization (%) | 9 (7.00) | 3 (8.60) | 6 (12.20) | 0.72 |
CV: cardiovascular: DM: diabetes mellitus; AMI: acute myocardial infarction; NS: not significant; PreDM: prediabetes; TLR: target lesion revascularization.
There was no difference in hospital admissions between the cohorts; total admissions, RR: 1.13, 95% CI: 0.73–1.73, p=0.57; admissions for cardiac causes, RR: 0.74, 95% CI: 0.35–1.55, p=0.42; admissions for vascular causes, RR: 1.48, 95% CI: 0.21–10.09, p=1.00; admissions for non-cardiovascular causes, RR: 1.91, 95% CI: 0.83–4.37, p=0.12.
Myocardial infarction and revascularization rates during follow-up were similar in both groups. AMI RR: 4.28, 95% CI: 0.46–39.52, p=0.30; target lesion revascularization, RR: 0.70, 95% CI: 0.18–2.61, p=0.72; target vessel revascularization, RR: 0.56, 95% CI: 0.11–2.72, p=0.69; other revascularization, RR: 0.70, 95% CI: 0.18–2.61, p=0.72.
DiscussionThe relationship between HbA1c and cardiovascular disease has already been established. HbA1c is a risk factor for cardiac and all-cause mortality in both diabetic and non-diabetic patients.24–26 Selvin et al.16 measured basal blood glucose and HbA1c in 11,092 patients with no history of DM or cardiovascular disease, and after a mean follow-up of 14 years found a significant association of HbA1c with cardiovascular disease and stroke, even with values <6.5%.
In patients with IHD, specifically with STE-ACS, a high-risk group, the identification of all the circumstances that may contribute to a worsening of that condition is of paramount importance. DM, and changes in carbohydrate metabolism in general, has been one of the main study objectives in this regard, because a prevalence of unknown DM ranging from 9.2% to 10.1% has been reported in groups of patients with STE-ACS.27
Previous studies have reported an association between blood glucose levels measured at admission and hospital mortality and major adverse cardiovascular events in the long term.28 There are, however, some aspects which have limited the use of blood glucose levels, such as the temporal relationship with the last intake. In addition, the pathophysiological response triggered by coronary disease may substantially increase serum glucose levels.29 This has led to HbA1c levels, rather than glucose levels, being used.
Gustafsson et al.,30 in an analysis of the OPTIMAAL study, divided 2346 patients with no history of DM into three groups based on their HbA1c levels (<4.9%, 4.9–5.1%, and >5.1%) and noted a progressive increase in long-term mortality (2 years and 6 months) to 13%, 17%, and 22% respectively. This population showed a higher mortality in our study, which is closely related to the fact that fibrinolysis, rather than percutaneous coronary intervention, was used as a reperfusion method in approximately 60% of patients.
As compared to the results of Timmer et al.,18 total mortality in our series was much lower (10% vs 3.2%) over a follow-up time of approximately three years in both groups. Similarly, in patients with the lowest HbA1c values, the survival rate was lower than that found in our study. The only difference between the populations was the greater presence of AMI in the territory of the anterior descending artery in the Timmer et al. series,18 which may be related to larger infarctions with poorer prognosis. These circumstances may explain the conflicting findings.
Chan et al.19 found no differences after a six-month follow-up. However, they used an HbA1c value of 7% as the cut-off point, and comparisons with our series would therefore be inappropriate, because patients with DM were studied. Special mention should be made of the high mortality in the group with HbA1c <7%, up to 7.3% taking all causes into account and 4% from cardiovascular causes, which is especially striking because cases of STE-ACS only represented 26.2%. It should be noted that the population in this study was older and had a greater prevalence of CVRFs.
The Lazzeri et al. study20 found results similar to ours. The population consisted of patients with STE-ACS only, and the cut-off point of HbA1c (6.5%) was the same as that used in our study. The impact of AMI, expressed as a decrease in the left ventricular ejection fraction, was small in both series.
In our study population, mainly consisting of patients with STE-ACS, no relationship was found between prediabetes and the long-term prognosis in patients with coronary revascularization using PCI, despite the fact that this was a cohort with a significant cardiovascular risk. However, this lack of association may be due to the low mortality rate found and the number of study subjects. Another limitation of this project is its single-center, retrospective nature. By contrast, one strength of this study was that a high number of potentially confounding clinical, demographic, and interventional factors were analyzed.
It should be noted that prior studies which showed a relationship with mortality consisted of enrolled populations with a greater CVRF burden, more severe AMI, or who were receiving fibrinolysis as reperfusion treatment, and it is possible that these factors influenced the prognosis. Despite the above limitations, it can be stated that prediabetes is not associated with a poorer clinical prognosis in patients with characteristics similar to those included in our study.
Taking all these results into consideration, but mainly the studies by Cicek et al.21 and Tian et al.,22 the evidence appears to suggest that no relationship exists between prediabetes and the short-term prognosis in patients undergoing percutaneous coronary revascularization, especially for STE-ACS.
The prediabetes rate found in our study (40.2%) was very similar to the rates reported by Cicek et al.21 and Tain et al.,22 ranging from 29.9% to 44.1%.
Adequate evidence appears to exist for not changing the medical, pharmacological, or interventional treatment of acute ACS in patients with prediabetes who are to undergo coronary revascularization procedures, because a higher risk of hospital cardiovascular events has not been shown. Controversy still exists as regards the long term prognosis, but this has mainly resulted from patient selection. The value of, and the need for, screening carbohydrate metabolism changes in this subgroup of high-risk patients in order to perform interventions that slow the progression to DM should, however, not be underestimated.31
ConclusionsIn our sample of patients undergoing coronary revascularization, the presence of prediabetes, defined on the basis of the HbA1c level, was not associated with increased cardiovascular events in the long term. However, although these findings agree with others previously reported, prospective studies on larger study samples are needed.
Conflicts of interestNone.
Please cite this article as: Cueva-Recalde JF, Ruiz-Arroyo JR, García-Blanco FR. Prediabetes y pronóstico clínico de los pacientes con cardiopatía isquémica y revascularización coronaria percutánea. Endocrinol Nutr. 2016;63:106–112.
This study was conducted in cooperation with the Department of Health Sciences Universidad Católica San Antonio in Murcia, in the setting of the Official Master in Cardiovascular Risk.