The aim of this study was to evaluate the association between biomass formation and the clinical characteristics and prognosis of Staphylococcus aureus infective endocarditis (IE).
MethodsWe prospectively studied 209 S. aureus strains causing IE. Biomass formation was examined using the crystal violet assay and quantified spectrophotometrically. The average (SD) optical density of the biomass was compared for each clinical, microbiological (methicillin-resistance, vancomycin MIC≥1.5μg/ml) and molecular (clonal complex, agr type and agr dysfunction) variable according to their presence or absence. The primary clinical endpoints studied were in-hospital death, severe sepsis, persistent bacteraemia, symptomatic peripheral embolisms and prosthetic valve IE.
ResultsMean age was 66.1 years, 61.5% of patients were male and the median age-adjusted Charlson comorbidity index was 5 points (IQR 3–8). In-hospital mortality was 37.3%. Strains belonging to CC5 and CC22 had optical biomass densities [mean (SD) 1.573 (1.14) vs 0.942 (0.98) p<0.001 and 1.720 (0.94) vs 1.028 (1.04) p=0.001, respectively]. Strains belonging to CC5 and CC22 had significantly higher optical biomass densities [1.369 (1.18) vs 0.920 (0.93) p=0.008]. No statistically significant differences were found in the clinical endpoints studied.
ConclusionsHigh biomass production was associated with CC5 and CC22 but not with higher hospital mortality, septic complications, type of endocarditis, methicillin-resistance, elevated vancomycin MIC or agr dysfunction.
La bacteriemia por Staphylococcus aureus es un problema de salud importante asociado a una elevada mortalidad. El objetivo de este estudio fue evaluar la asociación entre la capacidad de formación de biomasa y las características clínicas y el pronóstico de la endocarditis infecciosa (EI) por Staphylococcus aureus.
MétodosSe estudiaron de forma prospectiva 209 cepas de S. aureus causantes de episodios de EI. La formación de biomasa se estudió mediante la técnica de cristal violeta y se cuantificó por espectrometría. La media (DE) de la densidad óptica de la biomasa se comparó para cada variable clínica, microbiológica (resistencia a la meticilina, CMI de vancomicina ≥1,5μg/ml) y molecular (complejo clonal, tipo y disfunción de agr) según su presencia o ausencia. El criterio principal de valoración fue la mortalidad hospitalaria. Otras variables clínicas evaluadas fueron: septicemia grave, bacteriemia persistente, embolias periféricas sintomáticas y EI sobre válvula protésica.
ResultadosLa edad media (DE) fue de 66,1 (16,2) años, el 61,5% eran varones y la mediana del índice de comorbilidad de Charlson ajustado a la edad fue de 5 puntos (RIC 3-8). La mortalidad hospitalaria fue del 37,3%. Las cepas pertenecientes a CC5 y CC22 presentaron densidades ópticas de biomasa significativamente más elevadas (media [DE] 1,573 [1,14] frente a 0,942 [0,98] p<0,001 y 1,720 [0,94] frente a 1,028 [1,04]; p=0,001, respectivamente). Las cepas pertenecientes a los grupos agrII mostraron mayores densidades ópticas de biomasa (1,369 [1,18] frente a 0,920 [0,93]; p=0,008). No se observaron diferencias estadísticamente significativas en las variables clínicas estudiadas.
ConclusionesLa producción elevada de biomasa se asoció a determinados linajes clonales (CC5 y CC22), pero no se asoció a una mayor mortalidad hospitalaria, complicaciones sépticas, tipo de endocarditis, resistencia a la meticilina, CMI de vancomicina elevada o disfunción del agr.
Infective endocarditis (IE) is an uncommon and severe disease with in-hospital mortality around 20%,1 being greater than 30% in infections due to Staphylococcus aureus.2 The poor prognosis of S. aureus bacteremia is conditioned by factors associated with the host, the management of infection, and the intrinsic characteristics of the bacteria. Among the latter, there is a great need to determine whether factors other than antimicrobial resistance that are related to the prognosis can assist with decision making. In this sense, the production of biofilms is considered a variable that is associated with a worse prognosis although scientific evidence is scarce.
Recently, our group published a prospective study of 213 consecutive episodes of S. aureus IE with the aim of evaluating the impact of the phenotype and genotype on the clinical characteristics and prognoses of these patients.2 However, despite a recent study of 485 episodes of S. aureus bacteremia from different sources of infection found no relationship between biofilm production and a worse prognosis (30-day related mortality, infective endocarditis (IE), persistent bacteremia or recurrent bacteremia),3 we aimed to analyze the relationship between the biomass formation capacity and the clinical characteristics and prognosis of 209 consecutive episodes of S. aureus IE.
MethodsA detailed description of the study design can be found elsewhere.2 Briefly, a multicenter, longitudinal, prospective, observational study was performed in 15 hospitals with broad experience in IE. Between June 2013 and March 2016, consecutive adult patients (≥18 years) with a definite diagnosis of S. aureus IE were enrolled in the study.
Clinical, microbiological and prognostic variables were collected. The primary outcome was in-hospital death. Other clinical variables were healthcare-associated acquisition, previous use of antibiotics, severe sepsis/septic shock, persistent bacteremia 3 days after starting antimicrobial therapy, persistent bacteremia 5 days after starting antimicrobial therapy, symptomatic peripheral embolism (any), symptomatic osteoarticular involvement, native valve only IE, prosthetic valve IE, intracardiac device IE, surgery indicated and performed or indicated but nor performed, and a composite end-point including persistent bacteremia 3 or 5 days after starting antimicrobial therapy plus symptomatic peripheral embolism (any).
In-hospital death was defined as all-cause death during the hospital stay. For this study, persistent bacteremia was registered as a categorical variable (demonstration of positive blood cultures ≥3 and ≥5 days after initiation of active antimicrobial therapy).
The first isolate from each patient was included. Blood cultures were processed and bacterial identification and antimicrobial susceptibility testing were performed according to standard techniques. Vancomycin MICs were determined by the E-test method. Bacterial DNA was extracted using commercial extraction kits (Qiagen, Germany) according to the manufacturer's recommendations. DNA microarrays (Alere, Germany)4 covering species-specific markers, clonal complex (CC) and agr group typing markers were run for the entire collection of strains.
The functionality of the agr operon was measured by δ-hemolysin production as determined by streaking each S. aureus IE isolate adjacent to S. aureus strain RN4220 on a trypticase soy agar plate supplemented with 5% sheep blood and incubating overnight at 37°C.5
Biomass production by the bacterial strains was assessed according to the protocol described by Stepanović et al.6 with slight modifications. Briefly, biofilm growth was set up in 96-well polystyrene plates with the bacterial suspension (200μL containing 1.0×106colony-forming units/mL) in trypticase soy broth (TSB, Becton Dickinson and Company, Le Pont de Claix, France) supplemented with 1% glucose (Sigma-Aldrich Co., Madrid, Spain). The plates were incubated for 24h at 37°C under static conditions. Following incubation, the supernatant was discarded, and the wells were washed twice with distilled water. The plates were air-dried for 1h at 37°C and stained with 200μL/well of 0.1% crystal violet solution (Sigma-Aldrich Co., Madrid, Spain) at room temperature for 15min. The plates were washed twice with distilled water, and 200μL of 33% glacial acetic acid (VWR International Eurolab, Barcelona, Spain) was added to each well. The optical density (OD) was measured at 590nm using a microplate spectrophotometer (ELx800, Biotek, VT, USA). The assay was performed in triplicate.
The average optical density of the biomass was compared for each clinical, microbiological and molecular variable according to their presence or absence, using the Mann–Whitney test. Statistical significance was set at p<0.05, and the hypotheses were two-sided.
This study was approved by the Ethics Committee of each participating hospital and the Spanish Drug Agency (IRH-ANT 2013-01).
ResultsThe original study included 213 consecutive episodes of definitive S. aureus IE [mean age 66.1 years (SD 16.2), 61.5% male, median age-adjusted Charlson comorbidity index 5 points (IQR 3–8)]. In four of these episodes, the CC of the strain causing the infection could not be determined. Thus, we used 209 strains in the present study.
In the subgroups of patients analyzed, 111 (53.1%) infections were healthcare-associated, 143 (68.4%) affected native valves only, 39 (18.7) prosthetic valves, and 27 (12.9%) intracardiac devices. In 41 (19.6%) the infection was caused by a methicillin-resistant strain. Severe sepsis/septic shock was present in 95 (45.4%). After starting active treatment, persistent bacteremia at day 3 was demonstrated in 60 (28.7%) and at day 5 in 36 (17.2%). Symptomatic peripheral embolism was present in 103 (49.3%) and symptomatic osteoarticular metastasis in 25 (12.0%). Surgery was indicated in 133 (63.6%) but performed only in 79 (37.8%). In-hospital mortality was 37.3%.
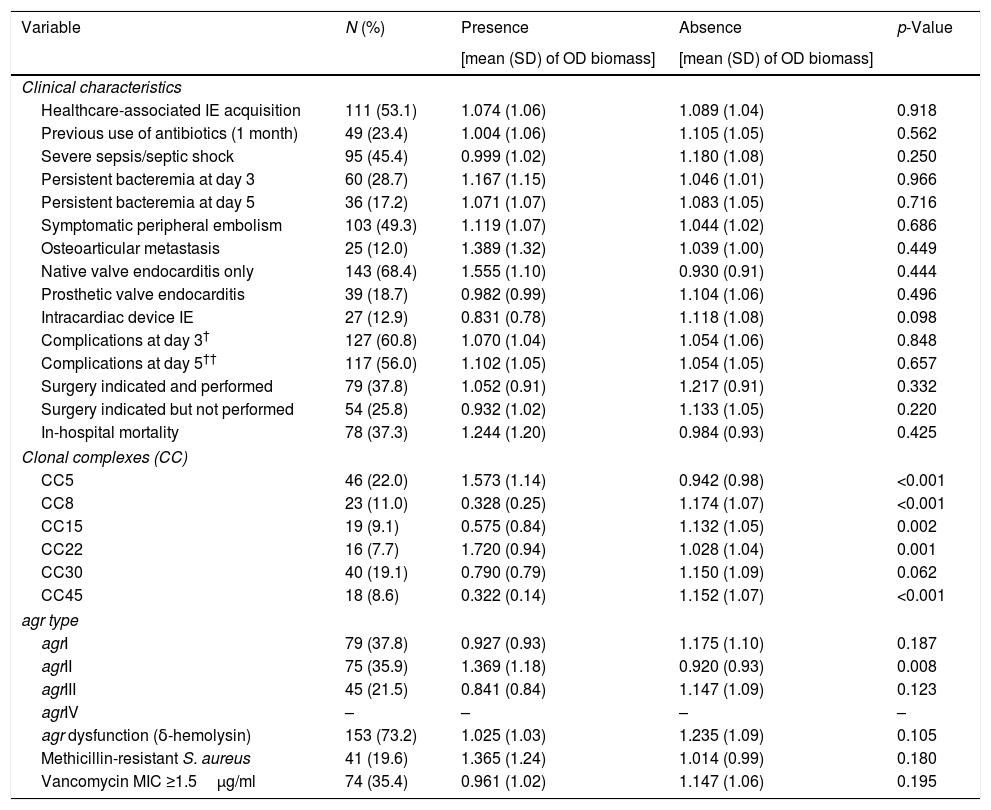
Table 1 compares the mean (SD) optical densities of biomass for the 209 S. aureus strains included in the study according to the presence or absence of clinical, microbiological and molecular variables. No statistically significant differences were found in the optical densities [OD (SD)] of the biomass between strains causing IE with and without clinical complications, including in-hospital death [1.244 (1.20) vs 0.984 (0.93), p=0.425]. Additionally, no statistically significant differences were observed in the optical densities of the biomass according to the type of endocarditis [native valve only 1.555 (1.10) vs 0.930 (0.91), p=0.444; prosthetic valve 0.982 (0.99) vs 1.104 (1.06), p=0.496; intracardiac device 0.831 (0.78) vs 1.118 (1.08), p=0.098], healthcare-associated infection [1.074 (1.06) vs 1.089 (1.04), p=0.918] or previous use of antibiotics [1.004 (1.06) vs 1.105 (1.05), p=0.562]. Strains belonging to CC5 and CC22 had significantly higher biomass optical densities [1.573 (1.14) vs 0.942 (0.98), p=<0.001 and 1.720 (0.94) vs 1.028 (1.04), p=0.001, respectively], which was in contrast to the strains belonging to CC8, CC15 and CC45, which had significantly lower optical densities [0.328 (0.25) vs 1.174 (1.07), p<0.001; 0.575 (0.84) vs 1.132 (1.05), p=0.002; and 0.322 (0.14) vs 1.152 (1.07), p<0.001, respectively]. The strains belonging to the agrII group showed a biomass optical density that was significantly higher than that of the rest of the agr groups [1.369 (1.18) vs 0.920 (0.93), p=0.008]. Finally, no differences were found in agr dysfunction, methicillin resistance or vancomycin MICs.
Comparison of the mean (SD) optical density of biomass according to the presence or absence of clinical, microbiological and molecular variables in 209 strains of S. aureus causing definite infective endocarditis.
| Variable | N (%) | Presence | Absence | p-Value |
|---|---|---|---|---|
| [mean (SD) of OD biomass] | [mean (SD) of OD biomass] | |||
| Clinical characteristics | ||||
| Healthcare-associated IE acquisition | 111 (53.1) | 1.074 (1.06) | 1.089 (1.04) | 0.918 |
| Previous use of antibiotics (1 month) | 49 (23.4) | 1.004 (1.06) | 1.105 (1.05) | 0.562 |
| Severe sepsis/septic shock | 95 (45.4) | 0.999 (1.02) | 1.180 (1.08) | 0.250 |
| Persistent bacteremia at day 3 | 60 (28.7) | 1.167 (1.15) | 1.046 (1.01) | 0.966 |
| Persistent bacteremia at day 5 | 36 (17.2) | 1.071 (1.07) | 1.083 (1.05) | 0.716 |
| Symptomatic peripheral embolism | 103 (49.3) | 1.119 (1.07) | 1.044 (1.02) | 0.686 |
| Osteoarticular metastasis | 25 (12.0) | 1.389 (1.32) | 1.039 (1.00) | 0.449 |
| Native valve endocarditis only | 143 (68.4) | 1.555 (1.10) | 0.930 (0.91) | 0.444 |
| Prosthetic valve endocarditis | 39 (18.7) | 0.982 (0.99) | 1.104 (1.06) | 0.496 |
| Intracardiac device IE | 27 (12.9) | 0.831 (0.78) | 1.118 (1.08) | 0.098 |
| Complications at day 3† | 127 (60.8) | 1.070 (1.04) | 1.054 (1.06) | 0.848 |
| Complications at day 5†† | 117 (56.0) | 1.102 (1.05) | 1.054 (1.05) | 0.657 |
| Surgery indicated and performed | 79 (37.8) | 1.052 (0.91) | 1.217 (0.91) | 0.332 |
| Surgery indicated but not performed | 54 (25.8) | 0.932 (1.02) | 1.133 (1.05) | 0.220 |
| In-hospital mortality | 78 (37.3) | 1.244 (1.20) | 0.984 (0.93) | 0.425 |
| Clonal complexes (CC) | ||||
| CC5 | 46 (22.0) | 1.573 (1.14) | 0.942 (0.98) | <0.001 |
| CC8 | 23 (11.0) | 0.328 (0.25) | 1.174 (1.07) | <0.001 |
| CC15 | 19 (9.1) | 0.575 (0.84) | 1.132 (1.05) | 0.002 |
| CC22 | 16 (7.7) | 1.720 (0.94) | 1.028 (1.04) | 0.001 |
| CC30 | 40 (19.1) | 0.790 (0.79) | 1.150 (1.09) | 0.062 |
| CC45 | 18 (8.6) | 0.322 (0.14) | 1.152 (1.07) | <0.001 |
| agr type | ||||
| agrI | 79 (37.8) | 0.927 (0.93) | 1.175 (1.10) | 0.187 |
| agrII | 75 (35.9) | 1.369 (1.18) | 0.920 (0.93) | 0.008 |
| agrIII | 45 (21.5) | 0.841 (0.84) | 1.147 (1.09) | 0.123 |
| agrIV | – | – | – | – |
| agr dysfunction (δ-hemolysin) | 153 (73.2) | 1.025 (1.03) | 1.235 (1.09) | 0.105 |
| Methicillin-resistant S. aureus | 41 (19.6) | 1.365 (1.24) | 1.014 (0.99) | 0.180 |
| Vancomycin MIC ≥1.5μg/ml | 74 (35.4) | 0.961 (1.02) | 1.147 (1.06) | 0.195 |
Data are expressed as the mean (standard deviation). IE: infective endocarditis.
In this study, high biomass production was not associated with higher crude in-hospital mortality, septic complications, type of endocarditis, healthcare-associated infections or previous use of antibiotics. Moreover, it was also not associated with methicillin resistance, elevated vancomycin MICs, or agr dysfunction. However, strains belonging to the clonal complexes CC5 and CC22 showed high biomass production, which was in contrast to the strains belonging to CC8, CC15 and CC45, which were related to lower biomass production. Finally, strains belonging to the agrII group showed a biomass optical density that was significantly higher than that of the rest of the agr groups.
Classically, the biofilm production capacity has been considered a key factor that explains the morbidity and mortality of infections caused by S. aureus.7 However, this hypothesis has not been proven through clinical studies.
S. aureus bacteremia is not a homogeneous entity but instead is a sign of the spread of an infection that can originate in very diverse locations. Unlike the study of Guembe et al.,3 which included bacteremia due to S. aureus of any origin and in which no subgroups were analyzed, our study included only S. aureus strains causing definite episodes of IE. However, our study confirms the hypothesis that a high biomass production capacity is not related to a worse prognosis.
In the present study, CC5 and CC22 were the clonal complexes related to higher biomass production. However, none of these complexes was associated with mortality or complications in the previous clinical study.2 These results are consistent with those of another study of clinically invasive isolates, in which CC5 proved to be a strong biofilm producer.8 Conversely, CC8 and CC15, which were clonal complexes that produced fewer biomass, were respectively associated with higher in-hospital mortality and higher early mortality (≤2 days). Finally, CC45, which was the other clonal complex that produced fewer biomass, was related to infections on prosthetic valves and intracardiac devices.2
S. aureus infective endocarditis is an acute infection.2 This characteristic could justify the absence of a clinical influence of biomass production showed in this study. Whatever the reason, our results are consistent with those of a recent study conducted on 159 S. aureus strains causing invasive infections, in which the strains causing endocarditis did not show a greater capacity for biofilm formation nor the production of biofilm was associated to complicated bacteremia, severe sepsis, 28-day mortality or recurrence.9
No study is exempt from limitations. First, although the crystal violet assay is the most widely used method to quantify biomass production, we did not evaluate the metabolic activity of biofilm by means of XTT assay.10 Moreover, features such as host blood flow, serum proteins and other components of the immune system are not accurately reflected in the in vitro model. Second, we only measured biomass production by the first isolate from each episode; therefore, we do not know whether biomass production would have occurred in the strains of persistent isolates. Third, the correlation between the phenotypic test for the detection of agr operon dysfunction and its expression is not always good, and therefore the most appropriate technique would be quantification of RNAIII expression (gene effector of the agr operon).11,12
In conclusion, in this study high biomass production was associated with some clonal lineages (CC5 and CC22) but was not associated with higher hospital mortality, septic complications or other predisposing factors.
InvestigatorsCoporació Sanitària Parc Taulí: D. Fontanals, O. Gasch. Hospital de Barcelona: Y. Meije, M. Sierra. Hospital de la Santa Creu i Sant Pau: E. Gil Olivas, M. Gurgui, M.K. Lamarca, B. Mirelis, A. Rivera. Hospital San Pedro: J.M. Azcona Gutiérrez, L. García-Alvarez, J.A. Oteo. Hospital Universitari de Bellvitge: C. Ardanuy, J. Carratalà, G. Cuervo, C. Pena. Hospital Universitari Germans Trias i Pujol: L. Mateu, S. Molinos. Hospital Universitari Vall d’Hebron: B. Almirante, J. Basas, N. Fernández-Hidalgo, Joan Gavaldà, J.J. González-López, M.N. Larrosa, A. Ribera. Hospital Universitario 12 de Octubre: F. Chaves, J. Origüen, D. Pérez-Montarelo, E. Viedma. Hospital Universitario Cruces: J.L. Hernández, M. Montejo, R. Rodríguez Alvarez. Hospital Universitario de la Princesa: C. de las Cuevas, C. Sáez Béjar, C. Sarrià Cepeda. Hospital Universitario Marqués de Valdecilla: M.C. Fariñas, M. Cobo Belaustegui, C. González-Rico, M. Gutiérrez-Cuadra, L. Martínez Martínez. Hospital Universitario Puerta de Hierro: E. Múñez, M. Muñoz Algarra, B. Orden, A. Ramos. Hospital Universitario Virgen de la Victoria: J. Ruiz-Morales, M.V. García López. Hospital Universitario Virgen del Rocío: A. de Alarcón, J.A. Lepe. Hospital Universitario Virgen Macarena: M. de Cueto, J. Gálvez-Acebal.
FundingThis work was supported by Fondo de Investigación Sanitaria (FIS), Ministerio de Sanidad, Instituto de Salud Carlos III (PI12/01719, PI12/01205, PI15/02013 and PI15/02125), the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, co-financed by the European Development Regional Fund A Way to Achieve Europe ERDF, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0003 and REIPI RD16/0016/0002), and CIBER de Epidemiología y Salud Pública (CIBERESP), group CB06/02/0009. The funders had no role in study design, data collection and interpretation, or the decisión to submit the work for publication.
Conflict of interestAll authors declare that they have no conflicts of interest.