This study compares the severity of SARS-CoV-2 infections caused by Alpha, Delta or Omicron variants in periods of co-circulation in Spain, and estimates the variant-specific association of vaccination with severe disease.
MethodsSARS-CoV-2 infections notified to the national epidemiological surveillance network with information on genetic variant and vaccination status were considered cases if they required hospitalisation or controls otherwise. Alpha and Delta were compared during June–July 2021; and Delta and Omicron during December 2021–January 2022. Adjusted odds ratios (aOR) were estimated using logistic regression, comparing variant and vaccination status between cases and controls.
ResultsWe included 5,345 Alpha and 11,974 Delta infections in June–July and 5,272 Delta and 10,578 Omicron in December–January. Unvaccinated cases of Alpha (aOR: 0.57; 95% CI: 0.46–0.69) or Omicron (0.28; 0.21–0.36) had lower probability of hospitalisation vs. Delta. Complete vaccination reduced hospitalisation, similarly for Alpha (0.16; 0.13–0.21) and Delta (June–July: 0.16; 0.14–0.19; December–January: 0.36; 0.30–0.44) but lower from Omicron (0.63; 0.53–0.75) and individuals aged 65+ years.
ConclusionResults indicate higher intrinsic severity of the Delta variant, compared with Alpha or Omicron, with smaller differences among vaccinated individuals. Nevertheless, vaccination was associated to reduced hospitalisation in all groups.
El objetivo es comprar la gravedad de las infecciones por las variantes Alfa, Delta y Ómicron del SARS-CoV-2 en periodos de co-circulación en España, y estimar la asociación entre vacunación y gravedad en cada variante.
MétodosLas infecciones por SARS-CoV-2 notificadas a la red nacional de vigilancia epidemiológica con información sobre la variante viral y el estado de vacunación se clasificaron como casos si habían requerido hospitalización, o como controles en caso contrario. Alfa y Delta se compararon durante junio-julio de 2021, y Delta y Ómicron durante diciembre de 2021-enero de 2022. Se estimaron odds ratios ajustadas (ORa) mediante regresión logística, comparando la variante y el estado de vacunación entre casos y controles.
ResultadosSe incluyeron 5.345 infecciones por variante Alfa y 11.974 por Delta en junio-julio y 5.272 infecciones por Delta y 10.578 por Ómicron en diciembre-enero. Los casos no vacunados por Alfa (aOR: 0,57; IC 95%: 0,46-0,69) u Ómicron (0,28; IC 95%: 0,21-0,36) tuvieron menor probabilidad de hospitalización comparados con Delta. La vacunación completa se asoció a menor hospitalización de forma similar para Alfa (0,16; IC 95%: 0,13-0,21) y Delta (junio-julio: 0,16; IC 95%: 0,14-0,19; diciembre-enero: 0,36; IC 95%: 0,30-0,44) pero menor para Ómicron (0,63; IC 95%: 0,53-0,75) y para individuos con 65+ años.
ConclusiónLos resultados indican una mayor gravedad intrínseca de la variante Delta comparada con Alfa u Ómicron, con menor diferencia entre personas vacunadas. La vacunación se asoció a menor hospitalización en todos los grupos.
Since SARS-CoV-2 emerged in December 2019, the virus has evolved resulting in a wide range of genetic variability. Variants associated to increased transmissibility or immune escape have rapidly expanded to become dominant worldwide. Indeed the protection conferred by vaccination against SARS-CoV-2 infection was found lower for Delta (B.1.617.2) and Omicron (B1.1.529) variants, compared to the previously circulating Alpha (B.1.1.7).1–7 In Spain, the first case of SARS-CoV-2 variant Alpha was described in December 2020, the first case of Delta in June 2021, and the first case of Omicron in December 2021.
The severity of COVID-19 has changed through the successive epidemic waves, likely due to increasing population immunity (caused both by vaccination and by ongoing virus circulation), to the shift of the epidemic to younger age groups and possibly to a different intrinsic virulence of SARS-CoV-2 variants. Disentangling the contribution of each factor is challenging.8 While Delta variant was generally (though inconsistently) associated to increased risk of severe disease compared to the previously dominant Alpha variant,9–11 results from South Africa,12,13 Canada,14 the US,15 the UK,10 Denmark,11 Norway,16 Sweden17 and Navarre (Spain)18 have pointed to a lower severity of Omicron. On the other hand, vaccine effectiveness (VE) against severe COVID-19 with Delta variant was found well preserved compared to Alpha,19,20 but evidence for severe COVID-19 with Omicron is less consistent.5–7,21–23
There are contextual factors that may affect the estimates of variant severity as well as the variant-specific vaccine effectiveness, such as the intensity of previous circulation of other SARS-CoV-2 variants in the territory or particular characteristics of the COVID-19 vaccination rollout. Thus, results from different contexts can add to existing evidence and confirm previous findings. The objective of our study is to evaluate the comparative severity, in terms of requiring hospital admission, of COVID-19 cases caused by Alpha, Delta or Omicron variants in periods where they co-circulated in Spain, as well as to estimate the association of vaccination with the evolution to severe disease, using nationally representative data from Spain.
Materials and methodsWe analysed data from the National Epidemiological Surveillance Network (RENAVE, by its Spanish acronym). Within RENAVE, Spanish regions notify, daily and exhaustively, every case of SARS-CoV-2 infection, together with basic clinical and epidemiological data, including the need of hospital admission due to COVID-19 (excluding hospital admissions with COVID-19, but not due to COVID-19). Genetic characterization by Whole Genome Sequencing is also collected when available. Finally, information on COVID-19 vaccination includes dates and type of every vaccine dose. Data was extracted on 19th April 2022.
We selected SARS-CoV-2 infections in people ≥12 years of age, with known SARS-CoV-2 variant in the RENAVE database. By system design, a random sample of SARS-CoV-2 positive tests are sent for sequencing within the National Laboratory Network for SARS-CoV-2 Sequencing (RELECOV). However, these are sampled separately in hospital settings and from primary health care, with pre-specified proportions. Moreover, other non-randomly selected sequenced samples were also be performed ad hoc by the centres, and these were not appropriately identified in the data. As a result, it is not possible to assess the risk of hospitalisation in the sample of SARS-CoV-2 infections with known variant information.
Instead, to address the study question, we designed a case–control study in which SARS-CoV-2 infections were classified as cases if they required hospital admission or as controls if they did not. SARS-CoV-2 infections with unknown information on hospitalisation were dropped. We selected only SARS-CoV-2 infections with reference date (date of symptom onset or, if missing, date of diagnosis minus 3 days) in periods of co-circulation of different SARS-CoV-2 variants: June and July 2021 for the comparison between Alpha and Delta variants, and December 2021 and January 2022 for the comparison between Delta and Omicron variants.24
At the reference date for each SARS-CoV-2 infection, we classified the vaccination status as: (1) non-vaccinated, if the person had never received any vaccine-dose or (2) completely vaccinated, if the case had received either 2 vaccine-doses (separated a minimum of 19 days for Pfizer vaccine or 21 days for Moderna and Astra Zeneca vaccines) or 1 vaccine dose of Janssen, and with a minimum elapsed time since the last vaccine dose (induction period of the vaccine effect) of 7 days for Pfizer vaccine or 14 days for the others. Persons completely vaccinated who had received a booster vaccine dose were analysed in the same category as those completely vaccinated without a booster dose as main analysis. SARS-CoV-2 infections with unknown vaccination status or incompletely vaccinated were dropped from the analysis.
We first analysed the association between SARS-CoV-2 variant and the need of hospital admission by comparing the distribution of SARS-CoV-2 variants between hospitalised cases and non-hospitalised controls. We performed this estimate separately for non-vaccinated and completely vaccinated persons in the study sample. We then analysed the association between vaccination status and the need of hospital admission (cases vs. controls) separately for each SARS-CoV-2 variant to compute variant-specific association of vaccination status and probability of hospital admission. We estimated the adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) by fitting logistic regression models adjusted by sex, age (restricted cubic splines), autonomous community and calendar week. Stratified models were implemented using interaction terms.
As sensitivity analyses: (1) we excluded, from the category of completely vaccinated, persons who had received a booster dose, to analyse the association of vaccination without any possible confounding due to the higher frequency of persons with a booster dose among the Omicron infections; (2) we considered that individuals in RENAVE with no information on hospitalisation were non-hospitalized and included them in the study as controls; (3) we considered that individuals in RENAVE with no information on vaccination were non-vaccinated and included them in the study in that category; (4) we restricted the sample of cases to hospitalised persons with date of diagnosis previous to date of admission, to increase the probability that the hospitalisation was due to COVID-19 and not with COVID-19 as an incidental finding; and (5) we adjusted all models by previous SARS-CoV-2 infection as reported in RENAVE, though completeness of this variable is low and many infections may not have been documented throughout the epidemic. We used Stata statistical software, release 17 (College Station, TX: StataCorp LLC).
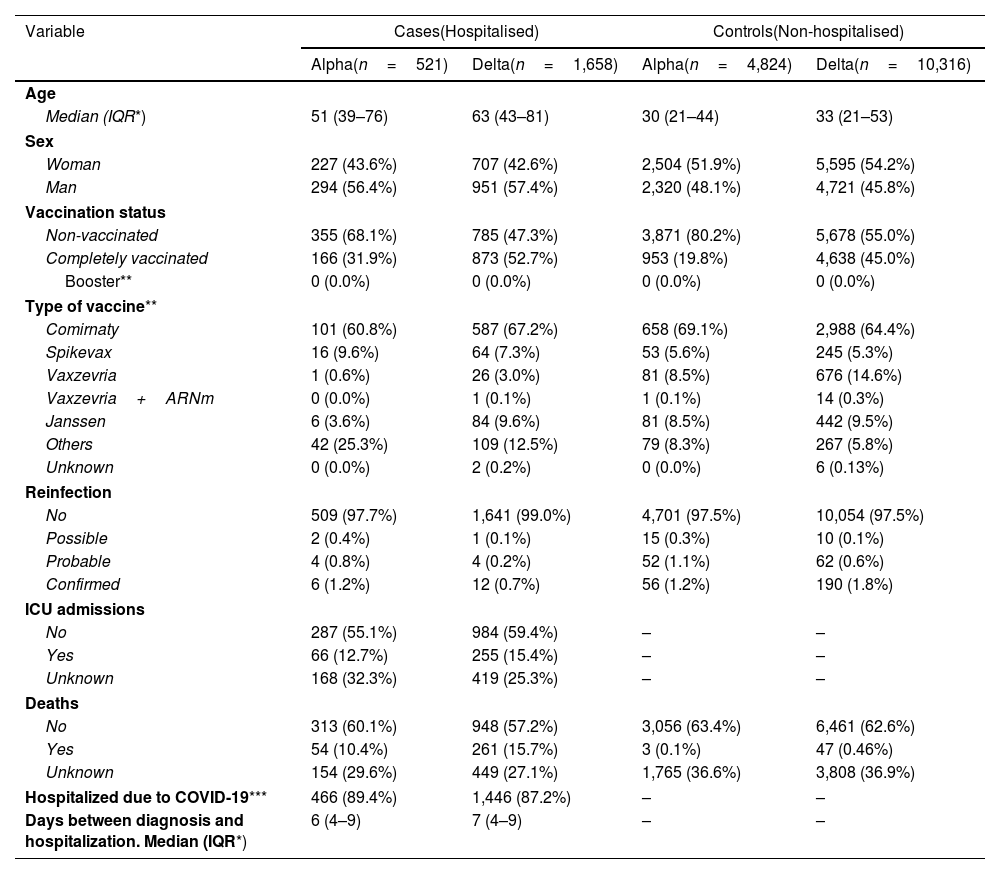
ResultsComparison of Alpha and Delta variants (June and July 2021)Of a total 869,284 SARS-CoV-2 notified infections in June and July 2021, 5,345 Alpha and 11,974 Delta infections were selected (Supplementary Figure S1a). Of total infections, 17.8% were excluded for missing data on hospitalisation and a further 16% for missing data on vaccination. Sequencing was more frequent among vaccinated (16.0% in hospitalized and 6.3% in non-hospitalized) than among unvaccinated (7.6% in hospitalized and 2.5% in non-hospitalized), likely explained by the policy to prioritise breakthrough infections for sequencing at that time. Included and not included patients were similar in key characteristics (Supplementary Table S1).
In the selected sample, 68% of (non-hospitalised) controls were Delta infections, compared to 76% of (hospitalised) cases (Table 1). Cases were more frequently male, had higher age and were more frequently vaccinated compared to controls, probably because the completely vaccinated population included people of higher risk. Delta infections had higher median age and an age distribution more skewed to higher ages (Supplementary Figure S2a). Complete vaccination was more frequent among Delta infections, probably because they occurred later into the study period (Supplementary Figure S3a).
Characteristics of the sample of hospitalized cases and non-hospitalised controls. Sample for comparison of Alpha and Delta variants in June 2021 and July 2021.
| Variable | Cases(Hospitalised) | Controls(Non-hospitalised) | ||
|---|---|---|---|---|
| Alpha(n=521) | Delta(n=1,658) | Alpha(n=4,824) | Delta(n=10,316) | |
| Age | ||||
| Median (IQR*) | 51 (39–76) | 63 (43–81) | 30 (21–44) | 33 (21–53) |
| Sex | ||||
| Woman | 227 (43.6%) | 707 (42.6%) | 2,504 (51.9%) | 5,595 (54.2%) |
| Man | 294 (56.4%) | 951 (57.4%) | 2,320 (48.1%) | 4,721 (45.8%) |
| Vaccination status | ||||
| Non-vaccinated | 355 (68.1%) | 785 (47.3%) | 3,871 (80.2%) | 5,678 (55.0%) |
| Completely vaccinated | 166 (31.9%) | 873 (52.7%) | 953 (19.8%) | 4,638 (45.0%) |
| Booster** | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Type of vaccine** | ||||
| Comirnaty | 101 (60.8%) | 587 (67.2%) | 658 (69.1%) | 2,988 (64.4%) |
| Spikevax | 16 (9.6%) | 64 (7.3%) | 53 (5.6%) | 245 (5.3%) |
| Vaxzevria | 1 (0.6%) | 26 (3.0%) | 81 (8.5%) | 676 (14.6%) |
| Vaxzevria+ARNm | 0 (0.0%) | 1 (0.1%) | 1 (0.1%) | 14 (0.3%) |
| Janssen | 6 (3.6%) | 84 (9.6%) | 81 (8.5%) | 442 (9.5%) |
| Others | 42 (25.3%) | 109 (12.5%) | 79 (8.3%) | 267 (5.8%) |
| Unknown | 0 (0.0%) | 2 (0.2%) | 0 (0.0%) | 6 (0.13%) |
| Reinfection | ||||
| No | 509 (97.7%) | 1,641 (99.0%) | 4,701 (97.5%) | 10,054 (97.5%) |
| Possible | 2 (0.4%) | 1 (0.1%) | 15 (0.3%) | 10 (0.1%) |
| Probable | 4 (0.8%) | 4 (0.2%) | 52 (1.1%) | 62 (0.6%) |
| Confirmed | 6 (1.2%) | 12 (0.7%) | 56 (1.2%) | 190 (1.8%) |
| ICU admissions | ||||
| No | 287 (55.1%) | 984 (59.4%) | – | – |
| Yes | 66 (12.7%) | 255 (15.4%) | – | – |
| Unknown | 168 (32.3%) | 419 (25.3%) | – | – |
| Deaths | ||||
| No | 313 (60.1%) | 948 (57.2%) | 3,056 (63.4%) | 6,461 (62.6%) |
| Yes | 54 (10.4%) | 261 (15.7%) | 3 (0.1%) | 47 (0.46%) |
| Unknown | 154 (29.6%) | 449 (27.1%) | 1,765 (36.6%) | 3,808 (36.9%) |
| Hospitalized due to COVID-19*** | 466 (89.4%) | 1,446 (87.2%) | – | – |
| Days between diagnosis and hospitalization. Median (IQR*) | 6 (4–9) | 7 (4–9) | – | – |
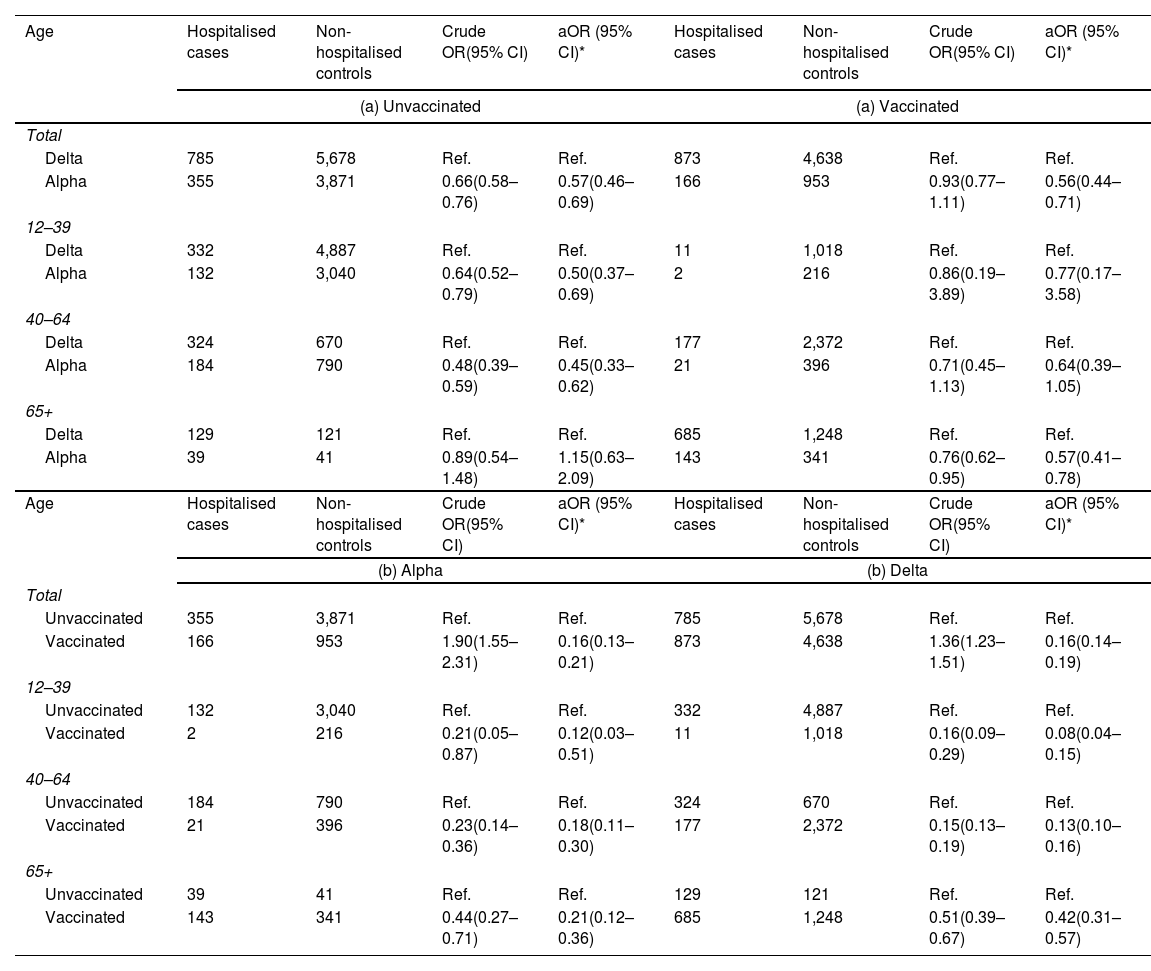
Among the unvaccinated, Alpha was associated to 43% lower probability of hospitalisation compared to Delta (aOR: 0.57; 95% CI: 0.46–0.69), except for people 65+ years where no difference between the variants was observed (Table 2). Among the vaccinated, the lower hospitalisation associated with Alpha infections compared to Delta was observed for all age groups and was 44% lower overall (aOR: 0.56; 95% CI: 0.44–0.71). The lower severity of Alpha in 65+ in the vaccinated (vs. no difference in the unvaccinated) is consistent with the slightly higher association of the vaccine with reduced hospitalisation in 65+ against the Alpha variant (79%; aOR:0.21; 0.12–0.36), compared to the Delta variant (58%; aOR: 0.42; 0.31–0.57).
Odds ratio (OR) and 95% confidence intervals (95% CI) for the association between: (a) SARS-CoV-2 variant Delta vs. Alpha and the odds of hospitalization, stratified by vaccination status and (b) between and vaccination status and the odds of hospitalization, stratified by SARS-CoV-2 variant (June and July 2021).
| Age | Hospitalised cases | Non-hospitalised controls | Crude OR(95% CI) | aOR (95% CI)* | Hospitalised cases | Non-hospitalised controls | Crude OR(95% CI) | aOR (95% CI)* |
|---|---|---|---|---|---|---|---|---|
| (a) Unvaccinated | (a) Vaccinated | |||||||
| Total | ||||||||
| Delta | 785 | 5,678 | Ref. | Ref. | 873 | 4,638 | Ref. | Ref. |
| Alpha | 355 | 3,871 | 0.66(0.58–0.76) | 0.57(0.46–0.69) | 166 | 953 | 0.93(0.77–1.11) | 0.56(0.44–0.71) |
| 12–39 | ||||||||
| Delta | 332 | 4,887 | Ref. | Ref. | 11 | 1,018 | Ref. | Ref. |
| Alpha | 132 | 3,040 | 0.64(0.52–0.79) | 0.50(0.37–0.69) | 2 | 216 | 0.86(0.19–3.89) | 0.77(0.17–3.58) |
| 40–64 | ||||||||
| Delta | 324 | 670 | Ref. | Ref. | 177 | 2,372 | Ref. | Ref. |
| Alpha | 184 | 790 | 0.48(0.39–0.59) | 0.45(0.33–0.62) | 21 | 396 | 0.71(0.45–1.13) | 0.64(0.39–1.05) |
| 65+ | ||||||||
| Delta | 129 | 121 | Ref. | Ref. | 685 | 1,248 | Ref. | Ref. |
| Alpha | 39 | 41 | 0.89(0.54–1.48) | 1.15(0.63–2.09) | 143 | 341 | 0.76(0.62–0.95) | 0.57(0.41–0.78) |
| Age | Hospitalised cases | Non-hospitalised controls | Crude OR(95% CI) | aOR (95% CI)* | Hospitalised cases | Non-hospitalised controls | Crude OR(95% CI) | aOR (95% CI)* |
| (b) Alpha | (b) Delta | |||||||
| Total | ||||||||
| Unvaccinated | 355 | 3,871 | Ref. | Ref. | 785 | 5,678 | Ref. | Ref. |
| Vaccinated | 166 | 953 | 1.90(1.55–2.31) | 0.16(0.13–0.21) | 873 | 4,638 | 1.36(1.23–1.51) | 0.16(0.14–0.19) |
| 12–39 | ||||||||
| Unvaccinated | 132 | 3,040 | Ref. | Ref. | 332 | 4,887 | Ref. | Ref. |
| Vaccinated | 2 | 216 | 0.21(0.05–0.87) | 0.12(0.03–0.51) | 11 | 1,018 | 0.16(0.09–0.29) | 0.08(0.04–0.15) |
| 40–64 | ||||||||
| Unvaccinated | 184 | 790 | Ref. | Ref. | 324 | 670 | Ref. | Ref. |
| Vaccinated | 21 | 396 | 0.23(0.14–0.36) | 0.18(0.11–0.30) | 177 | 2,372 | 0.15(0.13–0.19) | 0.13(0.10–0.16) |
| 65+ | ||||||||
| Unvaccinated | 39 | 41 | Ref. | Ref. | 129 | 121 | Ref. | Ref. |
| Vaccinated | 143 | 341 | 0.44(0.27–0.71) | 0.21(0.12–0.36) | 685 | 1,248 | 0.51(0.39–0.67) | 0.42(0.31–0.57) |
Complete vaccination was overall associated to 84% lower hospitalization, equally for Alpha and Delta, and with a trend to a higher magnitude of the association in the younger age groups.
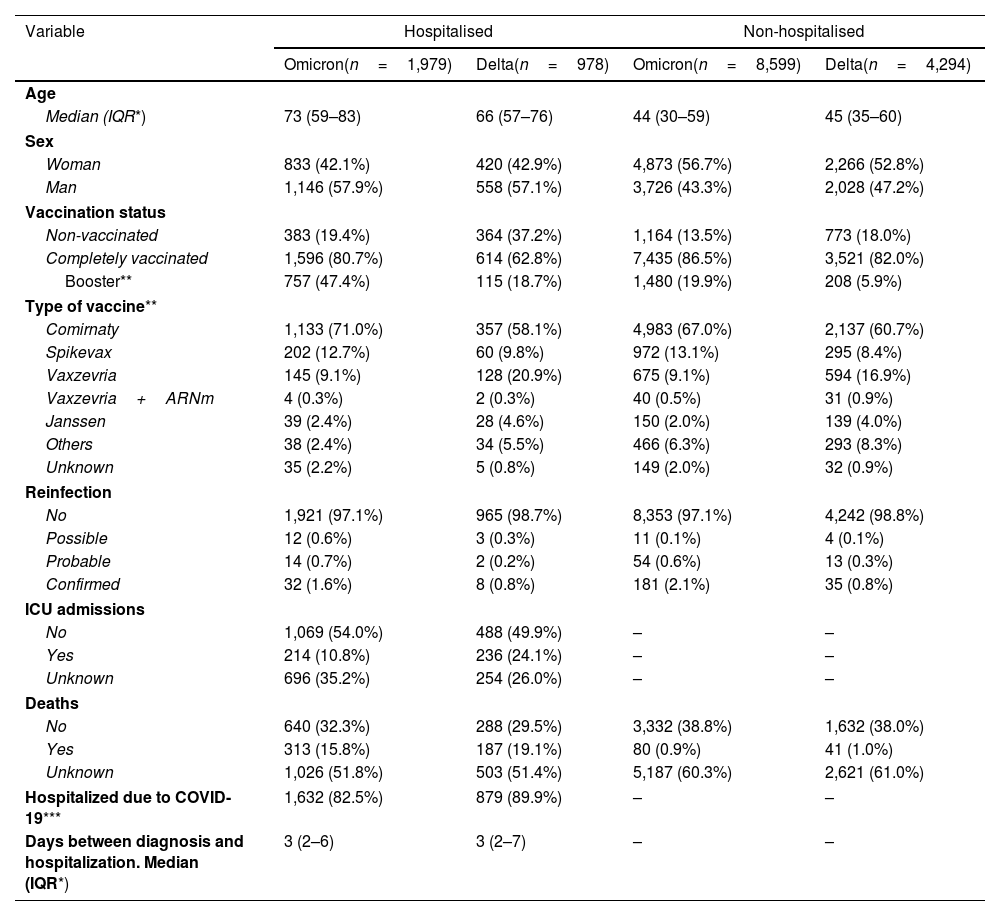
Comparison of Delta and Omicron variants (December 2021 and January 2022)Of 5,070,747 SARS-CoV-2 notified infections, 5,272 Delta and 10,578 Omicron infections were selected (Supplementary Figure S1b). Of total infections, 24.5% were excluded for missing data on hospitalisation and a further 3.4% due to missing data on vaccination among the hospitalised and 7.4% among non-hospitalised. Availability of sequencing results was similar between vaccinated (5.4% in hospitalized and 0.4% in non-hospitalized) and unvaccinated (4.6% in hospitalized and 0.5% in non-hospitalized). Included and not included patients were similar in key characteristics (Supplementary Table S1).
In the selected sample, 67% were Omicron infections, both among the (hospitalised) cases and the (non-hospitalised) controls (Table 3). Cases were more frequently male and had higher age compared to the controls, without meaningful differences between Omicron and Delta, which showed similar age distribution (Supplementary Figure S2b). The proportion of completely vaccinated was lower among cases (both for Delta and Omicron), as expected due to the protection of vaccination. Complete vaccination and vaccination with a booster dose was more frequent among Omicron infections, probably because they occurred later into the study period (Supplementary Figure S3b).
Characteristics of the sample of hospitalized cases and non-hospitalised controls. Sample for comparison of Delta and Omicron variants in December 2021 and January 2021.
| Variable | Hospitalised | Non-hospitalised | ||
|---|---|---|---|---|
| Omicron(n=1,979) | Delta(n=978) | Omicron(n=8,599) | Delta(n=4,294) | |
| Age | ||||
| Median (IQR*) | 73 (59–83) | 66 (57–76) | 44 (30–59) | 45 (35–60) |
| Sex | ||||
| Woman | 833 (42.1%) | 420 (42.9%) | 4,873 (56.7%) | 2,266 (52.8%) |
| Man | 1,146 (57.9%) | 558 (57.1%) | 3,726 (43.3%) | 2,028 (47.2%) |
| Vaccination status | ||||
| Non-vaccinated | 383 (19.4%) | 364 (37.2%) | 1,164 (13.5%) | 773 (18.0%) |
| Completely vaccinated | 1,596 (80.7%) | 614 (62.8%) | 7,435 (86.5%) | 3,521 (82.0%) |
| Booster** | 757 (47.4%) | 115 (18.7%) | 1,480 (19.9%) | 208 (5.9%) |
| Type of vaccine** | ||||
| Comirnaty | 1,133 (71.0%) | 357 (58.1%) | 4,983 (67.0%) | 2,137 (60.7%) |
| Spikevax | 202 (12.7%) | 60 (9.8%) | 972 (13.1%) | 295 (8.4%) |
| Vaxzevria | 145 (9.1%) | 128 (20.9%) | 675 (9.1%) | 594 (16.9%) |
| Vaxzevria+ARNm | 4 (0.3%) | 2 (0.3%) | 40 (0.5%) | 31 (0.9%) |
| Janssen | 39 (2.4%) | 28 (4.6%) | 150 (2.0%) | 139 (4.0%) |
| Others | 38 (2.4%) | 34 (5.5%) | 466 (6.3%) | 293 (8.3%) |
| Unknown | 35 (2.2%) | 5 (0.8%) | 149 (2.0%) | 32 (0.9%) |
| Reinfection | ||||
| No | 1,921 (97.1%) | 965 (98.7%) | 8,353 (97.1%) | 4,242 (98.8%) |
| Possible | 12 (0.6%) | 3 (0.3%) | 11 (0.1%) | 4 (0.1%) |
| Probable | 14 (0.7%) | 2 (0.2%) | 54 (0.6%) | 13 (0.3%) |
| Confirmed | 32 (1.6%) | 8 (0.8%) | 181 (2.1%) | 35 (0.8%) |
| ICU admissions | ||||
| No | 1,069 (54.0%) | 488 (49.9%) | – | – |
| Yes | 214 (10.8%) | 236 (24.1%) | – | – |
| Unknown | 696 (35.2%) | 254 (26.0%) | – | – |
| Deaths | ||||
| No | 640 (32.3%) | 288 (29.5%) | 3,332 (38.8%) | 1,632 (38.0%) |
| Yes | 313 (15.8%) | 187 (19.1%) | 80 (0.9%) | 41 (1.0%) |
| Unknown | 1,026 (51.8%) | 503 (51.4%) | 5,187 (60.3%) | 2,621 (61.0%) |
| Hospitalized due to COVID-19*** | 1,632 (82.5%) | 879 (89.9%) | – | – |
| Days between diagnosis and hospitalization. Median (IQR*) | 3 (2–6) | 3 (2–7) | – | – |
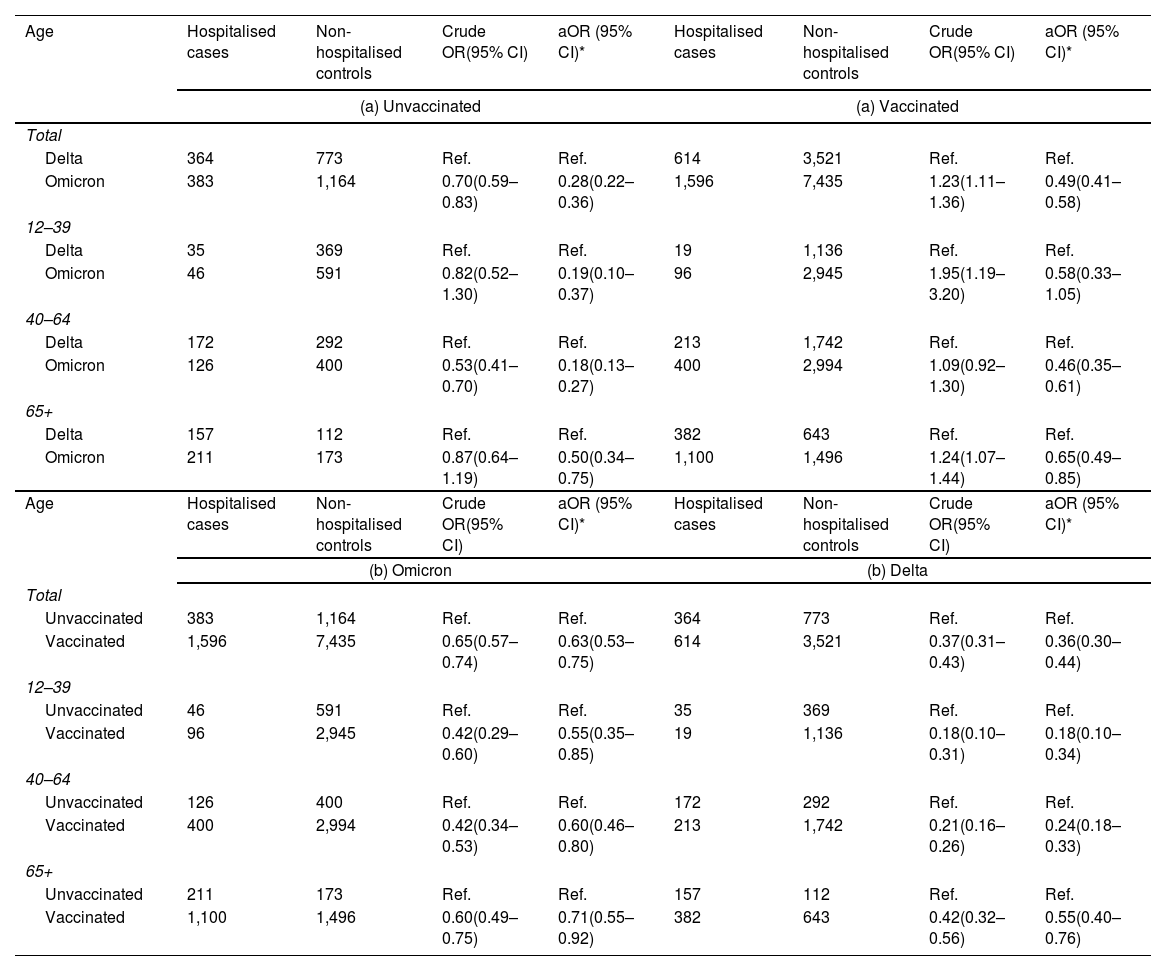
Among the unvaccinated, Omicron was associated to a 72% lower risk of hospitalisation compared to Delta (aOR: 0.28; 0.21–0.36), with a smaller difference among people 65+ (Table 4). The difference in severity between Omicron and Delta was smaller among the vaccinated, decreasing to 51% (aOR: 0.49; 95% CI: 0.41–0.58) overall, without significant differences between age groups.
Odds ratio (OR) and 95% confidence intervals (95% CI) for the association between: (a) SARS-CoV-2 variant Omicron vs. Delta and the odds of hospitalization, stratified by vaccination status and (b) between and vaccination status and the odds of hospitalization, stratified by SARS-CoV-2 variant (December 2021 and January 2022).
| Age | Hospitalised cases | Non-hospitalised controls | Crude OR(95% CI) | aOR (95% CI)* | Hospitalised cases | Non-hospitalised controls | Crude OR(95% CI) | aOR (95% CI)* |
|---|---|---|---|---|---|---|---|---|
| (a) Unvaccinated | (a) Vaccinated | |||||||
| Total | ||||||||
| Delta | 364 | 773 | Ref. | Ref. | 614 | 3,521 | Ref. | Ref. |
| Omicron | 383 | 1,164 | 0.70(0.59–0.83) | 0.28(0.22–0.36) | 1,596 | 7,435 | 1.23(1.11–1.36) | 0.49(0.41–0.58) |
| 12–39 | ||||||||
| Delta | 35 | 369 | Ref. | Ref. | 19 | 1,136 | Ref. | Ref. |
| Omicron | 46 | 591 | 0.82(0.52–1.30) | 0.19(0.10–0.37) | 96 | 2,945 | 1.95(1.19–3.20) | 0.58(0.33–1.05) |
| 40–64 | ||||||||
| Delta | 172 | 292 | Ref. | Ref. | 213 | 1,742 | Ref. | Ref. |
| Omicron | 126 | 400 | 0.53(0.41–0.70) | 0.18(0.13–0.27) | 400 | 2,994 | 1.09(0.92–1.30) | 0.46(0.35–0.61) |
| 65+ | ||||||||
| Delta | 157 | 112 | Ref. | Ref. | 382 | 643 | Ref. | Ref. |
| Omicron | 211 | 173 | 0.87(0.64–1.19) | 0.50(0.34–0.75) | 1,100 | 1,496 | 1.24(1.07–1.44) | 0.65(0.49–0.85) |
| Age | Hospitalised cases | Non-hospitalised controls | Crude OR(95% CI) | aOR (95% CI)* | Hospitalised cases | Non-hospitalised controls | Crude OR(95% CI) | aOR (95% CI)* |
| (b) Omicron | (b) Delta | |||||||
| Total | ||||||||
| Unvaccinated | 383 | 1,164 | Ref. | Ref. | 364 | 773 | Ref. | Ref. |
| Vaccinated | 1,596 | 7,435 | 0.65(0.57–0.74) | 0.63(0.53–0.75) | 614 | 3,521 | 0.37(0.31–0.43) | 0.36(0.30–0.44) |
| 12–39 | ||||||||
| Unvaccinated | 46 | 591 | Ref. | Ref. | 35 | 369 | Ref. | Ref. |
| Vaccinated | 96 | 2,945 | 0.42(0.29–0.60) | 0.55(0.35–0.85) | 19 | 1,136 | 0.18(0.10–0.31) | 0.18(0.10–0.34) |
| 40–64 | ||||||||
| Unvaccinated | 126 | 400 | Ref. | Ref. | 172 | 292 | Ref. | Ref. |
| Vaccinated | 400 | 2,994 | 0.42(0.34–0.53) | 0.60(0.46–0.80) | 213 | 1,742 | 0.21(0.16–0.26) | 0.24(0.18–0.33) |
| 65+ | ||||||||
| Unvaccinated | 211 | 173 | Ref. | Ref. | 157 | 112 | Ref. | Ref. |
| Vaccinated | 1,100 | 1,496 | 0.60(0.49–0.75) | 0.71(0.55–0.92) | 382 | 643 | 0.42(0.32–0.56) | 0.55(0.40–0.76) |
Complete vaccination was associated to lower risk of hospital admission, with a higher magnitude for Delta (64% lower, OR: 0.36; 95% CI: 0.30–0.44) compared to Omicron (37% lower, OR: 0.63; 95% CI: 0.53–0.75). Like in the first study period, there was a trend to a higher association of the vaccine with lower hospitalisation in the younger age groups. Interestingly, the effect of the vaccine against Delta infection was lower in this period (December 2021–January 2022), as compared to the previous one (June–July 2021), compatible with a waning protection with time since vaccination.
Results from sensitivity analysesResults were very similar in sensitivity analyses for both study periods, except when considering persons with missing vaccination status as unvaccinated when assessing the association between vaccination and hospitalisation in June–July 2021 (effect of the vaccine in the sensitivity analysis vs. main analysis of 71% vs. 84% in Alpha infections and 70% vs. 84% in Delta infections) (Supplementary Table S2). The decrease in the association could be due to a certain proportion of vaccinated people among the missing data. In December 2021–January 2022 no difference was observed, possibly due to higher completeness of vaccination data, and to people without information corresponding more likely to people not vaccinated (as seen in Figure S1). In December 2021–January 2022, slightly higher association of the vaccine with lower hospitalisation by the Omicron variant was found when assuming that individuals with missing data on hospitalisation were non-hospitalised (48% vs. 37% in the main analysis), with no differences otherwise (Supplementary Table S3).
DiscussionOur results suggest that unvaccinated persons infected with the Alpha or Omicron SARS-CoV-2 variants had lower probability of hospitalisation compared with the Delta variant. We also found that the probability of hospitalisation was lower in completely vaccinated cases compared with non-vaccinated cases for every variant.
Our results are consistent with previous studies addressing the intrinsic severity of the different SARS-CoV-2 variants. Previous studies have estimated a risk of hospitalisation between 1.85 and 3.0 times higher for Delta compared with Alpha.9–11 A recent study in Navarre (Spain), did not find this difference, but it had wide confidence intervals for this comparison and did not focus on unvaccinated populations.18 For Omicron, first estimates in South Africa showed a risk of hospitalisations 80% lower than for Delta.13 Estimates from Sweden found an approximate 40% lower risk for Omicron in the unvaccinated and about 71% in the vaccinated.17 However, both studies compared consecutive waves of the pandemic, rather than infectious occurring simultaneously, being subject to limitations due to secular changes in the context (see limitations). Using contemporary Delta and Omicron infections, studies in Scotland, England, Navarre (Spain), Norway, Canada and the US, have found a lower risk of hospitalisation for Omicron ranging 40–73%.14,16,18,25–27 Using European-level data, ECDC has estimated that Omicron infection was less likely to be reported with admission to hospital compared to infection with Delta (aOR 0.41; 95% CI: 0.37–0.46).28 All these estimates are in the range of the ones in our study. Also, similarly to our results, in Denmark, the lower risk of hospitalisation with Omicron was more pronounced among the unvaccinated (43% lower) than among people with two doses of vaccine (29% lower)29; and in England,27 the effect in the unvaccinated (70% reduction) was higher than in the full sample (57% reduction), likely because the vaccines masks the severity for Delta infection more than for Omicron. In England, like in our study, the difference in severity was attenuated with age.27 There is overall a considerable variability in the magnitude of the difference in severity between Delta and Omicron, possibly related to the different contexts.8
The association of complete vaccination and reduced hospitalisation was similar for Alpha and Delta. This is consistent with findings of preserved vaccine effectiveness against severe disease for Delta variant.20,30,31 In contrast, association between vaccination and reduced hospitalisation was lower for Omicron, despite the fact that Omicron infections included a higher proportion of people with a booster vaccine dose. Excluding these latter from the analysis yielded identical results. This may be explained by the reduced preventive potential in a less severe variant, and is consistent with previous studies. In the UK, effectiveness in preventing hospitalisation between 2 and 24 weeks of complete vaccination was 67% for Omicron (45–79%) and 90% for Delta (87–91%), decreasing to 51% (19–70%) and 85% (82–87%), respectively, after 25+ weeks.32 In Norway and the US, full vaccination with two or three doses was associated with an overall reduced risk of hospitalisation of 66% and 65%, respectively, for Omicron and 93% and 85% for Delta16,31; in contrast no difference was seen in risk reduction in Denmark.29 Estimates of the effect of the vaccine in our data were around half the one in these studies, although the relative difference between Omicron and Delta was more similar (around half effectiveness for Omicron than for Delta in our study).
Our study has some limitations. First, the proportion of sequenced infections in national surveillance was low, and was higher in SARS-CoV-2 infections requiring hospitalisation. The case–control approach avoids this bias, but assumes that sequencing was performed similarly regarding the underlying variant or the vaccination status within each of the two groups, hospitalised and non-hospitalised. The higher proportion of vaccinated cases that were sequenced and thus included in the study during the first study period makes these results less robust than in the second study period. Also, if sequencing was performed with higher probability in patients with a new variant (for example, following results of a specific PCR), this could also have affected our results. Second, although surveillance data should only collect hospitalisations due to COVID-19, we cannot exclude that an unknown proportion of hospitalisations with a positive result in the COVID-19 admission screening but due to other illness were also included. Third, deaths were not included as severe cases together with hospitalisation, as information on the cause of death in surveillance data was not considered sufficiently reliable. Fourth, some relevant variables such as socioeconomic status or comorbidities were not available in the study, with possible residual confounding. Finally, secular changes need to be taken into account, such as the higher level of overall immunity (both due to previous infection, even if not documented, or due to vaccination) with time. This would show a lower severity of infections that occurred later in time, irrespective of the infecting variant. This is particularly so if a variant is more likely to elude immunity and therefore affect more people with previous infection resulting in less severe forms, as is the case with Omicron.8 Also, incomplete ascertainment of infections due to changing testing recommendations and the increase in the use of self-tests, as has been the case in Spain in January 2022, could affect estimates of severity. In our study, only calendar weeks where infections by both variants were notified were included (as opposed to studies that compare different time periods), and the analysis was adjusted by week of infection, likely reducing these potential biases. In a sensitivity analysis we also adjusted by previous documented infection, with no change in results.
In conclusion, our results provide further evidence of a lower virulence of Omicron and Alpha variants and support vaccination as a key intervention to prevent severe COVID-19, even in the current Omicron-dominant epidemic.
Authors’ contributionsConceptualization, Esteban Aznar, Lucía García San Miguel Rodríguez-Alarcón, Inmaculada Casas Flecha, María José Sierra, Amparo Larrauri and Susana Monge; Data curation, Elena Varea Jimenez, Lorena Vega-Piris, María Iglesias-Caballero and Sonia Vázquez-Morón; Formal analysis, Elena Varea Jimenez; Methodology, Esteban Aznar, Lorena Vega-Piris, Clara Mazagatos, Elena Vanessa Martínez Sánchez, Amparo Larrauri and Susana Monge; Resources, Inmaculada Casas Flecha, María José Sierra and Amparo Larrauri; Supervision, Susana Monge; Validation, Susana Monge; Visualization, Esteban Aznar, Amparo Larrauri and Susana Monge; Writing – original draft, Elena Varea Jimenez; Writing – review & editing, Susana Monge, Esteban Aznar, Clara Mazagatos, Elena Vanessa Martínez Sánchez, Lucía García San Miguel Rodríguez-Alarcón, Inmaculada Casas Flecha, María José Sierra and Amparo Larrauri.
FundingIn this study the identification of variants by genomic sequencing has been partially supported by HERA-Incubator ECDC/GRANT/2021/024-Enhancing Whole Genome Sequencing (WGS) and/or Reverse Transcription Polymerase Chain Reaction (RT-PCR) national infrastructures and capacities to respond to the Covid-19 pandemic in Spain.
Conflicts of interestNone declared.
We acknowledge the contribution of all persons involved in the National Epidemiological Surveillance Network (RENAVE) and the National Laboratory Network for SARS-CoV-2 Sequencing (RELECOV) for their invaluable work in the generation of the data that made this study possible.
*Members of the Working group for the surveillance and control of COVID-19 in Spain:
Adrián Hugo Aginagalde-Llorente (Public Health Observatory of Cantabria, Santander), Alberto Malvar Pintos (Servicio de Epidemiología, DGSP, Santiago de Compostela, Galicia), Ana García-Fulgueiras (Servicio de Epidemiología, DGSPA, Murcia and CIBERESP), Ana Martínez Mateo (Subdirección General de Vigilancia y Respuesta a Emergencias de Salud Pública, Agencia de Salud Pública, CIBERESP, Catalunya), Ana Isabel Rivas Pérez (Servicio de Epidemiología, Consejería de Sanidad, Consumo y Gobernación, Ceuta), Nicola Lorusso (DGSP y Ordenación Farmacéutica, Sevilla, Andalucía), Araceli Alemán Herrera (DGSP, Islas Canarias), Juan Pablo Alonso (Servicio de Vigilancia en Salud Pública, DGSP, Zaragoza, Aragón), Aurelio Barricarte (Sección de Vigilancia de Enfermedades Transmisibles del Instituto de Salud Pública, CIBERESP, Pamplona, Navarra), Jaume Giménez Durán (DGSP i Participació, Illes Balears), Maria del Carmen Pacheco (Servicio de Epidemiología, DGSP, Castilla y León), Daniel Castrillejo (Servicio de Epidemiología, DGSP y Consumo, Melilla), Eva Martinez Ochoa (Servicio de Epidemiología y Prevención Sanitaria, DGSP, Consumo y Cuidados, La Rioja), Juan Antonio Linares Dopido (Subdirección Epidemiología, DGSP, Extremadura), Rosa Carbó Malonda (Servicio de Vigilancia y Control Epidemiológico, DGSPA, Comunidad Valenciana), Ismael Huerta Gonzalez (Servicio de Vigilancia Epidemiológica, DGSP, Asturias), Fernando González Carril (Servicio de Vigilancia y Vacunas, DGSPA, País Vasco), José Francisco Barbas del Buey (Subdirección General de Epidemiología, DGSP, Comunidad de Madrid), Sara García Hernández (Servicio Epidemiología, Castilla-La Mancha), Pedro Arias Bohigas, María Sastre García, Rocío Amillategui Dos Santos, Concha Delgado-Sanz, Jesús Oliva (Centro Nacional de Epidemiología, CIBERESP, ISCIII), Fernando Simón, Patricia Santágueda Balaguer, Mónica Fernández Gorostiza (Centro de Coordinación de Alertas y Emergencias Sanitarias, Ministerio de Sanidad)
**Members of National Laboratory Network for SARS-CoV-2 Sequencing (RELECOV):
Francisco Pozo (Centro Nacional de Microbiología, CIBERESP, ISCIII), Jose Antonio Lepe (Microbiología. Hospital Universitario Virgen del Rocio. Sevilla), Rafael Benito Ruesca (Hospital Clínico Universitario Lozano Blesa), Santiago Melón García (Hospital Universitario Central de Asturias), Carla López-Causapé (Servicio de Microbiología, Hospital Universitario Son Espases, IdISBa, CIBERINFEC), Oscar Díez Gil (Hospital Universitario Ntra. Sra de Candelaria (HUNSC), Jesus Rodríguez Lozano (Hospital Universitario Marqués de Valdecilla), Soledad Illescas (Hospital General Universitario de Ciudad Real), Marta Hernández (Consorcio LUCIA (SACYL,ITACYL UBU,UVa), Andrés Antón (Hospital Universitari Vall d’Hebron), Miguel Fajardo Olivares (Hospital Universitario de Badajoz), Sonia Pérez Castro (Hospital de Vigo), María de Toro Hernando (Plataforma de Genómica y Bioinformática), Darío García de Viedma (Hospital Universitario Gregorio Marañón), Laura Moreno Parrado (Hospital Universitario Virgen de la Arrixaca), Carmen Ezpeleta (Complejo hospitalario de Navarra), Jose Maria Marimon (Hospital Universitario Donostia), Fernando González Candelas (FISABIO CSIC-Epidemiología Molecular-Universidad de Valencia).