To compare the clinical characteristics, treatments, and evolution of critical patients with COVID-19 pneumonia treated in Intensive Care Units (ICU) after one year of pandemic.
MethodologyMulticenter, prospective study, which included critical COVID-19 patients in 9 ICUs in northwestern Spain. The clinical characteristics, treatments, and evolution of patients admitted to the ICU during the months of March-April 2020 (period 1) were compared with patients admitted in January-February 2021 (period 2).
Results337 patients were included (98 in period 1 and 239 in period 2). In period 2, fewer patients required invasive mechanical ventilation (IMV) (65% vs 84%, p < 0.001), using high-flow nasal cannulas (CNAF) more frequently (70% vs 7%, p < 0.001), ventilation non-invasive mechanical (NIMV) (40% vs 14%, p < 0.001), corticosteroids (100% vs 96%, p = 0.007) and prone position in both awake (42% vs 28%, p = 0.012), and intubated patients (67% vs 54%, p = 0.034). The days of IMV, ICU stay and hospital stay were lower in period 2. Mortality was similar in the two periods studied (16% vs 17%).
ConclusionsAfter 1 year of pandemic, we observed that in patients admitted to the ICU, CNAF, NIMV, use of the prone position, and corticosteroids have been used more frequently, reducing the number of patients in IMV, and the length of stay in the ICU and hospital stay. Mortality was similar in the two study periods.
Comparar las características clínicas, tratamientos, y evolución de los pacientes críticos con neumonía por COVID-19 atendidos en Unidades de Cuidados Intensivos (UCI) tras un año de pandemia.
MetodologíaEstudio multicéntrico, prospectivo, en el que se incluyó pacientes críticos COVID-19 en 9 UCIs del noroeste de España. Se compararon las características clínicas, los tratamientos, y la evolución de pacientes ingresados en UCI durante los meses de marzo-abril 2020 (periodo 1) con pacientes ingresados en enero-febrero 2021 (periodo 2).
ResultadosSe incluyeron 337 pacientes (98 en el periodo 1 y 239 en el periodo 2). En el periodo 2 menos pacientes requirieron ventilación mecánica invasiva (VMI) (65% vs 84%, p < 0,001), utilizándose con mayor frecuencia cánulas nasales de alto flujo (CNAF) (70% vs 7%, p < 0.001), ventilación mecánica no invasiva (VMNI) (40% vs 14%, p < 0,001), corticoides (100% vs 96%, p = 0,007) y posición de decúbito prono tanto en pacientes despiertos (42% vs 28%, p = 0,012), como intubados (67% vs 54%, p = 0,034). Los días de VMI, de estancia en UCI y hospitalaria fueron inferiores en el periodo 2. La mortalidad fue similar en los dos periodos estudiados (16% vs 17%).
ConclusionesTras 1 año de pandemia, observamos que en los pacientes ingresados en UCI se ha utilizado con mayor frecuencia CNAF, VMNI, uso del decúbito prono, y corticoides, disminuyendo los pacientes en VMI, y los tiempos de estancia en UCI y estancia hospitalaria. La mortalidad ha sido similar en los dos periodos a estudio.
Since the appearance of the SARS-CoV-2 coronavirus infection in Wuhan, China, in December 2019, it has spread rapidly worldwide.1,2 On 31 January, 2020, Spain confirmed its first patient with coronavirus disease 2019 (COVID-19), and Galicia, a region located in the north-west of the country, did so one month later. Since then, three waves have been documented. The first was between February and May 2020, the second was between September and November 2020, and the third between January and March 2021.
In recent months, various articles have been published related to the clinical characteristics of critically ill COVID-19 patients, the treatments used, disease-associated complications, the evolution of these patients in intensive care units (ICU) and their predictive factors.3–8 Most of these publications refer to the first half-year of the pandemic, in which there was hardly any scientific evidence due to the novelty of the infection. One year after the first documented case in Spain, different clinical trials and observational studies have led to modifications in the treatments used in these patients, particularly with regard to the use of corticosteroids,9,10 antivirals,11,12 anticoagulants,13 antibiotics14 or immunomodulators,15 as well as respiratory care: high-flow nasal cannulas (HFNC), non-invasive mechanical ventilation (NIMV), or prone positioning, both in patients undergoing spontaneous breathing ventilation and on invasive mechanical ventilation (IMV).16–19
Currently, few studies compare the clinical characteristics and the differences in the treatments used and the outcomes (complications, mortality) in critically ill COVID-19 patients throughout the year of the pandemic.20,21
Therefore, we conducted an observational, prospective study that included patients with COVID-19 infection presenting with severe respiratory failure and requiring admission to the ICU. Our objective was to compare the clinical characteristics, treatments used, complications and the evolution of the patients treated in an ICU in north-western Spain in two periods: the first period during March and April 2020, coinciding with the first wave of the pandemic, and the second period during January and February 2021, coinciding with the third wave.
MethodsIn March and April 2020, and in January and February 2021, we prospectively evaluated patients with acute respiratory failure due to COVID-19, confirmed by a positive result in a reverse transcriptase polymerase chain reaction (RT-PCR), admitted to the ICU of nine hospitals located in north-western Spain (Galicia): Complejo Hospitalario Universitario de Santiago (CHUS), Complejo Hospitalario Universitario de A Coruña (CHUAC), Complejo Hospitalario Universitario de Pontevedra (CHUP), Complejo Hospitalario Universitario de Ferrol (CHUF), Complejo Hospitalario Universitario de Ourense (CHUO), Complejo Hospitalario Universitario de Vigo (CHUVI), Hospital Universitario Lucus Augusti de Lugo (HULA), Hospital POVISA de Vigo and Hospital da Mariña en Lugo. The patients admitted to the ICU in March and April 2020 were considered to be from the initial period of the pandemic (period 1), and the patients admitted in January and February 2021 from the final period (period 2). The Galician Ethics Committee (code 2020–188) approved this study and, due to its characteristics and the pandemic situation, the need for patient informed consent was waived.
The following data were collected from all patients on admission to the ICU: age, sex, weight, height, concomitant diseases and home treatments; plus the Acute Physiology and Chronic Health Evaluation II (APACHE-II) score, arterial partial pressure of oxygen (PaO2), fraction of inspired oxygen (FiO2), PaO2/FiO2 ratio and initial laboratory tests (complete blood count, serum biochemistry, serum ferritin, procalcitonin, lactate dehydrogenase, D-dimer and C-reactive protein). Further evaluation consisted of the presence of coexisting infections and the time from onset of initial symptoms to hospital admission and ICU admission.
During the ICU stay, we evaluated the medications used (vasopressors, antibiotics, antivirals, corticosteroids, anticoagulants, neuromuscular blockers, immunosuppressants, antiplatelet agents), the mode of respiratory support (IMV, NIMV, HFNC), the use of renal replacement therapy, the use of prone positioning in awake or intubated patients, need for tracheostomy for prolonged mechanical ventilation, complications (ICU-acquired infection, thromboembolic complications, need for reintubation, pneumothorax, ICU readmission), and ICU outcome, including the number of patients who died, those discharged and those who remained in the ICU at the end of the follow-up on 21 March 2021.
The authors designed the trial, collected the data and performed the analysis. All authors reviewed the manuscript, attested to its accuracy and completeness of the data and approved the decision to submit the manuscript for publication.
Statistical analysisA descriptive analysis of the demographic and clinical characteristics of the patients treated in the two study periods was performed. Descriptive measures for categorical variables included absolute values and percentages; quantitative variables are described as measures of central tendency (mean or median) and measures of dispersion (standard deviation or interquartile range).
The chi-square statistic or Fisher's exact test was used to compare the frequency distributions between the two periods. The student's t-test or the Mann-Whitney test, as appropriate, were used to compare the quantitative variables. All tests were performed using a bilateral approach. A value of p < 0.05 was considered significant.
To explore risk factors associated with mortality in patients admitted to the ICU during the two study periods, a univariate and multivariate logistic regression analysis was performed, taking mortality-associated variables described in previous studies into account: age, APACHE-II severity score, obesity, PaO2/FiO2 on admission to the ICU and need for mechanical ventilation.
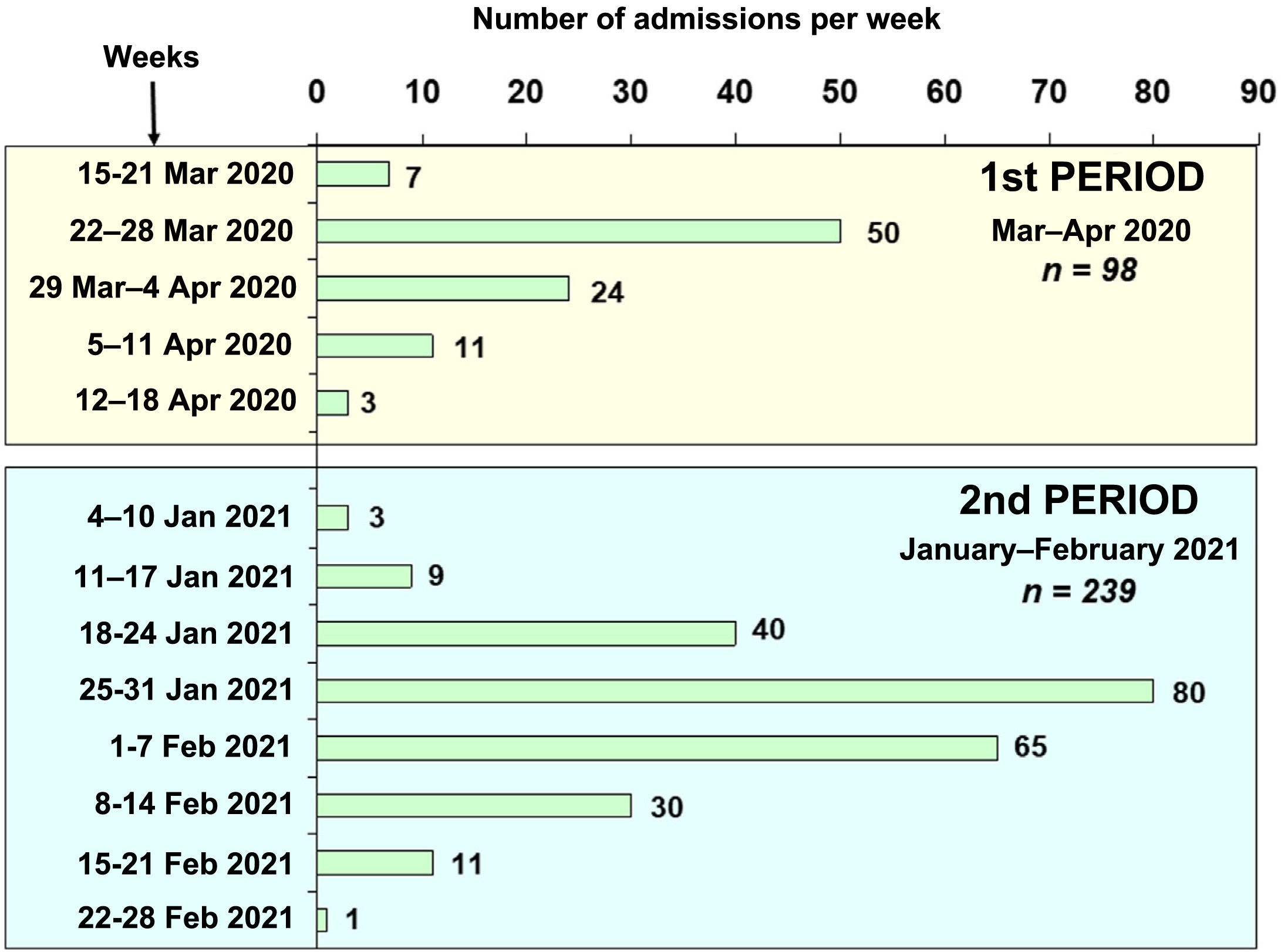
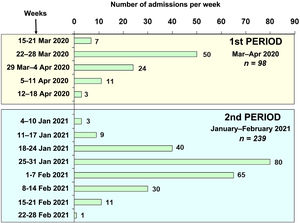
ResultsA total of 337 patients with acute respiratory failure due to COVID-19 were admitted to the ICUs of nine hospitals in north-western Spain during the two study periods (Fig. 1): 98 patients during period 1 (March–April 2020) and 239 patients during period 2 (January–February 2021). The percentage of hospitalised patients who required admission to a critical care unit varied between 10% and 15% in the centres included in the study. Table 1 shows the demographic characteristics, coexisting diseases, chronic medical treatments and laboratory data on admission to the ICU for the patients admitted in the two periods. The patients admitted in period 2 were younger than those in period 1 (63.88 [11.53] vs 66.94 [9.78] years, p = 0.037). Hypertension, hyperlipidaemia and obesity were the most common coexisting diseases during the two periods. Obesity (BMI > 30) was more common in period 2 than in period 1 (52% vs 40%, p = 0.022).
Demographic data, medical history and analytical data on admission to the ICU of the patients in the two study periods.
| Period 1March–April 2020n = 98 | Period 2January–February 2021n = 239 | p | |
|---|---|---|---|
| Age, years | 66.94 (9.78) | 63.88 (11.53) | 0.037 |
| Male gender, n (%) | 62 (63.3) | 161 (67.4) | 0.470 |
| Weight, kg | 83.50 (15.53) | 86.45 (19.73) | 0.337 |
| Height, cm | 166.14 (7.88) | 166.84 (8.64) | 0.393 |
| BMI, kg/m2 | 30.34 (5.04) | 30.97 (6.09) | 0.583 |
| Comorbidities, n (%) | |||
| Hypertension | 56 (57.1) | 124 (51.9) | 0.379 |
| Hyperlipidaemia | 45 (45.9) | 122 (51.0) | 0.393 |
| Diabetes | 22 (22.4) | 57 (23.8) | 0.783 |
| Asthma | 7 (7.1) | 21 (8.8) | 0.620 |
| COPD | 11 (11.2) | 17 (7.1) | 0.214 |
| Heart disease | 28 (28.6) | 35 (14.6) | 0.003 |
| Obesity: BMI ≥ 30 kg/m2. | 39 (39.8) | 128 (53.6) | 0.022 |
| Cancer | 7 (7.1) | 32 (13.4) | 0.104 |
| Home treatments, n (%) | |||
| ACE inhibitors | 29 (29.9) | 57 (23.9) | 0.258 |
| Anticoagulants | 5 (5.2) | 18 (7.5) | 0.434 |
| Antiplatelet agents | 23 (23.7) | 39 (16.3) | 0.113 |
| Statins | 38 (38.8) | 108 (45.2) | 0.281 |
| Corticosteroids | 6 (6.1) | 27 (11.3) | 0.147 |
| Immunosuppressants | 5 (5.2) | 13 (5.5) | 0.910 |
| Bronchodilators | 9 (9.2) | 28 (11.7) | 0.500 |
| Laboratory data on admission, median (IQR) | |||
| Leukocytes | 7,465 (5,577−11,602) | 8,170 (6,200−10,300) | 0.369 |
| Lymphocytes | 600 (400−930) | 570 (400−800) | 0.305 |
| Lactate dehydrogenase, U/l | 456 (363−633) | 454 (354−638) | 0.902 |
| D-dimer, ng/mL | 1,103 (737−2,121) | 889 (562−1,569) | 0.013 |
| C-reactive protein, mg/dl | 14.95 (10.28−34.75) | 10.00 (4.3−15.54) | <0.001 |
| Procalcitonin, ng/mL | 0.14 (0.09−0.37) | 0.13 (0.07−0.30) | 0.093 |
| Serum ferritin, μg/l | 927 (598−1,513) | 1,018 (533−1,665) | 0.958 |
| Creatinine, mg/dl | 0.86 (0.71−1.12) | 0.82 (0.66−1.05) | 0.265 |
| Urea, mg/dl | 43 (34−66) | 47 (38−65) | 0.165 |
ACE inhibitors, angiotensin-converting enzyme inhibitors; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit.
Values are displayed as a number (percentage) or median (interquartile range).
The clinical evolution of the patients, treatments and management of ventilation during the stay in the ICU are summarised in Table 2 and Figs. 2 and 3. The patients admitted in period 2, despite having a lower APACHE-II score (12 [12–20] vs 15 [12–20], p < 0.001), had lower PaO2/FiO2ratios at ICU admission (105 [83–132] vs 128 [100–170], p < 0.001). The time from symptom onset to hospital admission was similar in the two periods. However, the time from symptom onset to ICU admission was shorter in period 2 (9 [7–11] vs 10 [7–12] days, p = 0.047).
Clinical characteristics and treatments administered to patients admitted to the ICU during the two study periods.
| Period 1March–April 2020n = 98 | Period 2January–February 2021n = 239 | p | |
|---|---|---|---|
| APACHE-II | 15.00 (12.00−20.0) | 12.00 (10.00−16.00) | <0.001 |
| Time from onset of symptoms to hospital admission, days | 7.00 (5.00−10.00) | 7.00 (5.00−9.00) | 0.282 |
| Time from onset of symptoms to ICU admission, days | 10.00 (7.00−12.00) | 9.00 (7.00−11.00) | 0.047 |
| PaO2/FiO2 on admission to ICU, mg | 128.00 (99.75−170.00) | 105.00 (83.00−132.00) | <0.001 |
| PaO2 on admission to ICU, mg | 80.50 (66.50−97.00) | 72.00 (64.00−100.00) | 0.005 |
| FiO2 on admission to ICU, % | 60.00 (50.00−100.00) | 70.00 (60.00−100.00) | 0.007 |
| Hospital admission and ICU admission on the same day | 25 (25.5) | 62 (25.9) | 0.935 |
| ICU stay, days | 15.00 (10.00−24.00) | 12.00 (7.00−20.00) | 0.018 |
| Hospital stay, days | 29.00 (19.00−43.00) | 23.00 (15.00−36.00) | 0.001 |
| Coinfection on ICU admission | 18 (18.4) | 26 (10.9) | 0.064 |
| Oxygen therapy | |||
| High flow nasal cannula | 7 (7.1) | 166 (69.5) | <0.001 |
| Non-invasive mechanical ventilation | 14 (14.3) | 81 (33.9) | <0.001 |
| Invasive mechanical ventilation (IMV) | 82 (83.7) | 155 (64.9) | <0.001 |
| Time from ICU admission to orotracheal intubation, days | <0.001 | ||
| <24 h | 74 (90.2) | 96 (61.9) | |
| 24−48 h | 4 (4.9) | 26 (16.8) | |
| >48 h | 4 (4.9) | 33 (21.3) | |
| Days of mechanical ventilation in the total number of hospitalised patients | 12.00 (6.00−18.00) | 7.00 (0.00−15.00) | <0.001 |
| Days of mechanical ventilation in intubated patients | 13.00 (9.00−19.25) | 11.00 (8.00−20.00) | 0.318 |
| Patients who required tracheostomy | 23 (23.5) | 43 (18.0) | 0.250 |
| Time from IMV to tracheostomy, days | 16.00 (11.75−18.00) | 15.00 (10.75−19.00) | 0.611 |
| Use of prone positioning in awake patients | 27 (27.6) | 101 (42.3) | 0.012 |
| Use of prone positioning in ventilated patients | 53 (54.1) | 107 (67.3) | 0.034 |
| Renal replacement technique | 6 (6.1) | 13 (5.5) | 0.812 |
| ICU Medical treatments, n (%) | |||
| Lopinavir/ritonavir | 92 (93.9) | 0 (0.0) | <0.001 |
| Hydroxychloroquine | 97 (99.0) | 0 (0.0) | <0.001 |
| Remdesivir | 1 (1.0) | 18 (7.5) | 0.019 |
| Interferon | 41 (41.8) | 0 (0.0) | <0.001 |
| Tocilizumab | 57 (58.2) | 68 (28.5) | <0.001 |
| Corticosteroids | 94 (95.9) | 239 (100.0) | 0.007 |
| Antibiotics | 88 (89.9) | 218 (91.2) | 0.683 |
| Prophylactic dose anticoagulants | 16 (16.3) | 61 (25.5) | 0.068 |
| Intermediate dose anticoagulants (24 h) | 41 (41.8) | 104 (43.5) | 0.778 |
| High dose anticoagulants | 41 (41.8) | 74 (31.0) | 0.056 |
| Vasopressors | 64 (65.3) | 96 (40.2) | <0.001 |
| Muscle relaxants (in patients with IMV) | 57 (69.5) | 137 (88.4) | <0.001 |
APACHE-II, Acute Physiology and Chronic Health Evaluation II; FiO2, fraction of inspired oxygen; ICU, intensive care unit; PaO2, arterial partial pressure of oxygen.
Data are expressed as number (percentage), median (interquartile range).
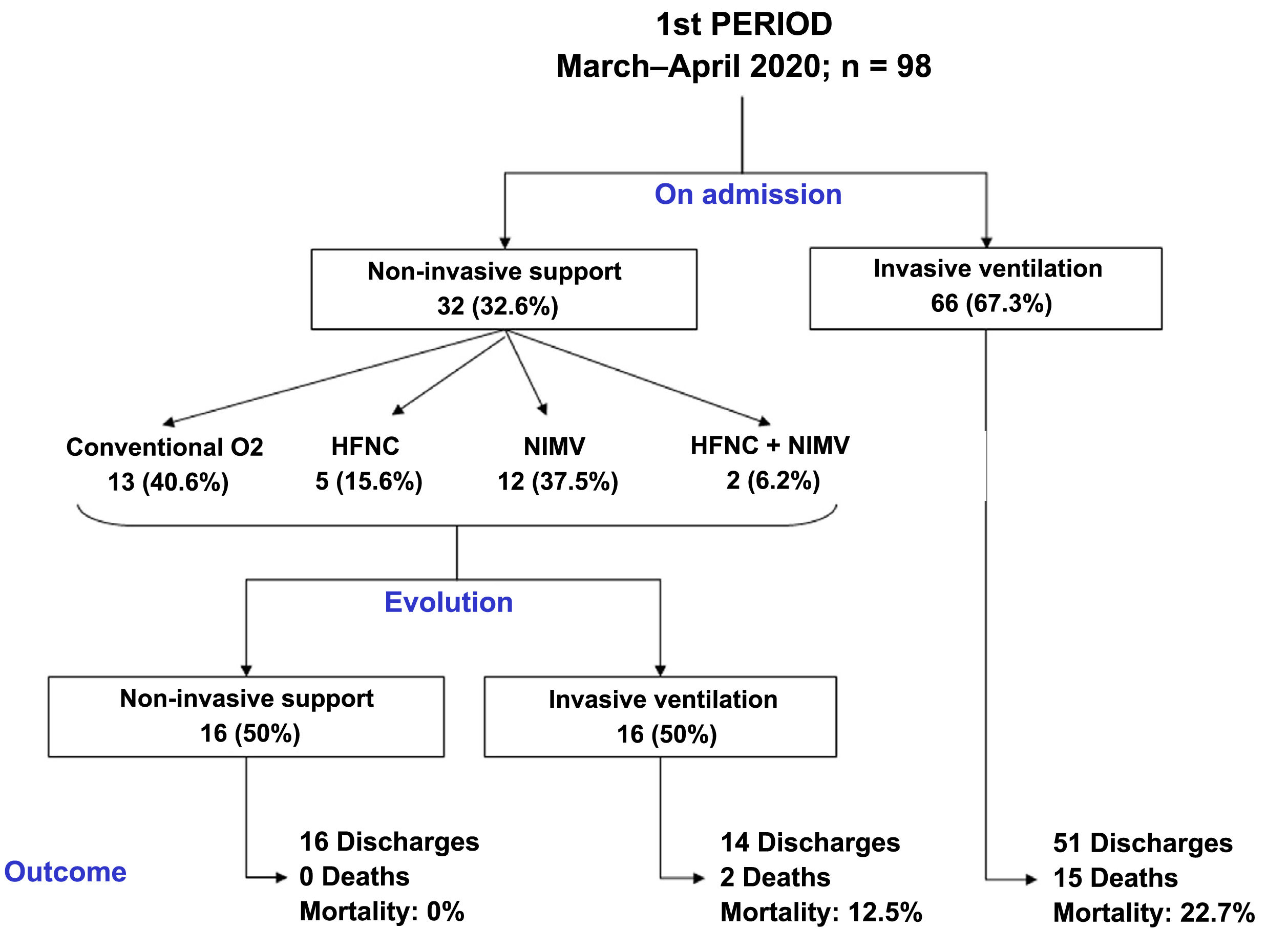
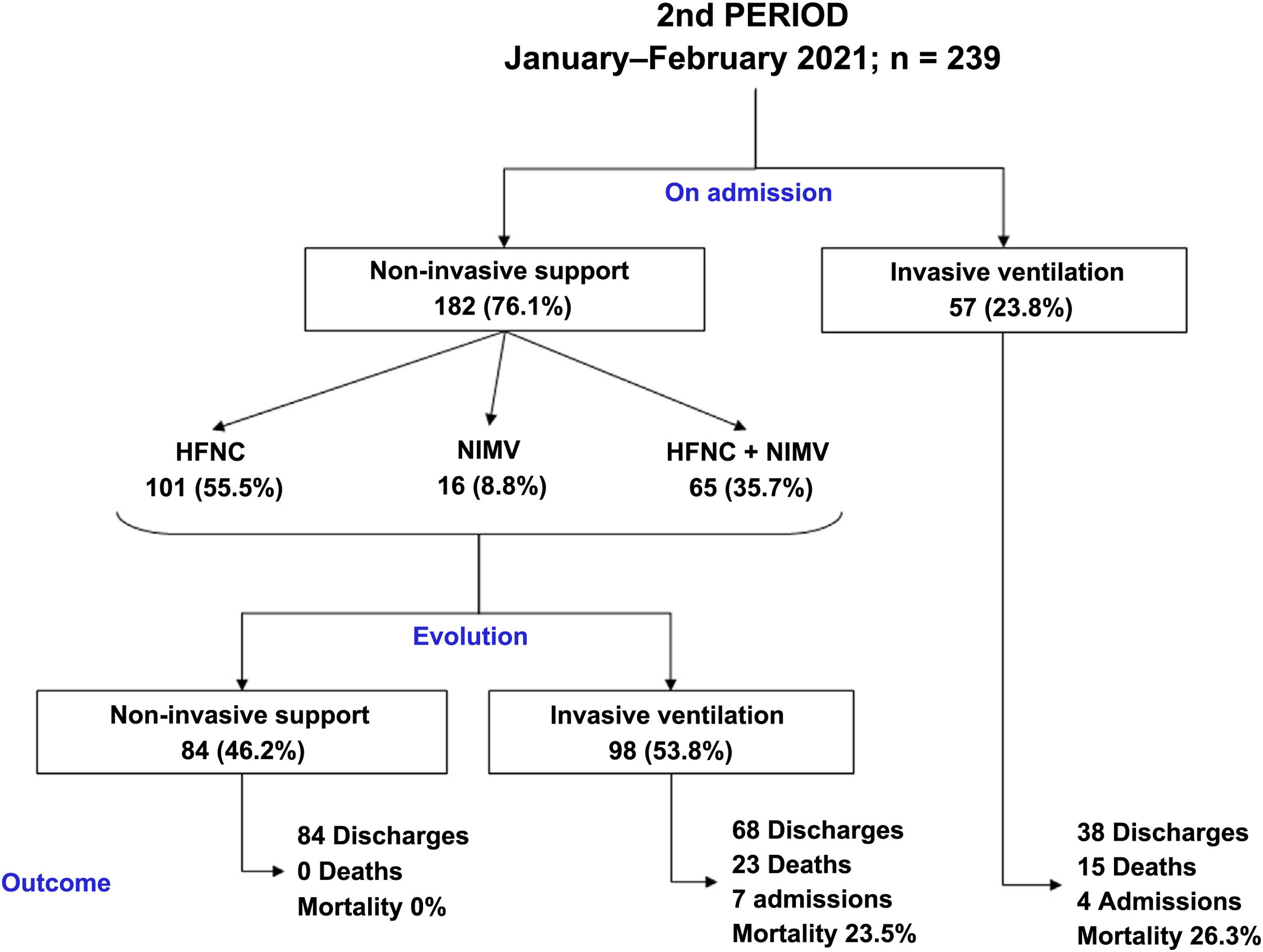
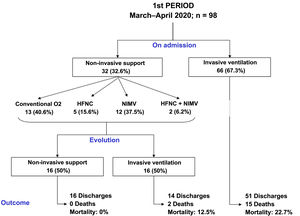
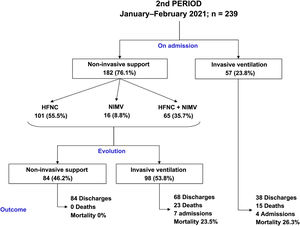
Compared to period 1, fewer patients in period 2 required IMV (65% vs 84%, p < 0.001) (Table 2). 90% of the patients who required IMV in period 1 were intubated in the first 24 h following admission to the ICU, compared to 63% in period 2 (p < 0.001). The start of IMV after admission to the ICU was later in period 2 (Table 2, p < 0.001). In relation to the days of IMV in intubated patients, no differences were observed between the two periods (13 [9–19] days vs 11 [8–20] days, p = 0.318). However, the days of IMV in the total number of patients admitted to the ICU were considerably lower in period 2 (7 [0–15] days vs 12 [6–18] days, p < 0.001).
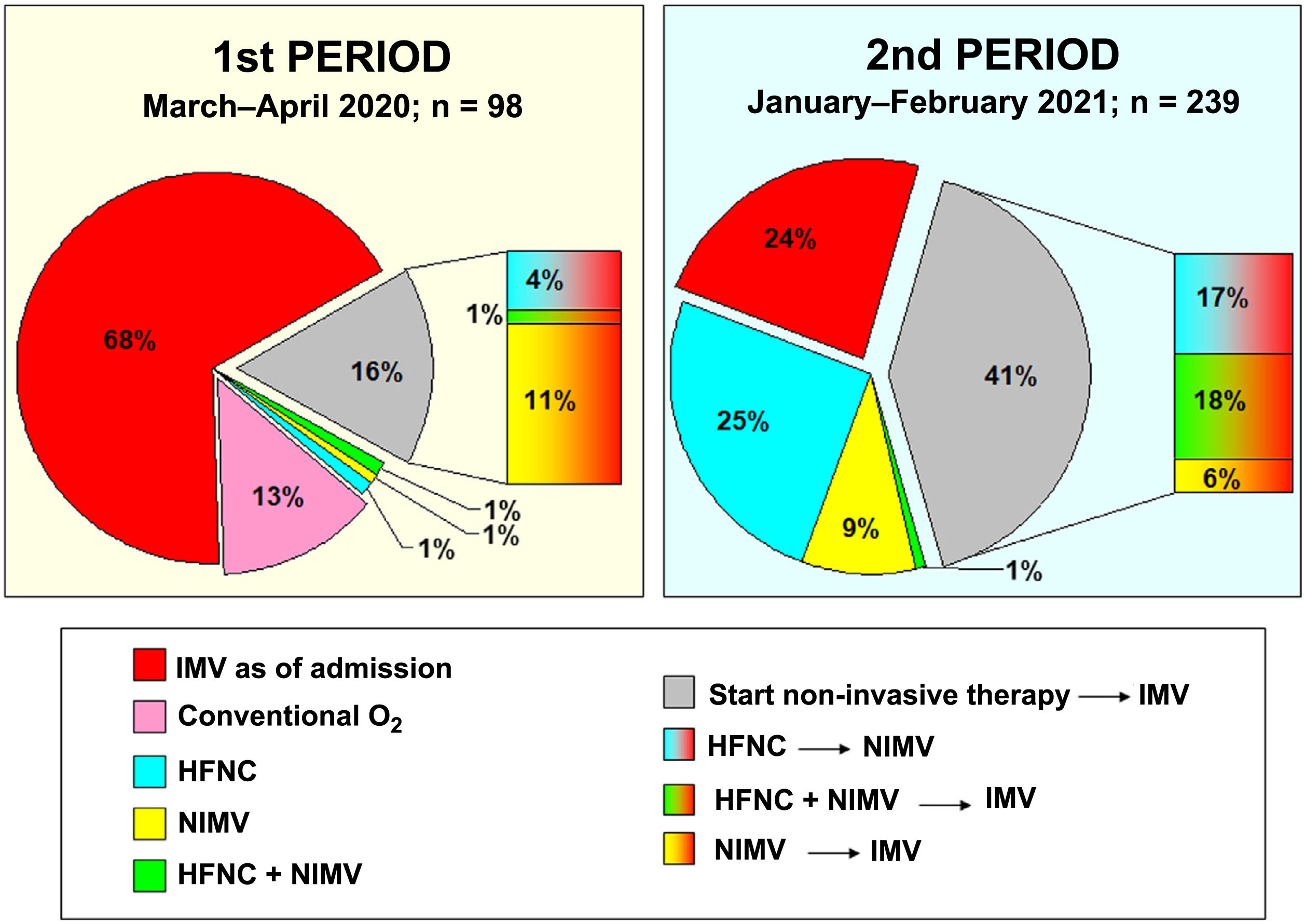
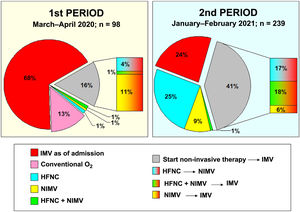
In period 2, a greater number of cases were managed with HFNC (70% vs 7%, p < 0.001), NIMV (40% vs 14%, p < 0.001) and prone positioning, both in awake patients (42% vs 28%, p = 0.012) and in intubated patients (67% vs 54%, p = 0.034) (Table 2). The management of the ventilation of patients on admission to the ICU and their evolution are described in Figs. 2, 3 and 4.
Table 2 shows the drugs used. Some drugs, such as lopinavir-ritonavir, hydroxychloroquine or interferon, were discontinued in period 2. Corticosteroids were used in 100% of patients in this second period.
No differences were found in relation to the mortality of patients admitted to the ICU between the two study periods (17% vs 16%, p = 0.756). However, the length of stay in the ICU (15 [10–24] vs 12 [7–20] days, p = 0.018) and the length of hospital stay (23 [15–36] vs 29 [19–43] days, p = 0.001) was lower in period 2 (Table 2).
Although we observed a trend towards a lower frequency of complications in patients with COVID pneumonia admitted to the ICU in period 2, we did not find significant differences in any of the complications studied (pneumothorax, reintubation, acute renal failure requiring renal replacement therapy, pulmonary thromboembolism or other thrombotic events, nosocomial infections, ICU readmissions, need for amines or death) (Tables 2 and 3).
Complications and outcome of Covid-19 patients treated in anaesthaesia ICUs during the two study periods.
| Period 1March–April 2020n = 98 | Period 2January–February 2021n = 239 | p | |
|---|---|---|---|
| Pneumothorax | 8 (8.2) | 11 (4.6) | 0.198 |
| Reintubation | 12 (12.2) | 17 (7.1) | 0.127 |
| Acute kidney injury requiring RRT | 6 (6.1) | 13 (5.5) | 0.812 |
| Pulmonary thromboembolism | 7 (7.1) | 13 (5.4) | 0.548 |
| Other thrombotic events | 5 (5.1) | 4 (1.7) | 0.128 |
| Nosocomial infection in patients on mechanical ventilation | 51 (62.2) | 102 (65.8) | 0.580 |
| Nosocomial infection in all hospitalised patients | 52 (53.1) | 113 (47.3) | 0.335 |
| ICU readmission | 4 (4.1) | 7 (3.0) | 0.598 |
| Death among patients who required MV | 16 (19.5) | 38 (24.7) | 0.369 |
| ICU Death | 17 (17.3) | 38 (16.0) | 0.756 |
| Intrahospital death | 20 (20.4) | 40 (16.8) | 0.433 |
ICU, intensive care unit; MV, mechanical ventilation; RRT, renal replacement therapy.
In the multivariate logistic regression analysis, in the first study period, age and the PaO2/FiO2 ratio were significantly associated with intrahospital mortality. In the second study period, being older and having a higher score on the APACHE-II scale were related to an increase in intrahospital mortality (Appendix B Table S1, Supplement).
DiscussionIn this study we investigated how the clinical characteristics, treatments received and outcomes of patients admitted for respiratory distress due to COVID-19 in nine ICUs in north-western Spain one year after the start of the pandemic varied. Comparing the first period of admissions in March and April 2020 with the last period in January and February 2021, in this second period we observed an increase in the use of non-invasive ventilatory support, a shorter IMV duration of patients admitted to the ICU and a shorter stay for patients, both in critical care units and in the hospital. However, we found no differences in terms of mortality.
In a similar study that compared the results of a smaller number of critical COVID-19 patients treated in a French ICU in the first and second waves of the pandemic, no differences were found in terms of mortality and length of stay in the ICU,20 although it is striking that they presented with a mortality rate close to 50%, much higher than that of our population.
During the first wave of the pandemic, coinciding with the first period of this study, we professionals faced a new disease that caused a large number of serious illnesses in a short period of time and led many ICUs to the point of saturation. In addition, there were questions regarding the forms of transmission of the disease, which conditioned respiratory support of patients in favour of IMV. The absence of specific treatments led to the use or avoidance of drugs based on the knowledge acquired in previous severe viral pneumonia epidemics.22,23 All these factors conditioned a high mortality and a high number of complications in the patients treated in this initial period. Greater knowledge of the pathophysiology and transmission of the disease, the development of clinical trials that tested the benefit or lack thereof of some therapies24 and the experience gained in the management of patients could give the impression that the mortality of patients admitted to the ICU almost a year later might be lower. However, in this study we found no differences in mortality in the second period of the pandemic compared to the first. Several factors could account for these results.
Firstly, in north-western Spain, unlike other regions, there was a greater healthcare burden during the second period (third pandemic wave) compared to the first period (first wave) (Fig. 1). This higher incidence of cases could be due to the increase in the British variant of the virus, which was predominant during the third wave of the pandemic in our region. System overload was one of the keys to COVID-19-associated mortality. In fact, mortality in the first wave for our anaesthesia ICUs was lower than that reported in studies published at the same time in other areas of our country with a greater healthcare burden.4,8,25
Secondly, corticosteroids, known to be effective in reducing the mortality of seriously ill patients with COVID-19,9,26 were already predominantly used in our area during the first study period, which could have contributed to a reduction in mortality compared to other areas during that stage and to a lesser positive impact of the treatment on our patients when the two periods studied are compared.
Regarding the clinical characteristics of the admitted patients, we observed, as other studies did, younger patients in the second period27, as well as less cardiac comorbidity. Both factors, and earlier ICU admission, may have contributed to the observation of a lower APACHE-II severity scale score, as well as some lower infection severity markers (CRP and D-dimers), and reduced need for vasopressors, compared to patients admitted a year ago. However, pulmonary pathology, reflected by gas exchange on admission, was more severe in patients in the second period.
One of the greatest differences in patient management was in the use of non-invasive ventilation therapies, restricted to a few cases at the beginning of the pandemic and the majority in the second period, an aspect that is also observed in other series.20,27 The early intubation or not of these patients continues to be a source of debate.28,29 Non-invasive support is intended to reduce the need for invasive ventilation and its associated complications. In the first period, most patients were managed with early IMV due to the open ICU structure and the risk of transmission between patients and professionals. In the second period studied, with more evidence that non-invasive therapies were safe, their use increased. The reduction in mortality with the use of these therapies compared to early IMV has not been proven, but they do seem to reduce the duration of mechanical ventilation and ICU stay,30 which could be take into account in situations of pandemic-induced overload. In fact, in our series, the duration of IMV, duration of ICU admission and duration of hospital admission were two, three and six days shorter, respectively, in the second period studied than in the first.
In contrast, a hypothetical delay in intubation may be associated with a worse subsequent prognosis, a circumstance observed in other cases of respiratory failure.31 However, this association has not been tested in patients with COVID-19.32 In our series, a longer delay between admission and intubation did not determine a longer duration of IMV in patients who were eventually intubated or a higher mortality in this subgroup of patients compared to those who were intubated at admission.
Placing the patient in the prone position for ventilation reduces mortality in severe distress.33 This technique has been used extensively since the start of the pandemic, even in spontaneous-breathing ventilated patients.16,17 Although its effectiveness is a source of debate in non-intubated patients,34 it was used more frequently in the second period in an attempt to reduce the need for intubation.
No drug other than corticosteroids has shown a mortality-reducing effect in patients with severe COVID 19. Many of those used predominantly at the beginning of the pandemic (hydroxychloroquine, lopinavir-ritonavir, interferon) were discontinued a year later due to the lack of evidence for their use.14,15 Only remdesivir, an antiviral that has demonstrated some effectiveness in improving clinical scores of infected patients,11,12 and the immunomodulator tocilizumab, whose effectiveness is still under discussion,35–37 were used in this second period.
Regarding the use of anticoagulants, in the series described, intermediate prophylaxis doses were predominantly administered in both periods, probably justified by the high percentage of obese patients. Despite the prothrombotic pathophysiology of COVID and the fact that some studies suggest a better outcome in patients in whom high doses of thromboprophylaxis are used,38 a randomised study found no difference between low- and intermediate-dose antithrombotic prophylaxis in a composite outcome of arterial or venous thrombotic events, the need for ECMO, or death, in patients admitted to the ICU,13 but there was an increase in the incidence of thrombocytopoenia in the group treated with intermediate doses. Currently, the use of high-dose prophylaxis is not recommended,39 except in specific populations, such as the obese.
We observed a non-significant trend towards a reduction in patient complications, reintubations and readmissions from the ward in the second period analysed. However, in line with other studies,40 the high percentage of cases with nosocomial infection continues to stand out, especially mechanical ventilation-associated pneumonia in patients with severe distress due to COVID-19.
This study has a number of limitations. This research only included patients with respiratory distress due to COVID-19 admitted to nine ICUs located in north-western Spain, hence the results may not reflect the experience of ICUs located in other regions of Spain or in other countries. Another limitation is that the design was observational, but the intention of the study was to analyse the change in therapeutic attitude and outcomes as knowledge about COVID-19 has increased after a year of pandemic.
In conclusion, patients admitted for respiratory distress due to COVID-19 in an ICU in north-western Spain, one year after the start of the pandemic, were younger, with worse gas exchange and less extrapulmonary pathology compared to the patients admitted in the first wave. More patients received non-invasive ventilation support and were placed in the prone position, both during IMV and during spontaneous breathing ventilation. We observed a reduction in the duration of IMV, in the length of ICU stay and length of hospital stay. The use of corticosteroids has been widespread and treatments tested in the initial phases of the disease and that were later proven to be ineffective have ceased to be used. Mortality on ICU admission did not present a significant reduction.
FundingNo funding was received for conducting this study. Support was provided solely from institutional and departmental sources.
AuthorsStudy concept: Pablo Rama, Yolanda Sanduende, Manuel Taboada.
Study design: Pablo Rama, Yolanda Sanduende, Manuel Taboada.
Data collection: all authors.
Data analysis: Teresa Seoane-Pillado.
Drafting of the manuscript: all the authors helped to review the draft manuscript.
Editing and approval of the manuscript: all authors.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank the doctors, nurses, auxiliaries, orderlies and staff involved in the treatment of COVID-19 patients during this very difficult year of the pandemic.