To perform a cost-effectiveness analysis of a molecular biology technique for the diagnosis of tuberculosis compared to the classical diagnostic alternative.
MethodsA cost-effectiveness analysis was performed to evaluate the theoretical implementation of a molecular biology method including two alternative techniques for early detection of Mycobacterium tuberculosis Complex, and resistance to rifampicin (alternative 1: one determination in selected patients; alternative 2: two determinations in all the patients). Both alternatives were compared with the usual procedure for microbiological diagnosis of tuberculosis (staining and microbiological culture), and was accomplished on 1972 patients in the period in 2008–2012. The effectiveness was measured in QALYs, and the uncertainty was assessed by univariate, multivariate and probabilistic analysis of sensitivity.
ResultsA value of €8588/QALYs was obtained by the usual method. Total expenditure with the alternative 1 was €8487/QALYs, whereas with alternative 2, the cost-effectiveness ratio amounted to €2960/QALYs. Greater diagnostic efficiency was observed by applying the alternative 2, reaching a 75% reduction in the number of days that a patient with tuberculosis remains without an adequate treatment, and a 70% reduction in the number of days that a patient without tuberculosis remains in hospital.
ConclusionThe implementation of a molecular microbiological technique in the diagnosis of tuberculosis is extremely cost-effective compared to the usual method. Its introduction into the routine diagnostic procedure could lead to an improvement in quality care for patients, given that it would avoid both unnecessary hospitalisations and treatments, and reflected in economic savings to the hospital.
Evaluar mediante un análisis de coste-efectividad la aplicación de una técnica de biología molecular al diagnóstico de tuberculosis frente a la alternativa diagnóstica clásica.
MétodosSe realizó un análisis de coste-efectividad para evaluar la aplicación teórica de un procedimiento de biología molecular que incluye 2 alternativas de una técnica para la detección precoz de Mycobacterium tuberculosis Complex y resistencia a rifampicina (alternativa 1: una determinación a pacientes seleccionados; alternativa 2: 2 determinaciones a todos los pacientes). Ambas alternativas se compararon con el procedimiento habitual de diagnóstico microbiológico de tuberculosis realizado a 1972 pacientes durante 2008-2012 (microscopia y cultivo). La medida de la efectividad se hizo en QALY y la incertidumbre se trató mediante análisis de sensibilidad univariable, multivariable y probabilístico.
ResultadosPara el método habitual se obtuvo un valor de 8.588 €/QALY. En la alternativa 1 el gasto fue de 8.487 €/QALY, mientras que en la alternativa 2 el cociente coste-efectivo ascendió a 2.960 €/QALY. La alternativa 2 fue la de mayor eficiencia diagnóstica, alcanzando una reducción del 75% del número de días que un paciente con tuberculosis permanece sin tratamiento adecuado, así como una reducción del 70% del número de días que un paciente sin tuberculosis permanece ingresado.
ConclusiónLa aplicación de una técnica microbiológica molecular en el diagnóstico de tuberculosis es sumamente coste-efectiva frente al método habitual. Su introducción en el procedimiento diagnóstico de rutina supondría una mejora en la calidad asistencial de los pacientes al evitar ingresos y tratamientos innecesarios, reflejándose en un ahorro económico al hospital.
Spain is a country with a low incidence of tuberculosis (TB), although approximately 5000 cases are diagnosed every year.1 It is therefore necessary to implement activities that ensure accurate and early diagnosis, as well as follow-up and adequate treatment compliance.2,3 Nevertheless, the microbiological diagnosis of TB is complex. A sputum smear microscopy (SSM) is a quick, simple and economical technique, but presents a low sensitivity and thus a high number of false negatives, with subsequent delayed diagnoses as well as some false positives.2 A delay in diagnosis increases the risk of passing on the infection and prolonging the disease in patients. A false positive result, on the other hand, may result in the prescription of an unnecessary treatment, pharmacological toxicity and the selection of resistant strains, as well as a delay in the correct diagnosis of chronic diseases, leading to increased morbidity and costs.4
The direct and real-time application of nucleic acid amplification techniques (NAATs) on clinical samples, such as the Xpert MTB/RIF® test (Xpert), facilitates the early detection of the Mycobacterium Tuberculosis Complex (MTBC) and resistance to rifampicin (RIF), with high sensitivity and specificity over the course of 2h.5,6 For this reason, both the World Health Organisation7 and the European Centre for Disease Prevention and Control8 recommend its use in bacilliferous pulmonary cases. Despite the relatively high financial cost of these techniques compared to conventional measures, their systematic application in patients with a high index of suspicion for TB would allow cost savings, particularly due to reduced hospital stays.9
Given that the costs of Xpert are presented as the main barrier to its implementation in TB diagnostic processes, the objective of this study was to evaluate, by means of a cost-effectiveness analysis, two alternatives for TB diagnosis, comprising the Xpert MTB/RIF® technology versus the conventional method used at our site.
MethodsBetween 1 January 2008 and 31 December 2012, a retrospective study was performed on 1972 patients attending the Hospital General La Mancha Centro (HGMC) with suspected TB. Among the admitted patients, the request of three sputum samples within a period of eight days was considered to be an inclusion criterion, from which the SSM and mycobacterial culture result is available, with the identification of the species and its susceptibility to various anti-TB drugs being obtained in positive cases.
A cost-effectiveness analysis was performed to evaluate three microbiological diagnosis strategies for TB. The first is based on the routine TB diagnosis method used at our hospital, while the other two strategies (alternative modes 1 and 2) are assessed through the theoretical application of Xpert MTB/RIF® technology (Sunnyvale, CA, USA) on the same patients. To compare the costs and effectiveness of the three strategies, a decision tree was constructed using the TreeAge Pro 2011® application (TreeAge Software Inc. Williamstown, MA, USA).
The routine TB diagnosis method used at the HGMC consists of requesting a chest X-ray, a Mantoux test and obtaining three consecutive sputum samples from each patient and carrying out a SSM (auramine-rhodamine) and culture in MGIT liquid medium (BD, S.A.), considering the isolation of MTBC as the diagnostic gold standard.3 While the SSM results are obtained, the patient remains in hospital in respiratory isolation. If a SSM result is positive, the patient may be discharged early (in under seven days), with empirical treatment and a follow-up consultation pending the results of the culture and antibiogram. Conversely, if the three SSMs are negative, two situations are distinguished:
- 1.
In patients with a high clinical suspicion of TB (HCSTB) (compatible clinical and epidemiological context and X-ray suggestive of TB), the study is completed with a computerised axial tomography (CAT) scan and the collection of bronchoalveolar lavage fluid and/or bronchial aspirate through a fibrobronchoscopy, without administering anti-TB treatment; if one SSM result is positive, the patient is treated empirically, having remained in isolation for an average of two weeks. Meanwhile, patients with a HCSTB and negative SSM results also start empirical treatment, but remain unisolated in hospital for an average of three weeks until the remaining diagnostic tests have been received (delay in imaging tests, anatomical pathology, cultures).
- 2.
If all three SSMs are negative without a HCSTB, the patient is admitted on an unisolated basis for an average of 11 days, during which his/her clinical course is observed. He/she is then discharged without anti-TB treatment, with or without the diagnosis of another respiratory disease.
The admission times for each situation were provided by the Internal Medicine Department and are an estimation based on routine clinical practice after 20 years of experience in managing TB-positive patients.
The first alternative proposed consists of incorporating early TB diagnosis by means of an Xpert test on a sputum sample in patients with a positive SSM result or HCSTB. The model follows the same procedure as the routine method, but does not consider collecting samples through invasive means. If one SSM result were positive, an Xpert test would be performed to confirm that MTBC is really present. If the Xpert result is negative and there is a HCSTB, the possibility of empirical treatment would be considered, with the patient remaining unisolated in hospital for an average of three weeks until his/her microbiological results have been obtained. If all three SSMs are negative and the patient presents a HCSTB, the last sputum sample would be analysed using the Xpert test, while in patients with three negative SSMs and no HCSTB, the conventional method would be applied. If the Xpert result is positive, irrespective of the SSM result, the patient would receive empirical treatment and an early discharge, as occurred with the routine method, albeit with the possibility of adjusting the anti-TB treatment in the event of data showing resistance to RIF.
The second alternative proposed includes the possibility of performing two tests with Xpert on all the study patients, irrespective of their clinical suspicion and SSM result. If the Xpert result is positive, the same procedure is followed as in the above alternative. If the Xpert result is negative, a second test would be performed with another sample. Patients with a positive SSM and two negative Xpert tests would be treated empirically until the culture and antibiogram result is obtained. Patients with three negative SSMs and two negative Xpert tests would be discharged without treatment until the culture result is obtained.
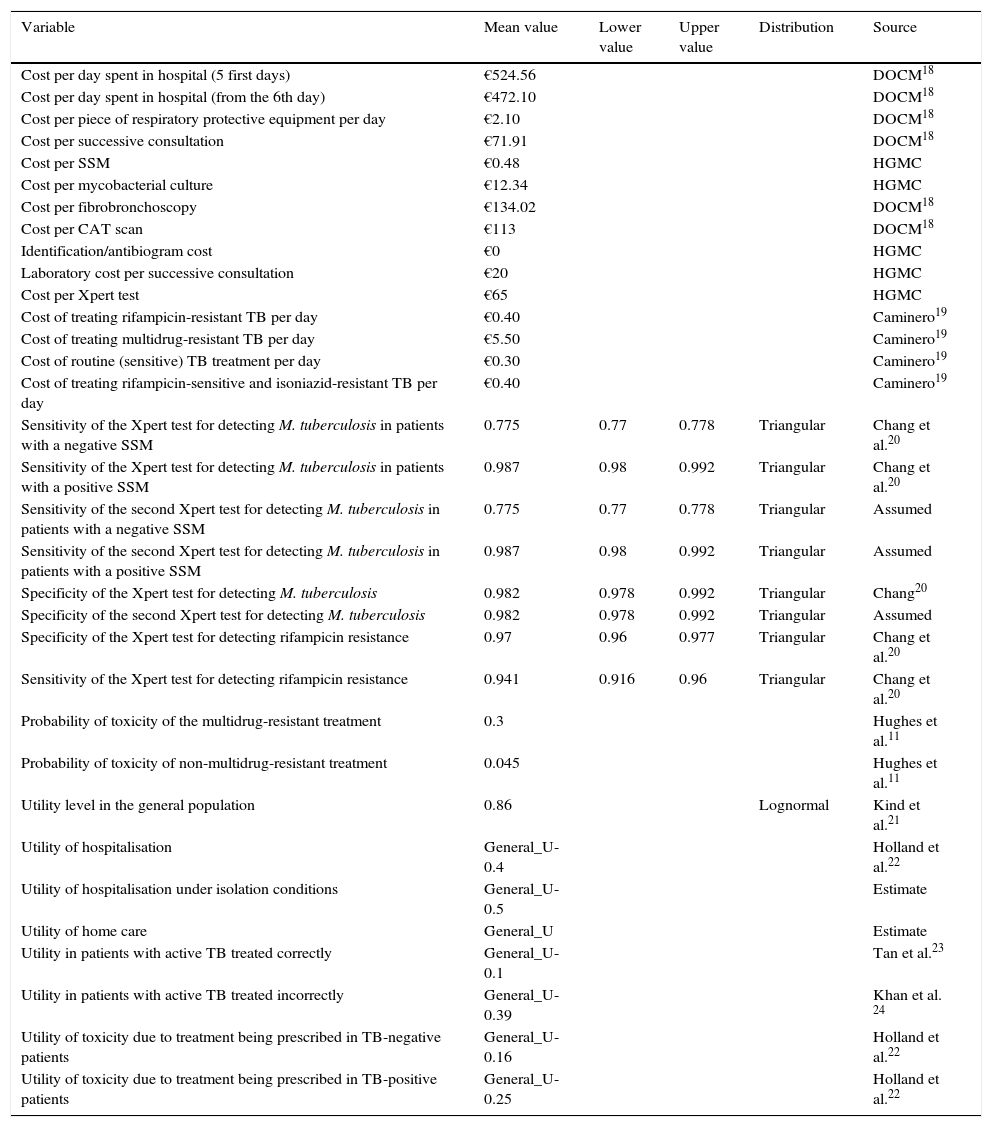
The model includes several types of variables. On the one hand, bibliographical variables are defined which are applied to all of the patients, such as costs, utilities and probabilities (Table 1). Numerical variables are also estimated due to routine clinical procedures being applied to each type of patient, for each branch of the decision tree (Table 2). Finally, variables that allow cost and utility to be estimated for each branch of the tree and which use bibliographical and numerical variables for their definition were calculated (Table 2). Mortality was not included as a variable to be measured due to the fact there were no TB-related deaths among the study population.
Variables used in the construction of the decision tree.
| Variable | Mean value | Lower value | Upper value | Distribution | Source |
|---|---|---|---|---|---|
| Cost per day spent in hospital (5 first days) | €524.56 | DOCM18 | |||
| Cost per day spent in hospital (from the 6th day) | €472.10 | DOCM18 | |||
| Cost per piece of respiratory protective equipment per day | €2.10 | DOCM18 | |||
| Cost per successive consultation | €71.91 | DOCM18 | |||
| Cost per SSM | €0.48 | HGMC | |||
| Cost per mycobacterial culture | €12.34 | HGMC | |||
| Cost per fibrobronchoscopy | €134.02 | DOCM18 | |||
| Cost per CAT scan | €113 | DOCM18 | |||
| Identification/antibiogram cost | €0 | HGMC | |||
| Laboratory cost per successive consultation | €20 | HGMC | |||
| Cost per Xpert test | €65 | HGMC | |||
| Cost of treating rifampicin-resistant TB per day | €0.40 | Caminero19 | |||
| Cost of treating multidrug-resistant TB per day | €5.50 | Caminero19 | |||
| Cost of routine (sensitive) TB treatment per day | €0.30 | Caminero19 | |||
| Cost of treating rifampicin-sensitive and isoniazid-resistant TB per day | €0.40 | Caminero19 | |||
| Sensitivity of the Xpert test for detecting M. tuberculosis in patients with a negative SSM | 0.775 | 0.77 | 0.778 | Triangular | Chang et al.20 |
| Sensitivity of the Xpert test for detecting M. tuberculosis in patients with a positive SSM | 0.987 | 0.98 | 0.992 | Triangular | Chang et al.20 |
| Sensitivity of the second Xpert test for detecting M. tuberculosis in patients with a negative SSM | 0.775 | 0.77 | 0.778 | Triangular | Assumed |
| Sensitivity of the second Xpert test for detecting M. tuberculosis in patients with a positive SSM | 0.987 | 0.98 | 0.992 | Triangular | Assumed |
| Specificity of the Xpert test for detecting M. tuberculosis | 0.982 | 0.978 | 0.992 | Triangular | Chang20 |
| Specificity of the second Xpert test for detecting M. tuberculosis | 0.982 | 0.978 | 0.992 | Triangular | Assumed |
| Specificity of the Xpert test for detecting rifampicin resistance | 0.97 | 0.96 | 0.977 | Triangular | Chang et al.20 |
| Sensitivity of the Xpert test for detecting rifampicin resistance | 0.941 | 0.916 | 0.96 | Triangular | Chang et al.20 |
| Probability of toxicity of the multidrug-resistant treatment | 0.3 | Hughes et al.11 | |||
| Probability of toxicity of non-multidrug-resistant treatment | 0.045 | Hughes et al.11 | |||
| Utility level in the general population | 0.86 | Lognormal | Kind et al.21 | ||
| Utility of hospitalisation | General_U-0.4 | Holland et al.22 | |||
| Utility of hospitalisation under isolation conditions | General_U-0.5 | Estimate | |||
| Utility of home care | General_U | Estimate | |||
| Utility in patients with active TB treated correctly | General_U-0.1 | Tan et al.23 | |||
| Utility in patients with active TB treated incorrectly | General_U-0.39 | Khan et al. 24 | |||
| Utility of toxicity due to treatment being prescribed in TB-negative patients | General_U-0.16 | Holland et al.22 | |||
| Utility of toxicity due to treatment being prescribed in TB-positive patients | General_U-0.25 | Holland et al.22 |
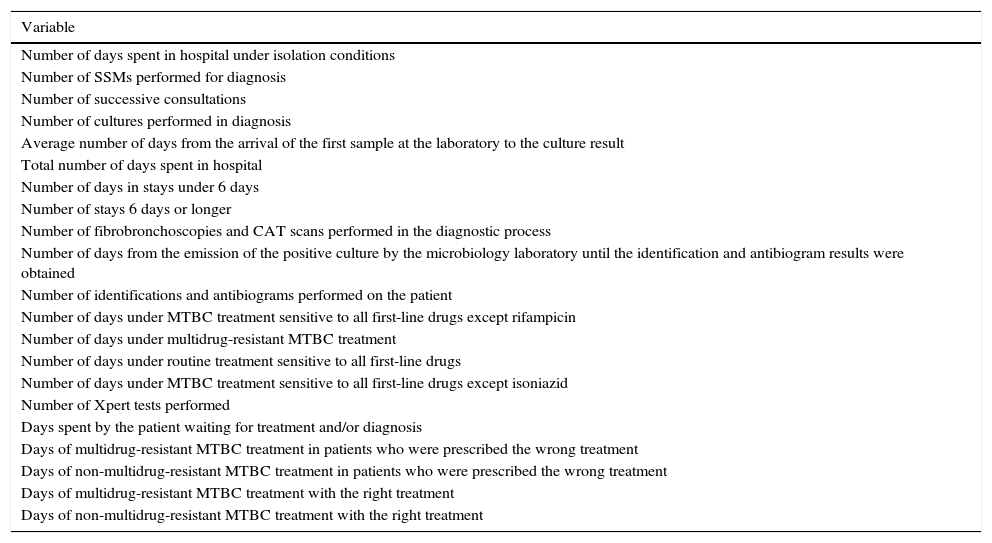
Variables measured for each patient from the decision tree.
| Variable |
|---|
| Number of days spent in hospital under isolation conditions |
| Number of SSMs performed for diagnosis |
| Number of successive consultations |
| Number of cultures performed in diagnosis |
| Average number of days from the arrival of the first sample at the laboratory to the culture result |
| Total number of days spent in hospital |
| Number of days in stays under 6 days |
| Number of stays 6 days or longer |
| Number of fibrobronchoscopies and CAT scans performed in the diagnostic process |
| Number of days from the emission of the positive culture by the microbiology laboratory until the identification and antibiogram results were obtained |
| Number of identifications and antibiograms performed on the patient |
| Number of days under MTBC treatment sensitive to all first-line drugs except rifampicin |
| Number of days under multidrug-resistant MTBC treatment |
| Number of days under routine treatment sensitive to all first-line drugs |
| Number of days under MTBC treatment sensitive to all first-line drugs except isoniazid |
| Number of Xpert tests performed |
| Days spent by the patient waiting for treatment and/or diagnosis |
| Days of multidrug-resistant MTBC treatment in patients who were prescribed the wrong treatment |
| Days of non-multidrug-resistant MTBC treatment in patients who were prescribed the wrong treatment |
| Days of multidrug-resistant MTBC treatment with the right treatment |
| Days of non-multidrug-resistant MTBC treatment with the right treatment |
Effectiveness was measured in Quality-Adjusted Life Years (QALYs). The final utility (equivalent to effectiveness) of each tree branch is calculated by weighing up the daily utilities for each patient over a year, from diagnostic suspicion to the end of treatment, or until a TB diagnosis is ruled out. To perform this calculation, the patient's utility per day is estimated until one year is reached. In the case of patients with a process duration exceeding one year, the yearly utility is deemed to be the same as total utility. Two final utilities are identified: utility in TB-positive and TB-negative patients.
Uncertainty was addressed using a univariate, multivariate and tornado-type sensitivity analysis.10
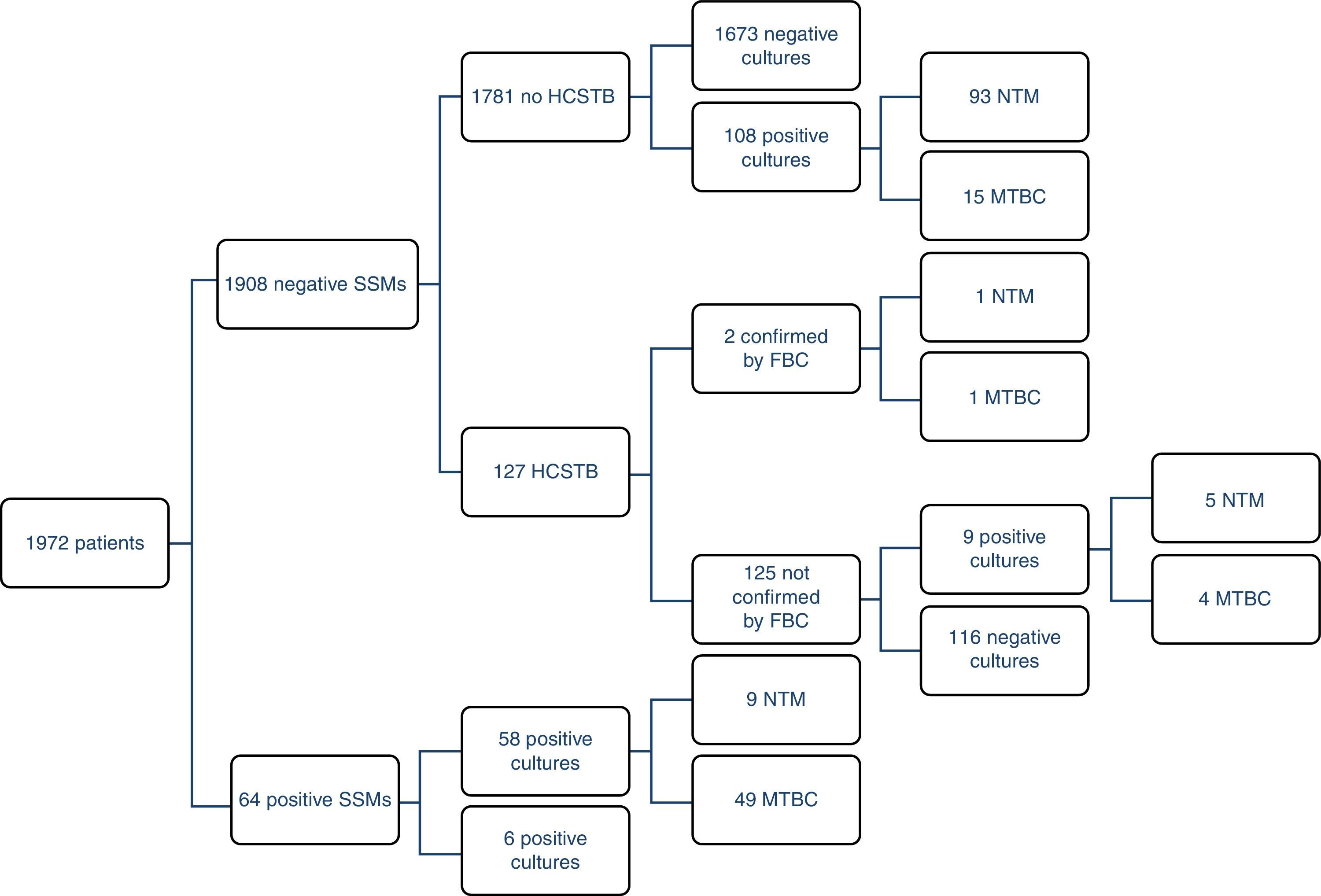
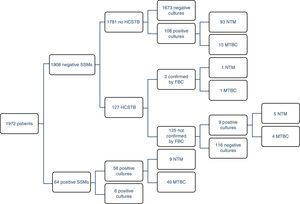
ResultsOf the 1972 patients studied, the disease was confirmed by cultures in 69 of them (3.5%), although there were 177 positive cultures; as such, 108 atypical mycobacteria were isolated. 71% (49/69) of the TB cases could be detected early using a SSM. There were four MTBC strains resistant to first-line anti-TB drugs (8%); two were resistant to isoniazid (INH), one to RIF and the other was multidrug-resistant. The largest group comprised patients with unconfirmed TB suspicion, no HCSTB and a negative SSM result, who remained in hospital for 11 days on average (three in isolation). Fig. 1 presents a detailed description of the results obtained with the routine method.
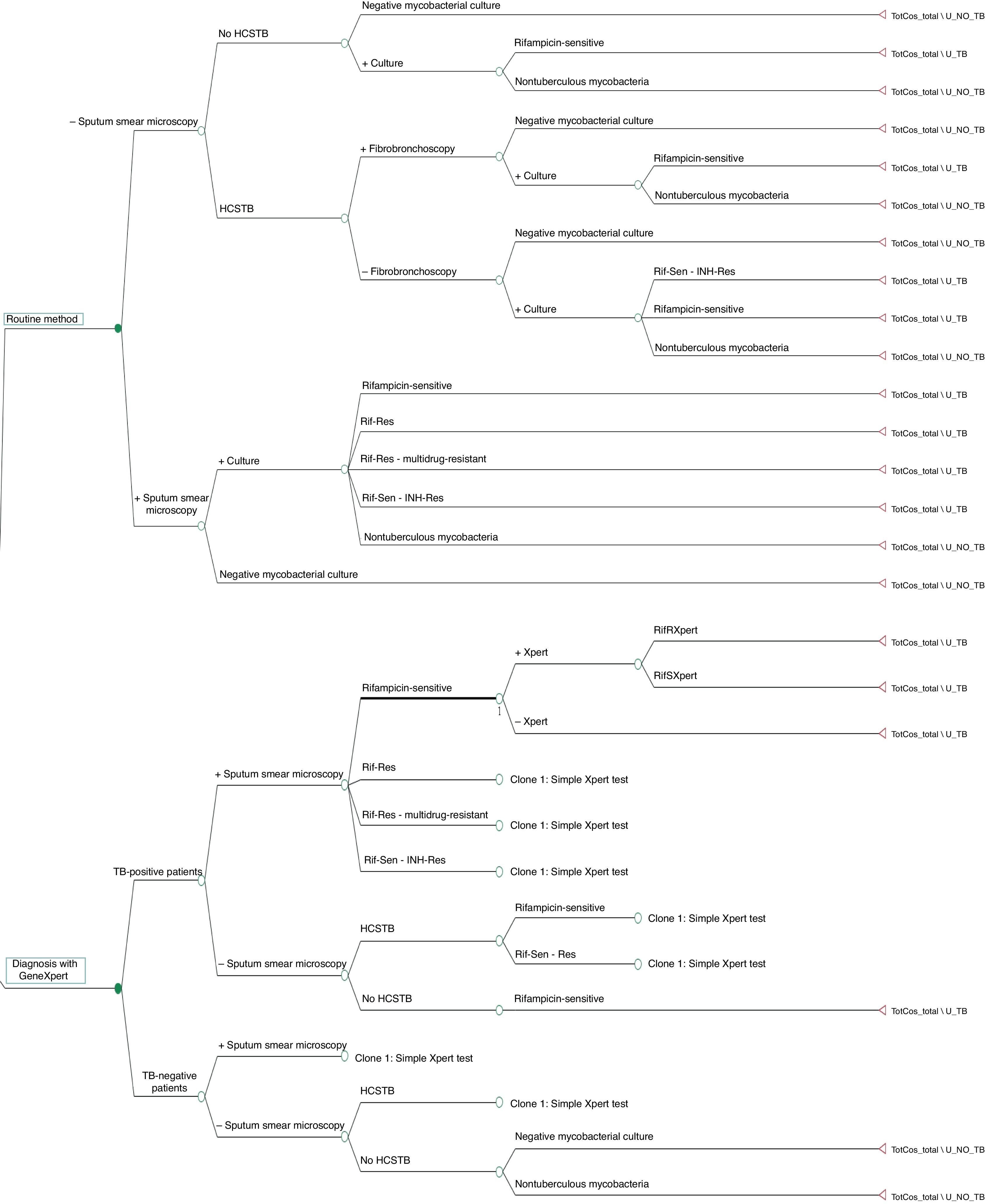
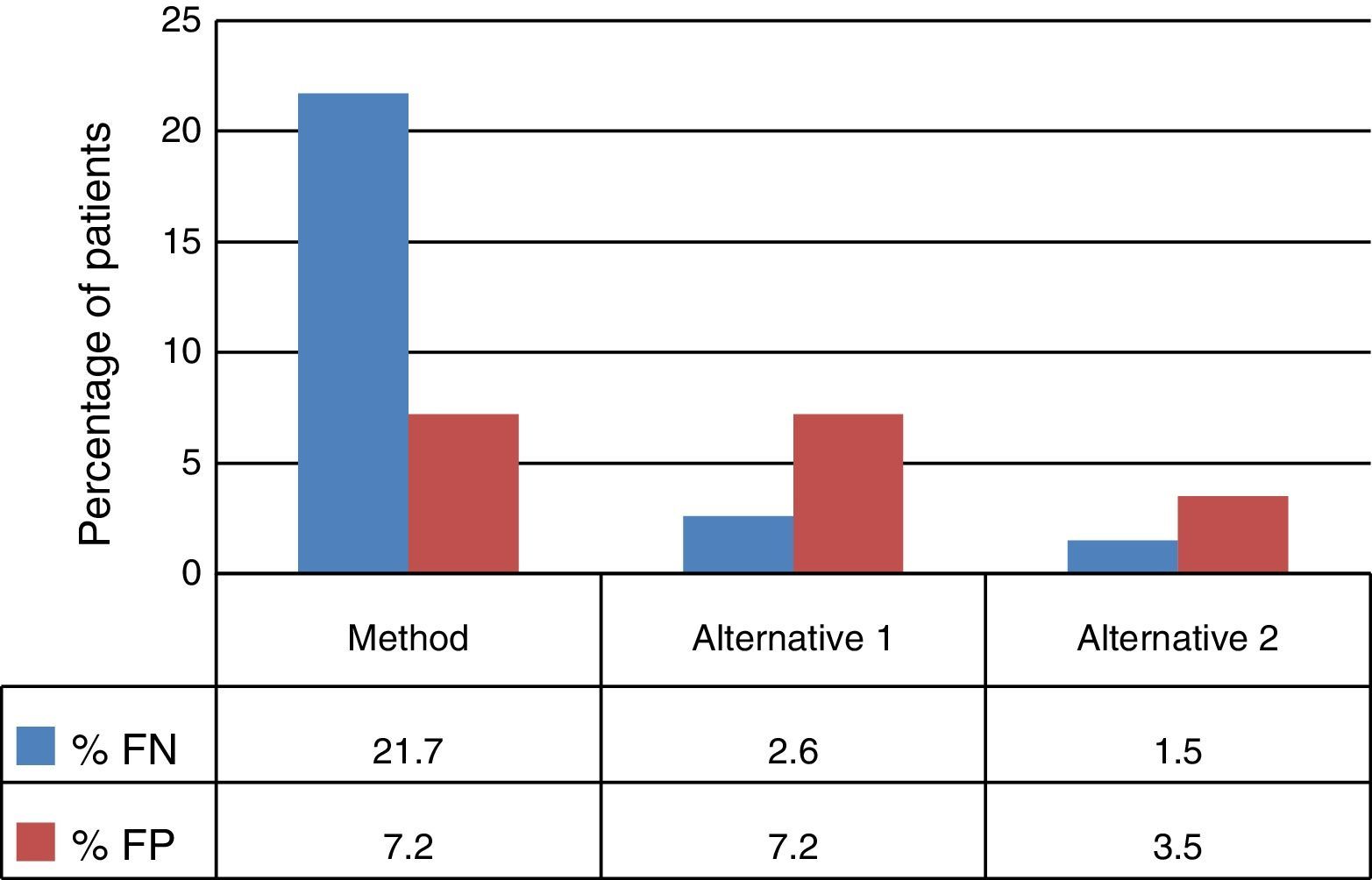
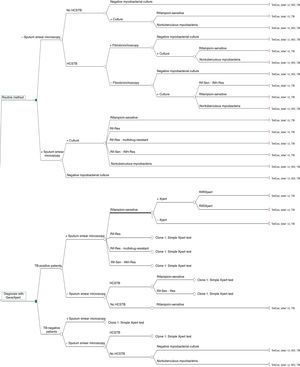
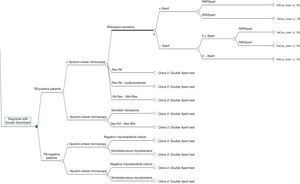
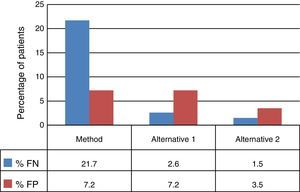
The decision tree with the three alternatives is shown in Fig. 2. Once the probabilities had been calculated for each branch of the decision tree, the use of resources by the 1972 patients on applying the different alternatives was estimated (Table 3). Of the three methods evaluated, the two alternatives to the routine method presented greater diagnostic efficiency, with a lower percentage of TB-positive patients who remained untreated until the culture result had been obtained (percentage of false negatives). Moreover, in alternative 2, a lower number of TB-negative patients who received inadequate treatment up until the culture and/or antibiogram results were obtained were observed (Fig. 3).
Estimates of resources used by the three alternatives evaluated.
| Routine method | Alternative 1 | Alternative 2 | |
|---|---|---|---|
| Total number of days spent without treatment by TB-positive patients | 522 | 403 | 126 |
| Total number of days where patients were prescribed the wrong routine treatment (for non-multidrug-resistant MTBC) | 5974 | 3177 | 2233 |
| Total number of days spent in isolation by the patient | 7533 | 7039 | 8200 |
| Number of successive consultations | 462 | 460 | 2135 |
| Total days spent in hospital | 29,124 | 28,807 | 8588 |
| Number of Xpert tests | 0 | 206 | 3847 |
For the routine method, a quotient of €8588/QALY was obtained. In TB-positive patients, the estimated cost was €6329/QALY and, in TB-negative patients, it was €8659/QALY. In alternative 1, a total cost of €8487/QALY is obtained, with the expenditure per TB-positive patient being €7222/QALY, and €8532/QALY per TB-negative patient. In alternative 2, a cost-effective quotient of €2969/QALY is observed. TB-positive patients presented an expenditure of €5510/QALY, while expenditure of the patients in whom a TB diagnosis was ruled out was €2878/QALY. As such, alternative 2 presents a dominant cost-effective ratio.
Sensitivity studyA tornado-type sensitivity analysis showed the parameter with the greatest influence on per-patient expenditure to be the admission cost over the first five days. The €300/day decrease represents a per-patient cost reduction of €1614/day on applying alternative 2, compared to the €6194 obtained on applying the routine method. The second variable with the greatest influence on the model was the cost of the protective measures. As possible values, a range of between €2.10 and €200 has been taken, where the minimum value corresponds to the cost of barrier measures (masks) and the maximum to remaining in an isolation room. The variation in this parameter had a greater influence on alternative 2, although this continues to be the dominant alternative. The variation in the cost of the Xpert test had a more notable, though moderate, influence on alternative 2. A sensitivity study was also performed for the utility values (QALY) of the general population, analysing the range of 0.80–1 QALY.
An acceptability curve was obtained following the application of the Monte Carlo method, indicating that alternative 2 is the most profitable, irrespective of the chosen threshold, with a probability of 100%.
DiscussionThe results of this study suggest that, in our field and with the assumptions made on the model, diagnosing TB with the Xpert technology is more cost-effective than the conventional procedure. Of the two alternatives to the current method, the one that includes the possibility of performing two Xpert tests proved dominant, in such a way that applying this technology as a screening method would lead to an improvement in the quality of life of TB-positive patients due to allowing adequate treatment to be initiated without delay, as well as reducing hospital costs by reducing the number of stays among TB-negative patients.
Studying the three branches of the decision tree shows differences between the QALYs measured for the three diagnostic procedures, with a higher number of QALYs gained through the application of alternative 2 (70% reduction in hospital stays and 75% reduction in days spent without adequate treatment). However, the most notable difference is in the reduction in per-patient costs, which were 65% lower in alternative 2 versus the routine method. This cost reduction would allow for a theoretical annual saving of 1.8 million euros due, above all, to the reduced number of hospital stays associated with TB suspicion.
Due to the fact that the sensitivity of the Xpert test in patients with negative SSMs is relatively low,6,10–12 its application is not recommended unless there is a HCSTB as, otherwise, the positive predictive value of the technique would be reduced.13 Some studies show an increase in the sensitivity of this test when a second determination is performed in patients with a negative result.14 In this sense, the use of Xpert as a screening technique would be justified, with the drawback of increasing laboratory costs and the number of false positives compared to alternative 1, although it would still result in fewer false positives compared to the current method (75 versus 200). Moreover, according to some authors, the damage of not treating a TB-positive patient is greater than treating a TB-negative patient with anti-TB drugs.15 In addition, after two negative Xpert results, the diagnosis of TB could be ruled out, and the conduct of the microbiological culture could even be abandoned, since the negative predictive value of the test is close to 100%,16 although this possibility has not been evaluated in our study. For this reason, it could appear contradictory to have considered prescribing empirical treatment to patients with a positive SSM and two negative Xpert results in alternative 2; however, this possibility was considered due to it being the worst-case scenario in which we could find ourselves, which also includes the potential resistance of certain clinics to stop treating a patient with a positive SSM, as well as the possibility of an infection caused by a nontuberculous mycobacterium (in this case it would be necessary to individualise therapy). Conducting a third Xpert test after two negative results could be inadvisable, as the number of false positives is estimated to be above 100.
Other cost-effectiveness studies on the Xpert test carried out in developing countries with a high TB burden show Xpert to be cost-effective, and its introduction could lead to a significant change in the morbidity and mortality of the infection through greater case detection and, thus, targeted treatments.5,6 The application of this technology in areas of low prevalence such as Spain would reduce its profitability, although its potential in situations of greater prevalence would be considerable (the immigrant population, homeless people, parenteral drug users, HIV patients and, generally speaking, where there is high index of TB suspicion).10 Our study shows that the theoretical application of this technique to any patient with suspected TB is more cost-effective than its application only in selected patients, as obtaining a reliable and fast result that rules out infection gives rise to hospital discharges, irrespective of the patient's real disease. Thus, the fundamental difference between alternative 2 and the routine method stems from the manner in which patients without a HCSTB are considered, in the sense that according to the routine method, these types of patients remain in hospital, while patients with two negative Xpert tests are discharged early.
One limitation of the study is the consideration of the cost of hospital stays without respiratory isolation conditions. However, in the sensitivity study, the possibility of an increased hospital cost due to the establishment of isolation conditions was taken into account, with this being the second most influential factor on the cost estimate. However, the quantification of the savings obtained with alternative 2 should be treated with caution, as the possibility of the patient being hospitalised to carry out the diagnosis and treatment of the nontuberculous disease was not taken into account. Moreover, it can be seen that the application of alternative 2 entails a significant increase in the number of successive consultations in order to ensure the review of patients that, despite having suspected TB, were discharged without a diagnosis and thus without treatment. This could, on the other hand, be interpreted as an improvement in patient healthcare due to increased vigilance towards a lower total cost, also contributing to the decongestion of hospital centres if follow-up were to be performed in primary care centres.2
In future studies, we feel that it would be useful to evaluate the real benefit that clinical practice with the application of Xpert technology would have on costs and quality of life among patients with suspected TB. There are also other NAATs on the market which may be evaluated as alternatives.17
In conclusion, this study suggests that the application of Xpert technology in the diagnosis of TB is extremely cost-effective compared to the conventional method. The impact of introducing Xpert technology covers both financial and healthcare aspects, in the sense that its application would lead to an improvement in patient healthcare quality due to avoiding unnecessary hospital stays and treatments, as well as allowing early, targeted therapy to be initiated, breaking the infection transmission chain and achieving a considerable financial saving for the hospital.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Herráez Ó, Asencio-Egea MÁ, Huertas-Vaquero M, Carranza-González R, Castellanos-Monedero J, Franco-Huerta M, et al. Estudio de coste-efectividad del diagnóstico microbiológico de tuberculosis mediante geneXpert MTB/RIF®. Enferm Infecc Microbiol Clin. 2017;35:403–410.
This project was awarded the 5th AEFA Prize for Quality and Innovation.