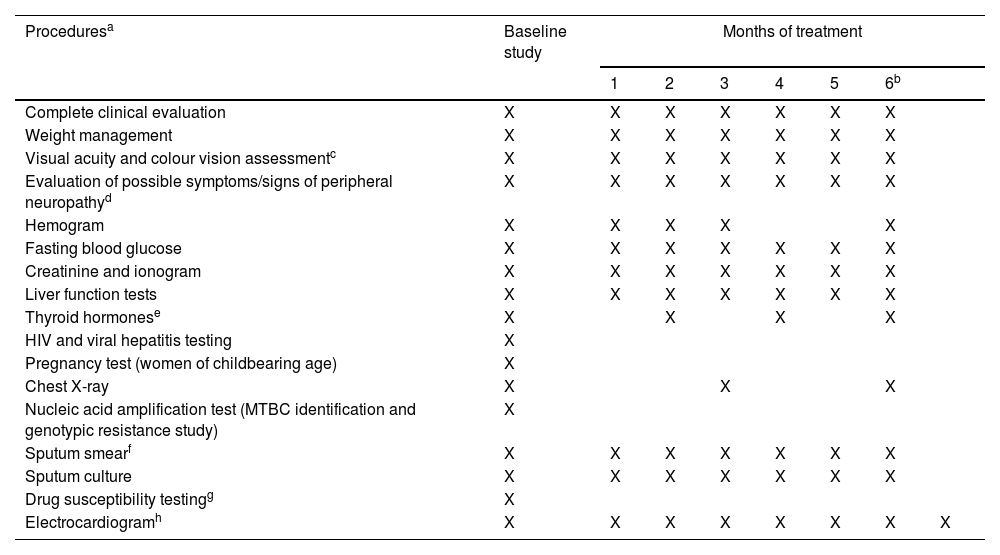
The Spanish Society of Pneumology and Thoracic Surgery (SEPAR) and the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) have developed together Clinical Practice Guidelines (GPC) on the management of people affected by tuberculosis (TB) resistant to drugs with activity against Mycobacterium tuberculosis. These clinical practice guidelines include the latest updates of the SEPAR regulations for the diagnosis and treatment of drug-resistant TB from 2017 to 2020 as the starting point. The methodology included asking relevant clinical questions based on PICO methodology, a literature search focusing on each question, and a systematic and comprehensive evaluation of the evidence, with a summary of this evidence for each question. Finally, recommendations were developed and the level of evidence and the strength of each recommendation for each question were established in concordance with the GRADE approach. Of the recommendations made, it is worth highlighting the high quality of the existing evidence for the use of nucleic acid amplification techniques (rapid genotypic tests) as initial tests for the detection of the M. tuberculosis genome and rifampicin resistance in people with presumptive signs or symptoms of pulmonary TB; and for the use of an oral combination of anti-TB drugs based on bedaquiline, delamanid (pretomanid), and linezolid, with conditional fluoroquinolone supplementation (conditioned by fluoroquinolone resistance) for six months for the treatment of people affected by pulmonary multidrug-resistant tuberculosis (MDR-TB). We also recommend directly observed therapy (DOT) or video-observed treatment for the treatment of people affected by DR-TB.
Artículo
Socio de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica

Para acceder a la revista
Es necesario que lo haga desde la zona privada de la web de la SEIMC, clique aquí
Para realizar los cursos formativos
La actividad estará abierta para socios de la SEIMC. IMPORTANTE, recuerde que requiere registro previo gratuito. Empezar aquí
Comprando el artículo el PDF del mismo podrá ser descargado
Precio 19,34 €
Comprar ahora