Detection and treatment of latent tuberculosis infection (LTBI) is an essential measure for tuberculosis (TB) control in low-incidence countries. However, such strategy is limited by the low predictive ability of the diagnostic tests for the development of active TB among infected people and the long-term and toxic treatment regimens. The in vitro interferon-gamma release assays are more specific and sensitive than the tuberculin skin test (TST), and enable a better selection of cases requiring treatment. Nonetheless, their capacity to predict development of TB is still poor. In addition, treatment regimens for LTBI are long, and compliance rates are low. This review discusses the use of the available diagnostic tests and the new approaches to the diagnosis of LTBI, as well as its management in different clinical scenarios.
La detección y tratamiento de la infección tuberculosa latente (ITBL) constituye una medida esencial para el control de la tuberculosis (TB) en países de baja incidencia. Esta estrategia está limitada, sin embargo, por la falta de capacidad predictiva de las pruebas diagnósticas para el desarrollo de TB activa entre las personas infectadas y la larga duración y toxicidad de las pautas de tratamiento. Las técnicas in vitro de liberación de interferón-gamma son más específicas y sensibles que la prueba de la tuberculina, y permiten seleccionar mejor los casos que requieren tratamiento. Aun así, su capacidad para predecir el desarrollo de TB sigue siendo pobre. Además, las pautas de tratamiento de ITBL son largas, y las tasas de cumplimiento, bajas. Esta revisión discute el uso de las técnicas diagnósticas disponibles y las nuevas aproximaciones al diagnóstico de la ITBL y su abordaje terapéutico en diferentes escenarios clínicos.
In addition to early treatment of patients with active tuberculosis (TB), the detection and treatment of latent tuberculosis infection (LTBI) has become an essential means of controlling TB in low-incidence countries, where the endogenous reactivation of a past infection is the principal source of new cases. Following primary infection, the immune system is capable of maintaining control in the majority of cases, giving rise to the persistence of viable bacilli that have the capacity to proliferate and develop into active TB in the future. Currently, this binary model of latency vs active disease is considered a simplification of a broader spectrum, stretching from elimination of the infection to clinical disease and passing through latent and clinically silent states. In practice, however, a pragmatic definition of LTBI as infection by Mycobacterium tuberculosis complex, based on reactivity to the tuberculin skin test (TST) or to an interferon-gamma release assay (IGRA), without evidence of active disease, is accepted.1 The risk of progression to active TB is estimated at 10% of infected individuals. Recent infection, age<5 years, cellular immune deficiency of any nature and never-treated pulmonary TB with residual lesions significantly increase the risk of progression.1 Between 1950 and 1960, the usefulness of isoniazid (INH) to prevent the development of TB in people exposed to patients with pulmonary TB was demonstrated. Since then, the same benefit has been demonstrated, with INH or alternative regimens, in other at-risk groups. This article discusses the diagnosis of and therapeutic approach to LTBI on the basis of current evidence.
Diagnosis of latent tuberculosis infectionLTBI is diagnosed using techniques that test the sensitivity of the individual to various M. tuberculosis antigens. Traditionally, the technique used has been the TST. This technique involves the intradermal inoculation of a purified protein derivative (PPD) containing a mix of more than 200 antigens present in M. tuberculosis, in the Bacillus Calmette-Guérin (BCG) vaccine strain and environmental mycobacteria.
Available techniques for the diagnosis of tuberculosis infectionThe TST has been in use for more than 100 years and represents a useful tool in the management of tuberculosis infection. However, it does have significant limitations. The main deficiency is due to its low specificity in individuals vaccinated with BCG and infected with environmental mycobacteria. Therefore, in vaccinated individuals or those sensitised with environmental mycobacterium, it is virtually impossible to distinguish a positive response due to a true reaction to M. tuberculosis from a reaction due to another cause. Moreover, the TST has reduced sensitivity in immunocompromised individuals and small children with immature immune systems, as they cannot mount an adequate response to antigen stimulation. In these cases, it is also difficult to discern between anergy and a true negative result.
Over a decade ago, the use of in vitro immunodiagnostic techniques was introduced in clinical practice, allowing tuberculosis infection to be diagnosed by means of laboratory tests. In short, these techniques involve in vitro stimulation of T-cells circulating in the blood, using antigens specific to the M. tuberculosis complex. If the individual is infected, their T-cells will respond by releasing a wide variety of cytokines that can be detected using immunological techniques. The cytokine detected in the commercially available techniques is interferon-gamma (IFN-γ). The main advantage of IGRAs stems from the antigens used to stimulate the T-cells: the proteins 6-kD M. tuberculosis early-secreted antigenic target (ESAT-6) and 10-kD culture filtrate protein (CFP-10) codified in region of difference 1 (RD1).2 These antigens are specific to M. tuberculosis and are not present in either the BCG vaccine strain or the majority of environmental mycobacteria. The two commercially available techniques are QuantiFERON®-TB Gold (QFT-G) (Qiagen, Düsseldorf, Germany) and T-SPOT®-TB (Oxford Immunotec, Oxford, UK). Both techniques have been approved by the U.S. Food and Drug Administration (FDA) and the European Commission (for use in Europe). Until recently, the version of QFT-G used was QuantiFERON-TB Gold In-Tube (QFT-GIT), which also included a third Ag (TB7.7) and detected the response of CD4+T cells. A new version is now in use, QuantiFERON®-TB Gold Plus (QFT-Plus), which is capable of identifying responses by CD4+T and CD8+T cells. Its other difference is that the TB7.7 Ag has been removed. The main difference between IGRAs is that whereas QFT-G stimulates whole blood and measures the quantity of IFN-γ released using ELISA, T-SPOT.TB measures the number of T-cells that produce IFN-γ in response to stimulation using ELISPOT. IGRAs offer a further potential advantage in the inclusion of negative and positive controls to identify possible non-specific reactions and false negative results.3 IGRAs can also be affected by immune deficiency and difficulty responding to M. tuberculosis antigens, although the studies carried out appear to suggest that the impact is less than with the TST.4
The primary objective of screening for tuberculosis infection is to be able to identify and threat those individuals who are infected and will progress to active disease. However, both the TST and IGRAs, to a greater or lesser extent, only detect sensitivity to antigens. The individuals identified may be those with recent infection and most risk of progression, those with distant infection and very low risk or even those whose risk of progression is null, but who still maintain an immune response to the mycobacterium. In other words, the capacity of the TST and IGRAs to predict the development of TB is very poor, as a large number of individuals with a positive result in the TST or IGRAs will not progress to clinical disease.5 Nonetheless, even with this limitation, IGRAs have significantly improved the diagnosis of tuberculosis infection. In spite of their similar predictive values, their increased specificity has allowed the number of unnecessary preventive treatments to be reduced without increasing the risk of developing active tuberculosis down the line, as has been demonstrated in a recent observational study6 and clinical trial.7 In addition, IGRAs have improved the detection of tuberculosis infection in immunocompromised patients.
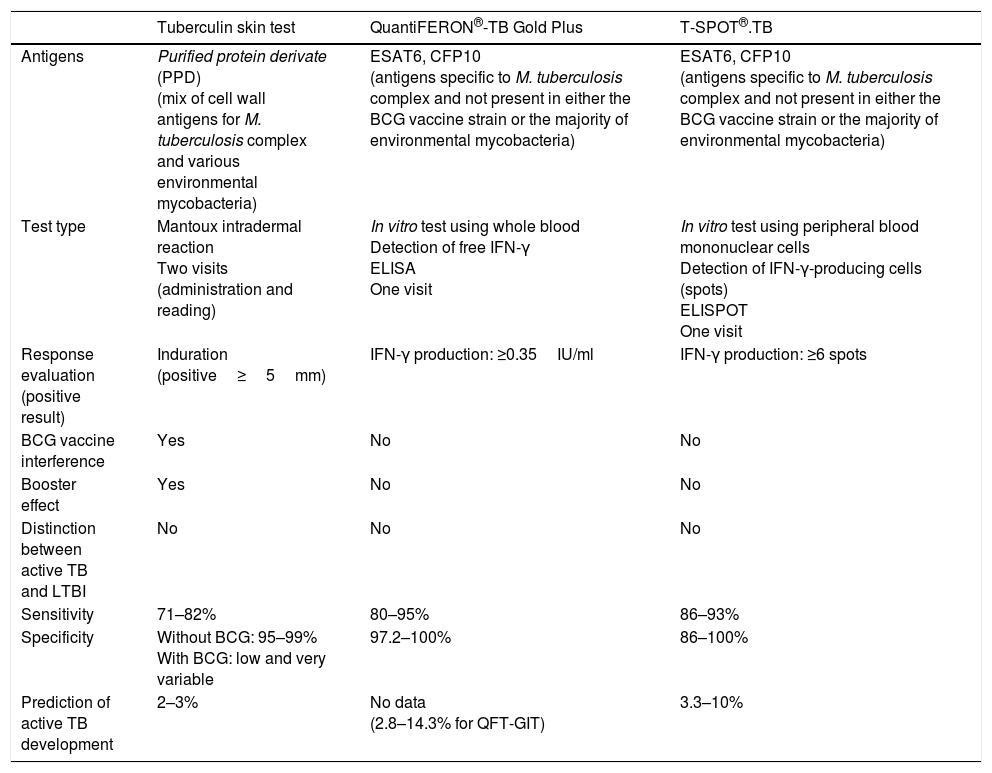
The potential capacity of QFT-Plus, the new version of QFT-GIT, to detect the specific response of CD4+T and CD8+T cells may increase our ability to identify infected individuals, especially those who are immunocompromised, since many immune deficiencies are associated with general lymphopenia or specific depletion of CD4+T cells. Moreover, CD8+T cells, which respond to short-chain peptides on RD1 antigens, are detected more frequently in patients with active TB than in those infected without active disease, and this response can therefore be associated with recent exposure to M. tuberculosis and a higher bacterial load.8 To date, it has been confirmed that QFT-Plus presents similar sensitivity and specificity to QFT-GIT, and it has been suggested that it might allow us to better identify those infected individuals at greater risk of progression to active disease.9,10 Even so, these data must be confirmed and evidence obtained of its use in managing the diagnosis of tuberculosis infection in the immunocompromised population. Table 1 shows the general differences between the TST and IGRAs.
Comparison between the available tests for latent tuberculosis infection.
| Tuberculin skin test | QuantiFERON®-TB Gold Plus | T-SPOT®.TB | |
|---|---|---|---|
| Antigens | Purified protein derivate (PPD) (mix of cell wall antigens for M. tuberculosis complex and various environmental mycobacteria) | ESAT6, CFP10 (antigens specific to M. tuberculosis complex and not present in either the BCG vaccine strain or the majority of environmental mycobacteria) | ESAT6, CFP10 (antigens specific to M. tuberculosis complex and not present in either the BCG vaccine strain or the majority of environmental mycobacteria) |
| Test type | Mantoux intradermal reaction Two visits (administration and reading) | In vitro test using whole blood Detection of free IFN-γ ELISA One visit | In vitro test using peripheral blood mononuclear cells Detection of IFN-γ-producing cells (spots) ELISPOT One visit |
| Response evaluation (positive result) | Induration (positive≥5mm) | IFN-γ production: ≥0.35IU/ml | IFN-γ production: ≥6 spots |
| BCG vaccine interference | Yes | No | No |
| Booster effect | Yes | No | No |
| Distinction between active TB and LTBI | No | No | No |
| Sensitivity | 71–82% | 80–95% | 86–93% |
| Specificity | Without BCG: 95–99% With BCG: low and very variable | 97.2–100% | 86–100% |
| Prediction of active TB development | 2–3% | No data (2.8–14.3% for QFT-GIT) | 3.3–10% |
BCG: Bacillus Calmette-Guérin; CFP10: culture filtrate protein 10; ESAT6: early-secreted antigenic target 6; IFN-γ: interferon-gamma; LTBI: latent tuberculosis infection; QFT-GIT: QuantiFERON®-TB gold in-tube; TB: tuberculosis; TST: tuberculin skin test.
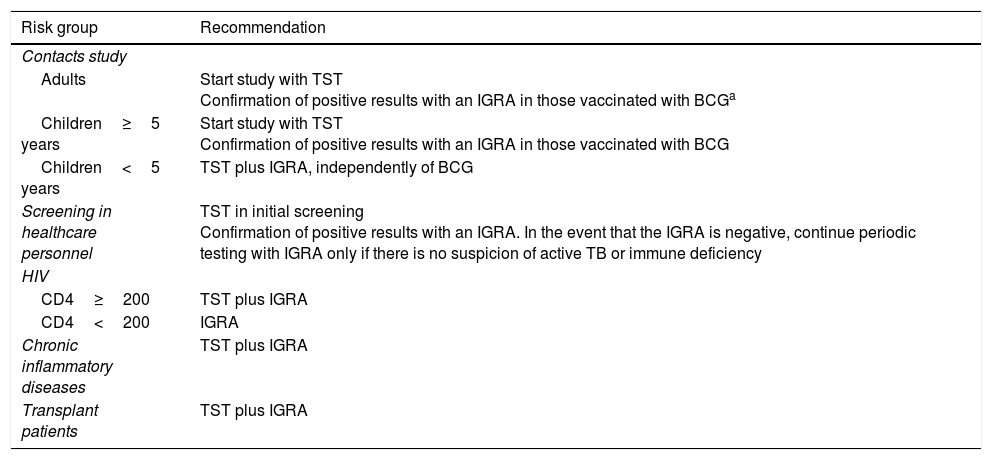
An evidence-based consensus document created by a panel of experts from the Mycobacteria Study Group (GEIM–Grupo de Estudio de Micobacterias) of the Spanish Society for Infectious Diseases and Clinical Microbiology (SEIMC–Socieded Española de Enfermedades Infecciosas y Microbiología Clínica) and the Spanish Society for Pulmonology and Thoracic Surgery (SEPAR – Socieded Española de Neumología y Cirugía Torácica) for the use of diagnostic techniques for tuberculosis infection was published in 2016.11 The recommendations are based on distinct population groups and their risk of progressing to active disease if infected (Table 2). When this document was being produced, the existing evidence for QFT-G corresponded only to the QFT-GIT version, as QFT-Plus had not yet been released. However, in view of the available data, the recommendations given below can also be applied to QFT-Plus. The principal recommendations for use are:
Recommendations for use of the tuberculin skin test and IGRAs in clinical practice11
| Risk group | Recommendation |
|---|---|
| Contacts study | |
| Adults | Start study with TST Confirmation of positive results with an IGRA in those vaccinated with BCGa |
| Children≥5 years | Start study with TST Confirmation of positive results with an IGRA in those vaccinated with BCG |
| Children<5 years | TST plus IGRA, independently of BCG |
| Screening in healthcare personnel | TST in initial screening Confirmation of positive results with an IGRA. In the event that the IGRA is negative, continue periodic testing with IGRA only if there is no suspicion of active TB or immune deficiency |
| HIV | |
| CD4≥200 | TST plus IGRA |
| CD4<200 | IGRA |
| Chronic inflammatory diseases | TST plus IGRA |
| Transplant patients | TST plus IGRA |
BCG: Bacillus Calmette-Guérin; IGRA: interferon gamma release assay; TB: tuberculosis; TST: tuberculin skin test.
The benefit of confirming a positive TST with QFT-G, even in contacts who have not received the BCG vaccine, has recently been demonstrated.7
Given that the TST continues to be much more widely available than IGRAs in healthcare centres, and that the percentage of patients with a negative result who do not develop TB is very high, it recommends that the study of contacts be initiated with the TST, although positive results will need to be confirmed with an IGRA in individuals vaccinated with BCG.
Screening in healthcare personnelThe TST is recommended for initial screening, as well as periodic testing for tuberculosis infection in healthcare personnel. However, it suggests that positive results be reconfirmed with an IGRA. In the event that the TST is positive but the IGRA is negative, it suggests continuing periodic testing with IGRAs only if there is no suspicion of active TB or immune deficiency.
Study of contacts in childrenAs in adults, it recommends initiating the study of contacts with the TST, but, in the case of children vaccinated with BCG, positive results must be confirmed with an IGRA. Due to the recognised low sensitivity of the techniques in children under 5 years of age, it suggests performing both the TST and IGRAs, regardless of BCG vaccination, with the aim of maximising the chances of detecting those infected children at greatest risk of progressing to clinical disease.
Patients with human immunodeficiency virus (HIV) infectionIt recommends using the TST and IGRAs in individuals infected with HIV. In addition, in individuals with HIV infection and a CD4 count below 200/ml, the panel suggests using only IGRAs, as the TST rarely detects infection in patients with advanced immune deficiency.
Patients with chronic inflammatory diseasesThe recommendation is aimed at screening for tuberculosis infection before patients begin biological therapies. These patients already receive other immunomodulator therapies that may reduce the sensitivity of screening techniques. It therefore recommends that both the TST and IGRAs be used to maximise the chances of detecting tuberculosis infection.
Transplant patientsThe recommendation in this at-risk population is also to perform screening prior to transplant using the TST and an IGRA.
Further considerations on the use of interferon-gamma release assaysThe use of IGRAs requires a certain infrastructure in order to perform them, which includes the relatively rapid transport of samples to laboratories (particularly important for the T-SPOT.TB) and the need to perform venipuncture, which makes studies of contacts in very large groups logistically more difficult to carry out.
The choice of one IGRA technique over another depends on diverse factors, beyond the inherent strengths of each, which, on the basis of the published evidence, can be considered comparable, and remains the user's decision. Some of the considerations that must be taken into account when choosing a technique are detailed below. On the one hand, T-SPOT.TB has the disadvantage of requiring more technical handling in the laboratory than QFT. But on the other, there is the perception that T-SPOT.TB offers greater sensitivity in the immunocompromised population and young children, although this perception is not solidly documented. An interesting fact is that the results of IGRAs can be affected by tobacco use.12 It has recently been demonstrated that there is a significant relationship between the number of cigarettes smoked by the patient and the possibility of a negative or undetermined IGRA result.13
There are also other logistical considerations, such as the fact that, in not requiring a second visit by the patient in order to obtain the results, as is the case with the TST, IGRAs may be preferable in those patients who are not expected to return for further visits.
Future approaches in the diagnosis of tuberculosis infectionWith the aim of overcoming the principal limitations of the available techniques–for example improving positive predictive value, capacity to distinguish between infection and disease, and between recent and remote infection – work is ongoing in the development of new approaches that will allow for global improvements in the diagnosis of tuberculosis infection.
Techniques that maintain the interferon-gamma release assay formatOn the one hand, new techniques that keep to the IGRA format, but aim to detect other cytokines, such as IP-10, have demonstrated that they may be a good alternative to the use of IFN-γ.14 IP-10 has demonstrated comparable results in various study groups,15–17 as well as being found to be very stable when dried on filter paper, which would facilitate the transport of samples to reference laboratories.17 On the other hand, other antigens to stimulate T-cells are being explored, such as dosR-regulon encoded proteins and IVE-TB antigens, which are expressed when the bacteria is latent. Despite the existence of results that could be considered promising, these have still not been completely finalised.18 A further proposal is a test similar to the TST involving intradermal inoculation of the specific antigens ESAT-6 and CFP-10 (C-Tb, Statens Serum Institut). This in vivo technique presents no cross-reactivity in individuals vaccinated with BCG and could be a real alternative to the conventional TST.19
The significance of the quantitative value of the IFN-γ response in the QFT-GIT, as a tool for predicting progression to disease, has been a controversial subject since IGRAs were first used. Recently, a study has found evidence that QFT-GIT conversions with elevated IFN-γ values have a strong prognostic value for the development of TB in children, which may help to improve decision-making in the prescription of preventive treatment.20,21 For this reason, more in-depth knowledge of the variability of these in vitro techniques, as well as conversions/reversions, may help to identify those persons at greatest risk of developing active TB.22 Lastly, transcriptome study could be a very useful tool to detect host RNA profiles that may be associated with the development of TB.23
Antibody glycosylation pattern detectionAlthough researchers have been trying for years to develop diagnostic techniques based on the detection of antibodies, until now these methods have been of little use in the diagnosis of TB. Recently, a study published by Lu et al. in the journal Cell24 has demonstrated that patients with active TB present IgG antibodies with patterns of glycosylation that differ from those of infected individuals. Concrete differences have been observed in the presence of galactose and sialic acid in IgG antibodies, such that infected individuals present much higher levels than those with active disease. It is known that the presence of certain galactose or sialic acid structures is associated with increased anti-inflammatory activity. These results therefore suggest that distinct glycosylation profiles may induce different types of effector response.25
Cell markersFinally, the search for new cell markers through cytometry is also a promising approach. It has been observed that the reduction in the expression of the cell marker CD27 on CD4+T cells specific to M. tuberculosis might represent a marker of active TB, as during infection with M. tuberculosis a series of CD4+T cells with low CD27 expression is produced in the blood as well as an increase of this cell type at the site of the infection.26,27 This process is known as homing, in reference to the migratory action of the cells that express specific homing receptors such that they are directed to the infected tissue.
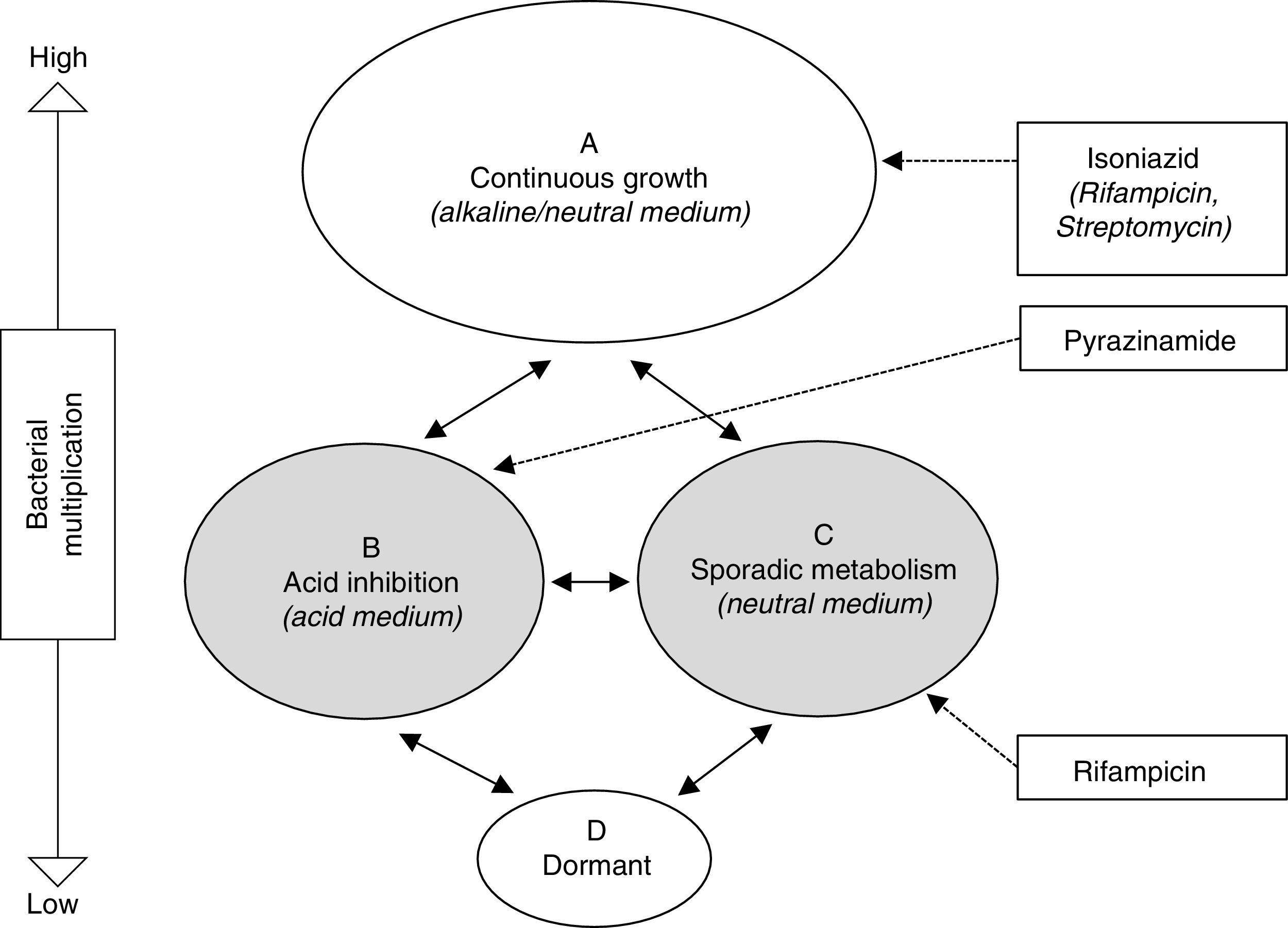
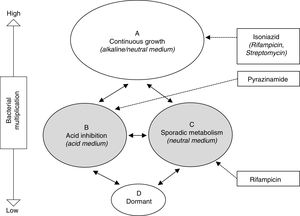
Treatment of latent tuberculosis infectionThe grounds for antibiotic treatment for the prevention of active TB are the elimination of the latent bacterial population, made up of bacilli with low metabolic activity, with the aim of avoiding a later reactivation. This population encompasses both the intracellular (macrophages) and extracellular in the caseum, as described by Mitchinson28 (Fig. 1). Paradoxically, while INH is very active against a constantly multiplying bacillus population, its activity against bacilli with low metabolic activity is marginal. The widespread use of INH with this objective is motivated by the excellent bactericidal activity demonstrated in the treatment of active TB prior to this knowledge. Specifically, the sterilising activity of rifampicin (RMP) and pyrazinamide (PZA), through its activity against this latent population, has prompted studies in animal models and clinical trials for the treatment of LTBI.
Schematic representation of hypothetical bacterial populations in active tuberculosis and the activity of anti-tuberculosis drugs.28 Populations B and C (shaded) represent metabolically less active types, which are essentially susceptible to pyrazinamide and rifampicin, respectively.
The efficacy of INH was established in clinical trials started in the 1950s and completed in the 1960s by the U.S. Public Health Service with more than 100,000 subjects, including children, TB contacts, institutionalised patients and those with positive TSTs.29,30 They compared INH against a placebo in these subjects for 12 months. The efficacy of INH varied between 25% and 92% (90% in subjects who adhered to treatment). Efficacy was correlated with treatment duration, gradually increasing up to 9–10 months and stabilising from that point. This observation prompted the majority recommendation of a duration of 9 months. Nonetheless, in a later meta-analysis including 73,375 subjects, INH for 6–12 months reduced the risk of TB by 60%, with no significant differences between a 6-month (relative risk: 0.44; 95% CI: 0.27–0.73) and 12-month (relative risk: 0.44; 95% CI: 0.27–0.73) duration.31 On the other hand, a study in 28,000 people with fibrotic pulmonary lesions compatible with previous TB demonstrated greater effectiveness of INH for 12 months (75%) compared to 6 months (65%), which increased when only those subjects who adhered to the treatment were taken into account (69% for 6 months and 93% for 12 months). Overall, the protective effect of preventive treatment with INH in these studies persisted for a number of decades.30 Isoniazid was also effective for the treatment of LTBI in patients infected with HIV.32 Its protective effect appears to the prolonged, as was demonstrated in a recent study in Brazil,33 but gradually decreases with time in high-incidence countries.
Hepatic toxicity is potentially the most serious adverse effect of INH. In its early days, when this effect was not well-known, there were serious cases of hepatitis, and even deaths.29,30 Rates of clinical hepatitis varied between 0.0% and 2.9% in a meta-analysis of 38,257 subjects. These cases arose primarily in the first three months of treatment, and age was a significant risk factor, varying between 0% in the under 20s and 2.3% in adults between 50 and 64 years of age. Contemporary studies, however, have given much lower rates of symptomatic hepatitis, varying between 1 and 3 per 1000.34 This is likely due to closer follow-up of patients and early suspension of treatment, thereby avoiding progression to more serious forms of hepatitis.
Alternatives to isoniazidDespite its demonstrated efficacy, INH's 6–9 month treatment regimens are still long and compliance is suboptimal. As alternatives to INH, attention has focussed on RMP and PZA, drugs that have allowed treatment for active TB to be shortened to 6 months. A recent meta-analysis concluded that regimens which include RMP for 3 or more months were equally as effective as, if not more than, INH for 6–9 months.35
Rifampicin in combination with pyrazinamideA regimen consisting of RMP and PZA for 2 months was effective for the prevention of TB in patients with HIV infection, and was recommended by the ATS/CDC in 2000. However, after its use was extended, cases of severe hepatotoxicity, including death, were reported in people without HIV infection, due to which its use is not recommended.29,30,34
Rifampicin in combination with isoniazid (RH)The combination of RMP with INH is a valid alternative to INH, and is in widespread use. Its efficacy has been proven, in particular in patients with HIV infection in Africa.29 It was evaluated in immunocompromised subjects in a clinical trial in patients with silicosis, which included INH for 6 months, RMP for 3 months and the combination of RMP and INH for 3 months vs placebo.36 All three regimens reduced the risk of TB with respect to placebo, but there were no significant differences between them. Considering only subjects who adhered to treatment, efficacy was estimated at 48% for INH and 41% for RMP in combination with INH.
Rifampicin monotherapyMonotherapeutic treatment of LTBI with RMP was studied in the clinical trial cited above, in which its effectiveness was estimated at 63% vs 48% for INH.36 RMP monotherapy has been associated with fewer serious adverse effects and better compliance rates than INH. The treatment duration usually recommended for RMP is 3–4 months.
Rifapentine in combination with isoniazidRifapentine (RPT) a medium- to long-acting rifamycin, has demonstrated good activity against LTBI in murine models when administered in combinations with INH, and its clinical efficacy was demonstrated in a clinical trial comparing RPT 600mg and INH 900mg, administered weekly for 3 months under directly observed therapy, with 9 months of self-administered INH 600mg/day.37 The regimen of RPT with INH was as effective as INH alone (0.19% and 0.43% of cases for RPT/INH and INH, respectively), had a higher rate of compliance (82.1% vs 69%) and caused less hepatotoxicity (0.4% vs 2.7%), although treatment was suspended due to adverse effects in more cases (4.9% vs 3.7%). Similar results were obtained in children and patients with HIV infection.38,39 Owing to these results, this regimen was approved by the FDA for the treatment of LTBI under directly observed therapy in persons≥2 years of age and, in 2014, the Centers for Disease Control and Prevention and the World Health Organisation included it as an alternative to INH. Rifapentine does not, however, have approval in Europe.
Treatment of latent tuberculosis infection in different clinical scenariosToday, the need to improve control of LTBI in order to meet the overall goal of control and elimination of TB is widely accepted. A significant reduction in the pool of LTBI carriers would have a real impact on controlling its spread at a population level. Nonetheless, this strategy is limited by two factors: firstly, the lack of diagnostic tests with the ability to adequately predict the development of active TB or biomarkers of LTBI reactivation, meaning that many unnecessary treatments must be administered in order to prevent a small number of cases of TB, and, secondly, the long duration of the treatment regimens and low rates of compliance. We are therefore faced with the dilemma of needing to treat more LTBI cases in order to have a real impact on TB control, while also needing to focus our efforts on the groups at most risk, in whom this strategy has been found to be cost-effective. The strategies and other programmatic aspects of TB control fall outside the scope of this article and can be consulted in other sources. Below we discuss relevant aspects of treatment in clinical scenarios in which it is routine practice in accordance with the recommendations in clinical practice guidelines.
Recent tuberculosis infectionThe basis for treatment due to recent TB infection is the increased risk of progression to active disease in the first years after the primary infection. Any person with a recent change in TST or IGRA results, or contact with a patient suffering from pulmonary and/or laryngeal TB and a TST≥5mm or positive IGRA, once active disease has been ruled out, should be offered treatment for tuberculosis infection (Table 3).
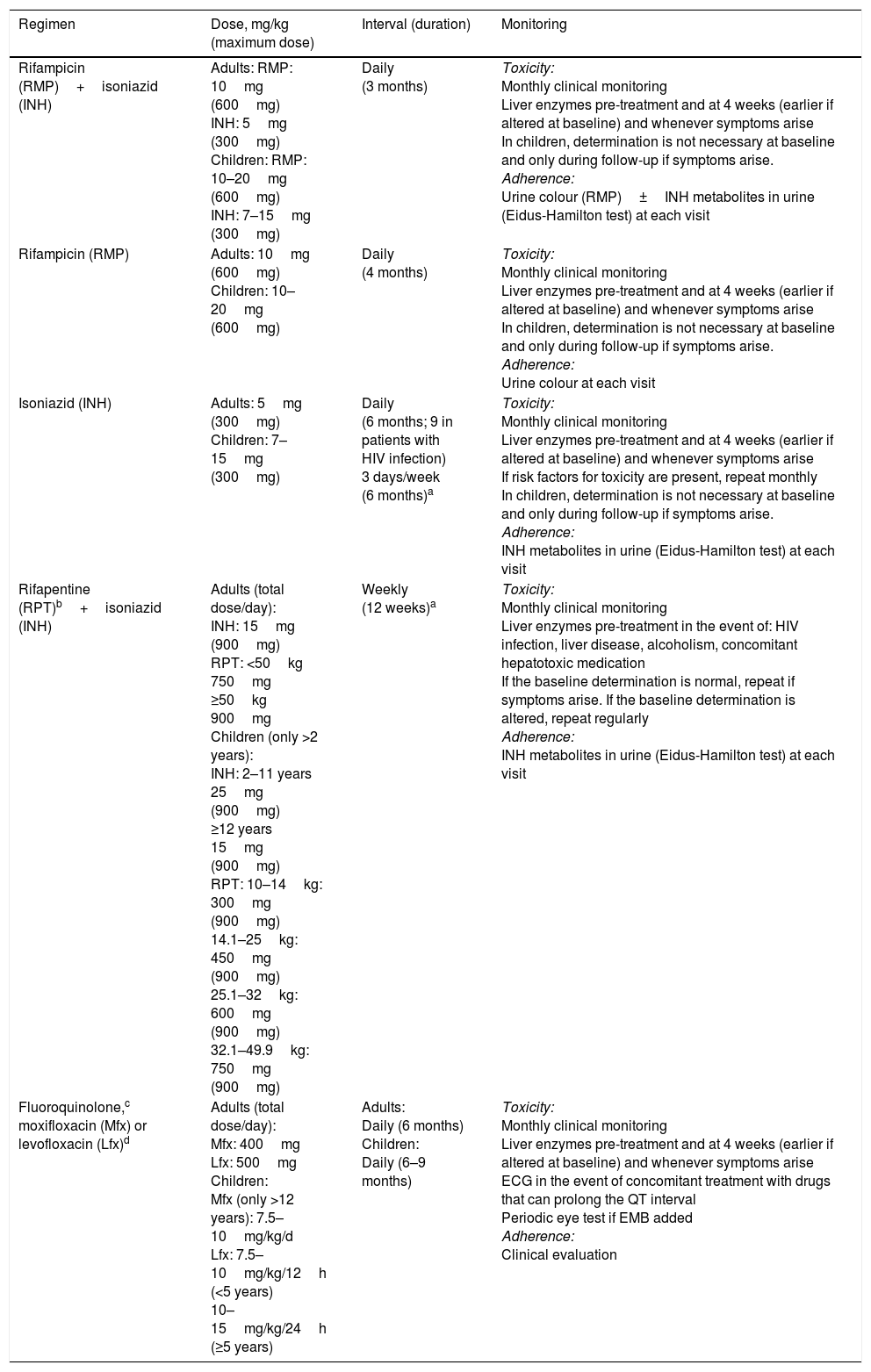
Treatment regimens recommended for the treatment of latent tuberculosis infection.
| Regimen | Dose, mg/kg (maximum dose) | Interval (duration) | Monitoring |
|---|---|---|---|
| Rifampicin (RMP)+isoniazid (INH) | Adults: RMP: 10mg (600mg) INH: 5mg (300mg) Children: RMP: 10–20mg (600mg) INH: 7–15mg (300mg) | Daily (3 months) | Toxicity: Monthly clinical monitoring Liver enzymes pre-treatment and at 4 weeks (earlier if altered at baseline) and whenever symptoms arise In children, determination is not necessary at baseline and only during follow-up if symptoms arise. Adherence: Urine colour (RMP)±INH metabolites in urine (Eidus-Hamilton test) at each visit |
| Rifampicin (RMP) | Adults: 10mg (600mg) Children: 10–20mg (600mg) | Daily (4 months) | Toxicity: Monthly clinical monitoring Liver enzymes pre-treatment and at 4 weeks (earlier if altered at baseline) and whenever symptoms arise In children, determination is not necessary at baseline and only during follow-up if symptoms arise. Adherence: Urine colour at each visit |
| Isoniazid (INH) | Adults: 5mg (300mg) Children: 7–15mg (300mg) | Daily (6 months; 9 in patients with HIV infection) 3 days/week (6 months)a | Toxicity: Monthly clinical monitoring Liver enzymes pre-treatment and at 4 weeks (earlier if altered at baseline) and whenever symptoms arise If risk factors for toxicity are present, repeat monthly In children, determination is not necessary at baseline and only during follow-up if symptoms arise. Adherence: INH metabolites in urine (Eidus-Hamilton test) at each visit |
| Rifapentine (RPT)b+isoniazid (INH) | Adults (total dose/day): INH: 15mg (900mg) RPT: <50kg 750mg ≥50kg 900mg Children (only >2 years): INH: 2–11 years 25mg (900mg) ≥12 years 15mg (900mg) RPT: 10–14kg: 300mg (900mg) 14.1–25kg: 450mg (900mg) 25.1–32kg: 600mg (900mg) 32.1–49.9kg: 750mg (900mg) | Weekly (12 weeks)a | Toxicity: Monthly clinical monitoring Liver enzymes pre-treatment in the event of: HIV infection, liver disease, alcoholism, concomitant hepatotoxic medication If the baseline determination is normal, repeat if symptoms arise. If the baseline determination is altered, repeat regularly Adherence: INH metabolites in urine (Eidus-Hamilton test) at each visit |
| Fluoroquinolone,c moxifloxacin (Mfx) or levofloxacin (Lfx)d | Adults (total dose/day): Mfx: 400mg Lfx: 500mg Children: Mfx (only >12 years): 7.5–10mg/kg/d Lfx: 7.5–10mg/kg/12h (<5 years) 10–15mg/kg/24h (≥5 years) | Adults: Daily (6 months) Children: Daily (6–9 months) | Toxicity: Monthly clinical monitoring Liver enzymes pre-treatment and at 4 weeks (earlier if altered at baseline) and whenever symptoms arise ECG in the event of concomitant treatment with drugs that can prolong the QT interval Periodic eye test if EMB added Adherence: Clinical evaluation |
ECG: electrocardiogram; EMB: ethambutol; HIV: human immunodeficiency virus.
In the absence of treatment, the natural history of MDR-TB is essentially the same as that of susceptible M. tuberculosis infection, and the risk of infection and disease after exposure to MDR-TB is well documented. From this, we can infer that the diagnosis and treatment of infection in contacts of MDR-TB is an important factor to avoid transmission of the infection. The only data available to date come from observational studies.40 These include a study on 104 contacts who received levofloxacin (in subjects≤12 years) or moxifloxacin (in subjects>12 years) for 12 months, in combination with ethambutol or ethionamide.41 None of the contacts treated developed active TB, while 3 of the 15 who refused treatment fell ill. Another attractive combination is levofloxacin (or ofloxacin) with PZA. However, these combinations have been poorly tolerated, leading to discontinuation of treatment in a high proportion of cases.40
Due to a lack of clinical trials, the World Health Organisation recommends periodic follow-up of MDR-TB contacts for the first two years, without antimicrobial treatment. Nonetheless, a consensus of experts recommends administering fluoroquinolone in high-risk contacts.42 There are currently two ongoing clinical trials comparing levofloxacin with placebo (VQUIN and TB CHAMP) and another comparing delamanid with INH (PHOENIx), the results of which are expected in 2020.
Patients with human immunodeficiency virus infectionHIV infection increases the risk of active TB in people with LTBI, and this risk rises further as the immune deficiency progresses. The effectiveness of LTBI treatment in HIV sufferers has been widely demonstrated.32 All patients with HIV infection with demonstrated LTBI should be offered treatment for the LTBI once active TB has been ruled out (Table 3). Shorter regimens with rifamycins have the disadvantage of frequent interactions with many antiretroviral drugs, which represents an important drawback for their use in these patients.
Transplant patientsThe incidence of TB in patients who have received organ transplants is higher than in the general population.43 The risk depends on the type of transplant (prevalence of 1.3–6.5% in lung transplants vs 0.05–0.26% in autologous haematopoietic stem cell transplants) and the endemic situation in the region (prevalence between 0.48% in low-incidence countries and 15.2% in high-incidence countries).43 Although the evidence of the benefit of LTBI treatment in transplant patients is limited, screening for infection and treatment is routine practice and forms part of clinical practice guidelines. The treatment of any transplant patient with a positive TST or IGRA is recommended. Experience is limited almost exclusively to INH in kidney and liver transplants, thus INH for 6 to 9 months is commonly the recommended treatment43 (Table 3). Treatment is particularly difficult in patients with advanced liver disease due to the potential hepatotoxicity of the drugs. For this reason, some groups advocate treating after transplant, a strategy which improves rates of treatment completion. Rifamycins appear to be better tolerated, although they have the drawback of interactions with immunosuppressants. A clinical trial assessed the efficacy and safety of levofloxacin, comparing it with INH in liver transplant candidates.44 The trial had to be halted prematurely due to an unexpectedly high incidence of severe tenosynovitis associated with levofloxacin (18%). Even so, 55% of the subjects treated with levofloxacin completed 9 months of treatment, vs 44% of those treated with INH, and there were no cases of TB after a mean follow-up period of 9 months. A later retrospective study had more favourable results with regard to the toxicity of levofloxacin.45
Patients with chronic inflammatory diseases on biological therapiesIn parallel with the improved quality of life and prognosis offered to patients with immune-mediated inflammatory diseases by biological therapies, a risk of TB has become apparent in these patients, particularly in relation to anti-tumour necrosis factor-alpha (anti-TNF-α) agents. The effectiveness of systematic screening and treatment of LTBI was also quickly demonstrated in this population.46,47 The reported results are predominantly with INH for 6–9 months, although shorter regimens with RMP with/without INH appear to be equally effective48 (Table 3).
Paediatric populationChildren exposed to bacilliferous pulmonary TB, when considered to be indicated, or if there is evidence of LTBI, are treated with INH for 6–9 months, RH for 3 months or, in children>12 years, RPT and INH for 12 weeks under directly observed therapy (Table 3). RMP for 4 months would be indicated in patients with toxicity or contraindications to INH or in the event of exposure to cases with strains resistant to INH. Regarding exposure to and (presumed) infection with MDR-TB, just as in adults, there is no consensus, but a regimen of 6–9 months of fluoroquinolone (moxifloxacin only if >12 years) in combination with EMB or ethionamide, depending on the susceptibility tests, is the most recommended regimen.
The most widely accepted regimen is FQ 6–9 months (moxifloxacin only if >12 years) in combination with another drug (EMB or ETO), considering the association of INH at high doses. For a more detailed knowledge of the approach to TB exposure in children, indications of diagnostic tests for TB infections and treatment indications and monitoring, we refer the reader to an update on the treatment of TB in children by the Spanish Society of Paediatric Infectious Disease (Socieded Española de Infectología Pediátrica).49
Other high-risk situationsOther situations exist in clinical practice in which immune deficiency induced by drugs and other debilitating chronic conditions – advanced kidney and liver disease, diabetes mellitus, cancer, malnutrition, alcoholism and drug addiction – lead to an increased risk of TB. In view of the lack of evidence regarding the cost-benefit ratio in these situations, there are not generally any explicit regulations in this regard. However, the possibility should be contemplated, weighing up the pros and cons in each case. In particular, but not exclusively, this should take into account the type and duration of the immune deficiency (for example dose and duration of a steroid therapy), risk of toxicity of the treatment (age, comorbidities, concomitant medication) and the pre-existence of factors for poor treatment adherence. In the absence of better evidence, when the risk-benefit ratio is considered to be favourable, screening for TB infection should be carried out and treatment indicated if it is found.
Adherence control measures and adverse effects monitoringAdherence control is an essential measure if LTBI treatment is to be successful. There is no consensus on the method to use. Poor adherence is associated with factors such as poor housing conditions, lack of family support, immigration and, in general, lack of perception of LTBI as a health problem and risk of illness. In these circumstances, health education becomes especially important, as well as supporting treatment for its duration. In the experience of the TB Unit of the Hospital Universitari de Bellvitge, measures such as initial health education by specialist nursing personnel (explaining the concept of tuberculosis infection/disease, reasons for treatment and side effects), easy access to the therapeutic team (direct telephone number), repeated contact in the event of failure to attend visits, and monitoring INH metabolites in urine or urine colour for treatment with RMP at each visit have allowed us to complete LTBI treatment in >80% of cases.50
Tolerance of the treatment must be monitored for its duration. Aside from detecting potentially serious problems, clinical assessment of mild symptoms attributed to the treatment by the patient serves as support to continue the treatment. Although it is not well defined how and how often this should be done, a good practice would consist of determining liver enzymes pre-treatment and at least one month from the start. An exception to this might be children, in whom liver toxicity is very rare, and it would be acceptable to start treatment without this analysis and to perform it only if symptoms arise. Conversely, in patients with an underlying disease such as transplant patients, closer monitoring may be required (Tables 3 and 4).
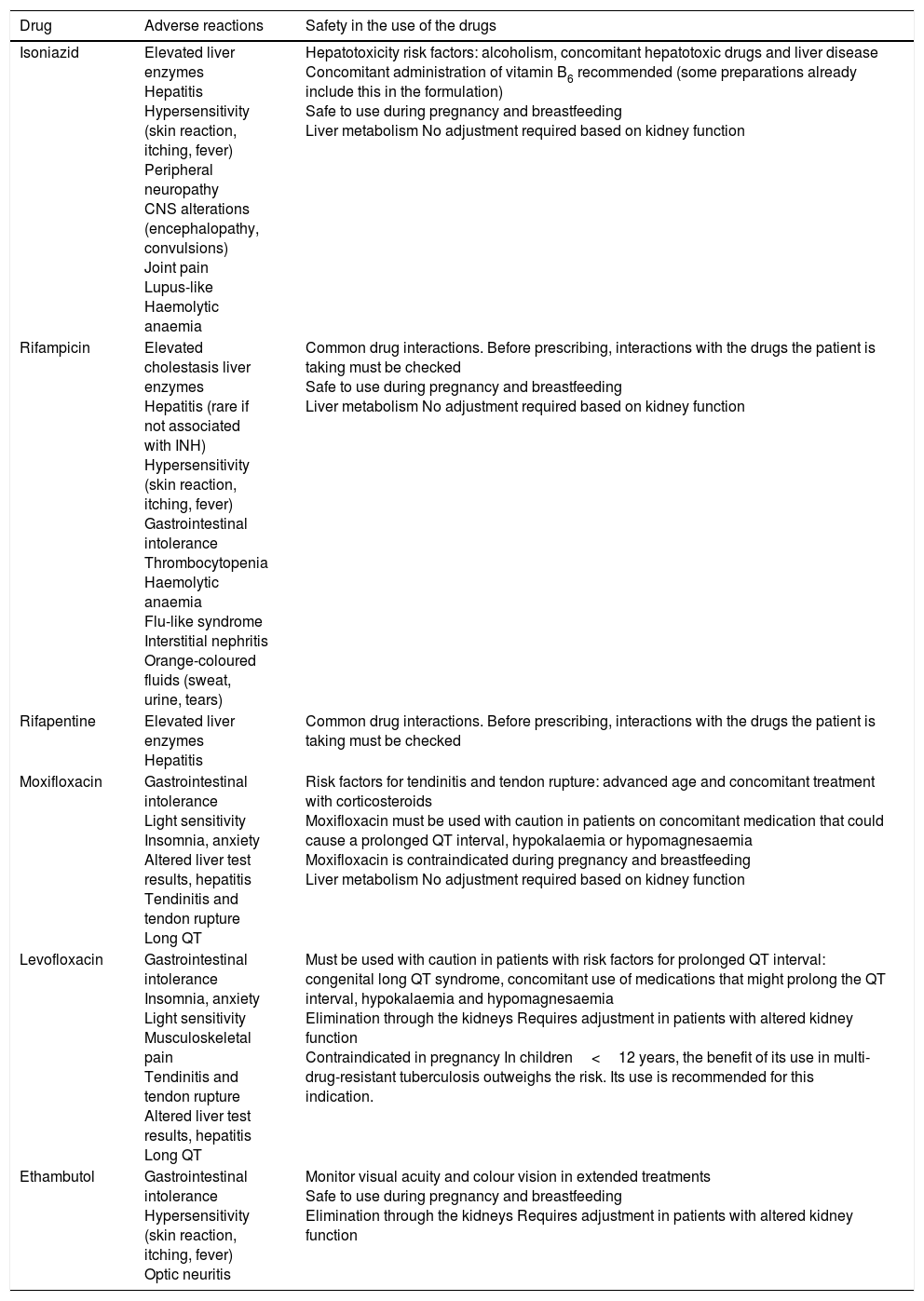
Anti-tuberculosis drugs used to treat latent tuberculosis infection and their adverse reactions.
| Drug | Adverse reactions | Safety in the use of the drugs |
|---|---|---|
| Isoniazid | Elevated liver enzymes Hepatitis Hypersensitivity (skin reaction, itching, fever) Peripheral neuropathy CNS alterations (encephalopathy, convulsions) Joint pain Lupus-like Haemolytic anaemia | Hepatotoxicity risk factors: alcoholism, concomitant hepatotoxic drugs and liver disease Concomitant administration of vitamin B6 recommended (some preparations already include this in the formulation) Safe to use during pregnancy and breastfeeding Liver metabolism No adjustment required based on kidney function |
| Rifampicin | Elevated cholestasis liver enzymes Hepatitis (rare if not associated with INH) Hypersensitivity (skin reaction, itching, fever) Gastrointestinal intolerance Thrombocytopenia Haemolytic anaemia Flu-like syndrome Interstitial nephritis Orange-coloured fluids (sweat, urine, tears) | Common drug interactions. Before prescribing, interactions with the drugs the patient is taking must be checked Safe to use during pregnancy and breastfeeding Liver metabolism No adjustment required based on kidney function |
| Rifapentine | Elevated liver enzymes Hepatitis | Common drug interactions. Before prescribing, interactions with the drugs the patient is taking must be checked |
| Moxifloxacin | Gastrointestinal intolerance Light sensitivity Insomnia, anxiety Altered liver test results, hepatitis Tendinitis and tendon rupture Long QT | Risk factors for tendinitis and tendon rupture: advanced age and concomitant treatment with corticosteroids Moxifloxacin must be used with caution in patients on concomitant medication that could cause a prolonged QT interval, hypokalaemia or hypomagnesaemia Moxifloxacin is contraindicated during pregnancy and breastfeeding Liver metabolism No adjustment required based on kidney function |
| Levofloxacin | Gastrointestinal intolerance Insomnia, anxiety Light sensitivity Musculoskeletal pain Tendinitis and tendon rupture Altered liver test results, hepatitis Long QT | Must be used with caution in patients with risk factors for prolonged QT interval: congenital long QT syndrome, concomitant use of medications that might prolong the QT interval, hypokalaemia and hypomagnesaemia Elimination through the kidneys Requires adjustment in patients with altered kidney function Contraindicated in pregnancy In children<12 years, the benefit of its use in multi-drug-resistant tuberculosis outweighs the risk. Its use is recommended for this indication. |
| Ethambutol | Gastrointestinal intolerance Hypersensitivity (skin reaction, itching, fever) Optic neuritis | Monitor visual acuity and colour vision in extended treatments Safe to use during pregnancy and breastfeeding Elimination through the kidneys Requires adjustment in patients with altered kidney function |
CNS: central nervous system; QT: QRS complex width.
Funded projects related to the content of this article: Spanish Society for Pulmonology and Thoracic Surgery (SEPAR – Socieded Española de Neumología y Cirugía Torácica), Catalan Pulmonology Foundation (FUCAP – Fundació Catalana per la Pneumologia), Instituto de Salud Carlos III (ISCIII – the main Public Biomedical Research Body in Spain) (PI13/1546, PI16/1912) included in the Spanish National R&D&I Plan (Plan Nacional de I + D + I) and co-financed by the ISCIII's General Sub-directorate for Evaluation and the European Regional Development Fund (ERDF); the Spanish Ministry of Health, Social Policy and Equality (assistance to promote the transfer to therapeutic application of medicinal products for human use, orphan drugs and advanced therapies, SAS/2481/2009), and the Catalan Regional Government's CERCA Programme. José Domínguez is an investigator in the Instituto de Salud Carlos III's Miguel Servet programme.
Conflicts of interestMiguel Santin is the principal investigator in a clinical trial on QuantiFERON®-TB Gold In-tube (QFT-GIT) in the study of contacts, for which Cellestis, Inc. (Carnegie, Australia) provided the QFT-GIT tubes.
Please cite this article as: Domínguez J, Latorre I, Santin M. Diagnóstico y abordaje terapéutico de la infección tuberculosa latente. Enferm Infecc Microbiol Clin. 2018;36:302–311.