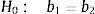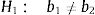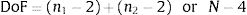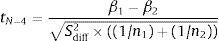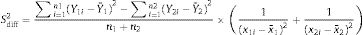To evaluate if a specific pediatric defined daily dose (PeDDD) can be replaced with the defined daily dose (DDD) indicated by World Health Organization (WHO).
MethodsThe 50th percentile of body weight for age of children admitted from 2016 to 2020 at Istituto Giannina Gaslini, Genoa, Italy, was used to calculate PeDDD for vancomycin at 40mg/kg and meropenem at 60mg/kg. Data obtained were then used to calculate the PeDDD number based on the amount of drugs delivered quarterly from 2012 to 2016. Subsequently the DDD number was calculated for vancomycin at 2g and meropenem at 3g. With these results two curves were generated which were then compared for parallelism and area under the curve (AUC).
ResultsPeDDD was found to be 2.6 times DDD for both drugs, but both curves obtained were parallel and the AUCs were identical
ConclusionsDDD according to WHO definition could be adopted in pediatrics to measure antibiotic consumption and therefore no specific PeDDD could be needed.
Evaluar si una dosis diaria definida pediátrica específica (PeDDD) puede ser reemplazada por la dosis diaria definida (DDD) indicada por la Organización Mundial de la Salud (OMS).
MétodosEl 50 percentil del peso por la edad media de los niños admitidos desde 2016 hasta 2020 al Istituto Giannina Gaslini, Génova, Italia, fue utilizado para calcular el PeDDD con vancomina a 40mg/kg y meropenem a 60mg/kg. Luego los datos obtenidos fueron utilizados para calcular el número de PeDDD basado en la cantidad de medicamentos entregados trimestralmente desde 2012 hasta 2016. Posteriormente, el número de DDD fue calculado con vancomicina a 2g y meropenem a 3g. Con los resultados, se generaron 2 curvas que fueron comparadas con paralelismo y área bajo la curva (AUC).
ResultadosPeDDD resultó ser 2,6 veces DDD por ambos medicamentos, pero ambas curvas obtenidas eran paralelas y las AUC eran idénticas.
ConclusionesDDD, según la definición de la OMS, podría adoptarse en Pediatría para medir el consumo de antibióticos y, por lo tanto, no podría ser necesario un PeDDD específico.
Evaluation of antibiotic use is pivotal in an era of increasing infections due to resistant pathogens and measurement of their consumption is one of the tools for a correct antibiotic stewardship. Drug consumption can be expressed in cost, number of units, number of prescriptions or by the physical quantity of drugs. However these variables can vary between regions and countries and over time. To address this, a technical unit of measurement, the defined daily dose (DDD), was created by the World Health Organization (WHO)1,2 and became a standard to quantify antibiotic use in different settings and contexts. The DDD is the average standard daily dose of a drug used in a 70kg adult for the most common indication2; it does not represent the recommended nor the actually dispensed dose, but is only an “unit of measure” for drug disposal. Therefor it should not be considered an exact picture of actual use, but a rough estimate of consumption, providing a fixed unit of measurement independent of price, currencies, package size and strength enabling the researcher to assess trends in drug utilization and to perform comparisons.1 Consequently, its use allows to examine changes in drug utilization over time and different spaces, including the effect of interventions on drug use and changes in the use of drugs classes or their prescribing patterns.1
Unfortunately, its use in pediatrics has been always considered difficult to applicate since in children drugs are generally administered on the base of age and body weight.2,3 Recently, a methodology for identification of a pediatric DDD (PeDDD) has been implemented.4 Briefly, the mean body weight of children admitted in 10 Spanish hospitals in 2013 was retrospectively estimated using the 50th percentile according to the WHO weight for age graphs in pediatrics.5 Overall cohort weight was the mean between female and male weights. PeDDD was then calculated by multiplying the “mean” body weight (kg) for the antibiotic dose administered for the most common indication that was previously agreed by via Delphi method. This method is very similar to the already evaluated recommended daily dose (RDD) in mg/kg.6
We performed a study to analyze if there was correspondence between the number of PeDDD calculated with this pediatric-specific method,4 and the number of DDD calculated according to WHO indication, using the amount of antibiotics delivered by Pharmacy service to all hospital wards7 in the period 2012–2020 in a tertiary care Italian pediatric hospital.
Materials and methodsThe Istituto Giannina Gaslini (IGG), Genoa, Italy, is a tertiary care children's hospital in North-West Italy serving as local pediatric hospital for the Genoa area, but also representing a referral hospital nationwide and for many foreign countries.
For evaluation of antibiotic use, patients’ mean weight was calculated according to previous description4,5 on the base of admissions in the period 2012–2020. PeDDD was then estimated for vancomycin at a dose of 40mg/kg/day and meropenem administered at 60mg/kg/day, two of the most frequently prescribed antibiotics in hospitalized children with severe, antibiotic resistant infections. Data on quarterly antibiotic distribution to the whole hospital for both drugs in the same period were extracted from the Pharmacy database and reported in grams (g) per quarter. This approach was selected since electronical record on antibiotic treatments were not uniformly available at patient level for all the hospital wards.8 The number of PeDDD was then calculated by dividing the amount of each drug delivered in each quarter by the estimated PeDDD, while the number of DDD was calculated dividing the total amount of drug delivered in a quarter by the daily dose indicated by WHO.
Statistical analysisQuarterly PeDDD and DDD were inserted in a calculation sheet where the X axis was the quarters present in the study period (n=36) and the Y the number of PeDDD or DDD; then curves (straight lines) were generated.
The first step in the analysis was the evaluation of the parallelism of the curves. For each quarter the beta coefficients were calculated for both straight lines (one referring to DDD and the other to PeDDD, respectively).
The regression coefficients of the straight lines of DDD were called b1 and the ones of PeDDD were called b2, the first ones with a sample of n1 observations and the second ones with a sample of n2 observations.
The comparison of the parallelism was performed by means of the Student's t-test to verify the following null hypothesis:
Against the alternative 2-sided hypothesis:
This test has the following number of degrees of freedom (DoF):
And the value of the test is:
where:Subsequently, the area under the curve (AUC) of each quarter was calculated using the trapezoid method.
The ratios between PeDDD and DDD values and their AUC (quarterly and for the total of the study period) where then calculated to compare PeDDD and DDD AUCs.
Each calculation was performed by means of a Microsoft Excel 365 for Mac version 16.55 (Microsoft Corporation, Redmond, WA, USA).
ResultsIn the period 2012–2020 the mean body weight of patients admitted at IGG was 14.25kg for those aging 0 to ≤14 years and 58.75kg for 15–18 years old. Since the group 0–14 represented more than 75% of admitted patients we adopted the mean weight of this group to establish a PeDDD, that resulted 0.77g for vancomycin, to be compared with a 2g DDD for adults, and 1.16g for meropenem, to be compared with a 3g DDD for adults. Starting from the amount of each drug distributed by Pharmacy to the whole hospital during the 36 quarters present in the period 2012–2020, PeDDD and DDD were then calculated for each quarter.
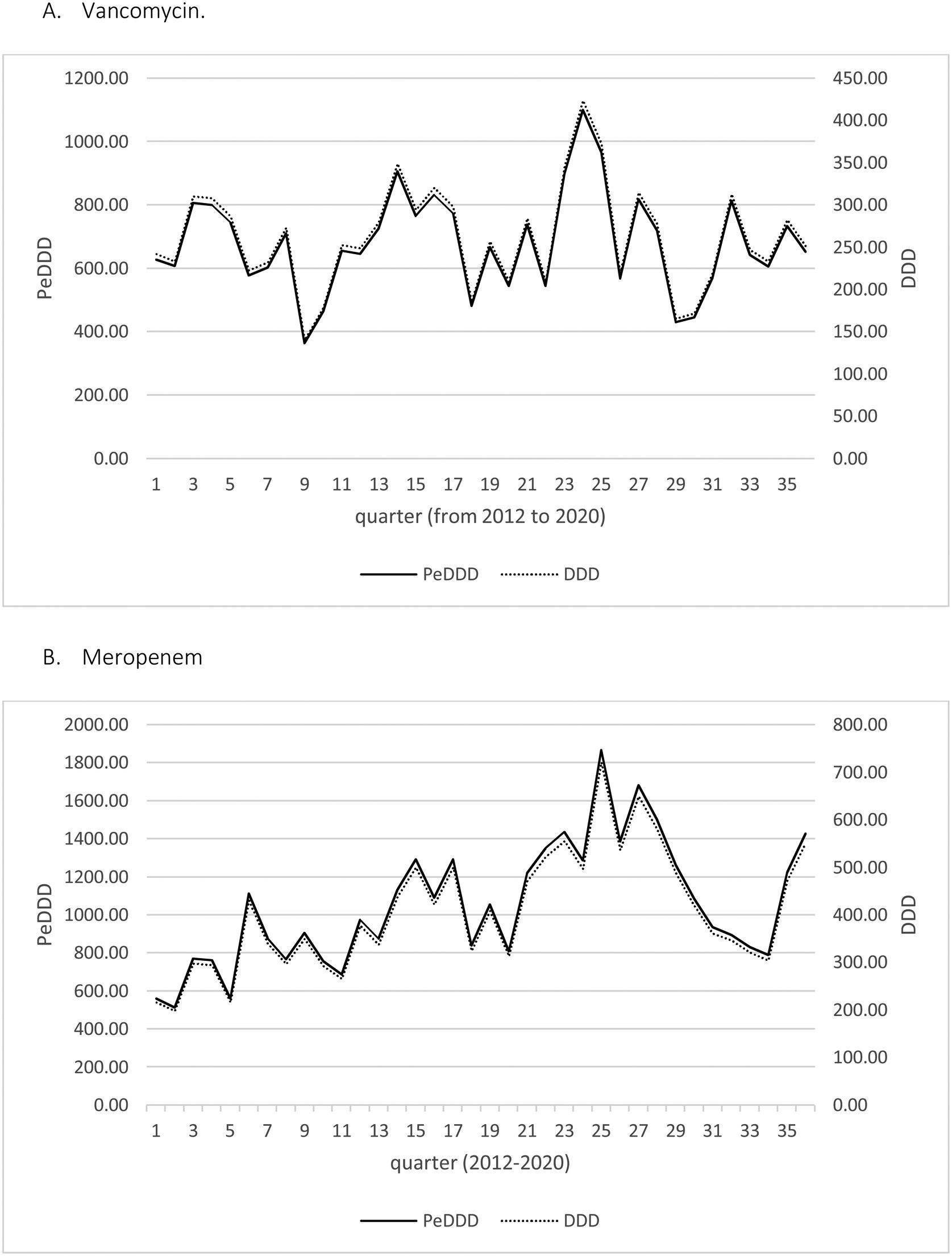
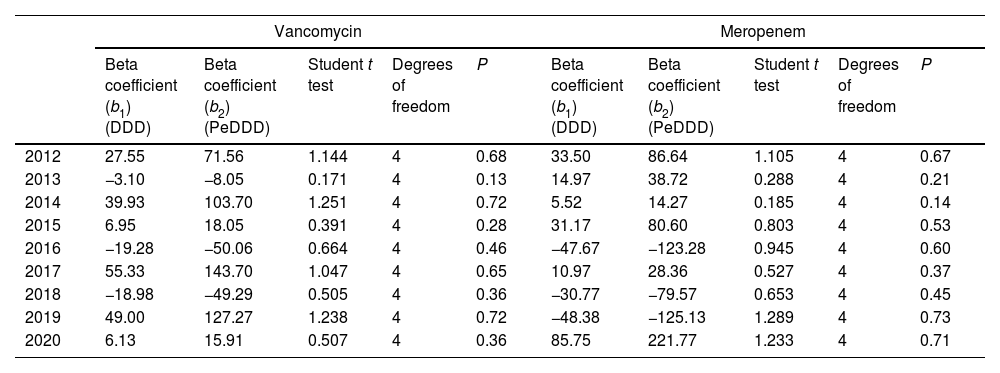
Fig. 1 displays the quarterly consumption of vancomycin of meropenem during the study period expressed in DDD and PeDDD. As shown in Table 1 none of the tests performed to evaluate curves parallelism turned out to be statistically significant, demonstrating parallelism for each of the segments (values measured in each quarter of year) of the straight lines drawn for each drug. The ratio between the values of PeDDD and DDD was 2.6 for vancomycin in all the quarters during the study period, and the same value was observed for the ratio between the two AUCs (PeDDD and DDD). For meropenem we observed some variations in the ratio between numbers of PeDDD vs. DDD, but the median value was 2.6 (95% confidence interval 2.5–2.8, range 1.7–3.1) and also in this case the ratio between AUCs was 2.6.
Comparison of beta coefficients (parallelism test) of curves based on DDD vs curves based on PeDDD.
| Vancomycin | Meropenem | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta coefficient (b1) (DDD) | Beta coefficient (b2) (PeDDD) | Student t test | Degrees of freedom | P | Beta coefficient (b1) (DDD) | Beta coefficient (b2) (PeDDD) | Student t test | Degrees of freedom | P | |
| 2012 | 27.55 | 71.56 | 1.144 | 4 | 0.68 | 33.50 | 86.64 | 1.105 | 4 | 0.67 |
| 2013 | −3.10 | −8.05 | 0.171 | 4 | 0.13 | 14.97 | 38.72 | 0.288 | 4 | 0.21 |
| 2014 | 39.93 | 103.70 | 1.251 | 4 | 0.72 | 5.52 | 14.27 | 0.185 | 4 | 0.14 |
| 2015 | 6.95 | 18.05 | 0.391 | 4 | 0.28 | 31.17 | 80.60 | 0.803 | 4 | 0.53 |
| 2016 | −19.28 | −50.06 | 0.664 | 4 | 0.46 | −47.67 | −123.28 | 0.945 | 4 | 0.60 |
| 2017 | 55.33 | 143.70 | 1.047 | 4 | 0.65 | 10.97 | 28.36 | 0.527 | 4 | 0.37 |
| 2018 | −18.98 | −49.29 | 0.505 | 4 | 0.36 | −30.77 | −79.57 | 0.653 | 4 | 0.45 |
| 2019 | 49.00 | 127.27 | 1.238 | 4 | 0.72 | −48.38 | −125.13 | 1.289 | 4 | 0.73 |
| 2020 | 6.13 | 15.91 | 0.507 | 4 | 0.36 | 85.75 | 221.77 | 1.233 | 4 | 0.71 |
DDD: defined daily dose in adults; PeDDD: defined daily dose calculated in pediatrics.
Data employed for all these calculations are available as supplementary materials.
DiscussionIn the present study we used a pediatric-specific method4 to calculate the number of PeDDD for vancomycin and meropenem consumed quarterly in a tertiary care Italian pediatric hospital7 from 2012 to 2020 and compared the results with that obtained using “standard” DDD.2 The results showed that PeDDD was parallel to DDD (with a coefficient of 2.6), meaning that DDD can be used to estimate antibiotic use also in pediatrics. Despite some criticism,9 the methodology we adopted in this study4,7 can be considered sufficiently robust and applicable, both in children and neonates.4,10,11 Moreover, though derived at a single center, the use of validated methods4,7,10,11 and the long period of observation12 in our opinion ensure the reproducibility of our results.
Antimicrobial stewardship represents a mandatory task also in pediatric patients,13 especially in an era of increasing antibiotic resistance. Evaluation of DDD is a well-known method to measure antibiotic consumption in adults,2 but its use in pediatrics has been always excluded since (especially youngest) children receive drug doses based on age and weight,3,14 and not in number of daily vials, as in the DDD system.2 Different studies have been performed applying different measures of antibiotic consumption in pediatrics,6 but in many cases they were restricted to specific populations as neonates15 or required the collection of many data, most of them at the patient level.16,17 However, all these systems, even if “elegant”, are time-consuming (need of meetings to define the dose and/or specific data extraction at patient level, not always easily available) and may suffer of differences in dose prescription attitudes in different countries and even in different hospitals in the same region.18 Therefore, we performed a study to proof the concept that DDD can be used also in pediatrics, aware of possible caveats or limitations associated with using an adult based DDD,1 and showed that DDD and PeDDD4 gave similar measurements. We agree that this study has at least 3 major limitations: 1. It is single center: but a single-center approach with a long follow up has been successfully adopted in other studies analyzing antibiotic consumption in pediatrics,12 and we think that the length of our study guarantees the reliability of the results17; 2. It is based on the evaluation of 2 drugs only, even if they are frequently used in severe pediatric infections, and therefore further studies should be performed on other drugs; 3. The study is focused at hospital-level, and therefore further studies should be performed at the level of single wards or group of patients.
In conclusion, stemming from our results we believe that our study “proofs the principle” that DDD provided by WHO can be adopted to monitor antibiotic consumption also in pediatric patients, even if further studies could be needed in specific pediatric populations and drugs, together with analyses on the appropriate use of antibiotics. DDD should not be considered an exact picture of actual use, but a “measure unit” to estimate drug consumption and providing a fixed unit to perform comparisons,1 and like all measurement systems it requires to clearly establish the “reference system”: indeed, a distance between two points is always the same even if measured with different metric systems (e.g., meters or yards). At the most, the order of magnitude changes, generally based on a “fixed” coefficient.
Conflicts of interestThe authors confirm that there are no conflicts of interest.
We gratefully thank the Ministero della Salute - Ricerca Corrente 2022 for its support.