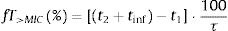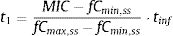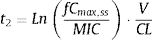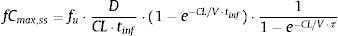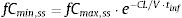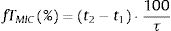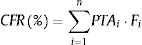In Europe, non-typeable H. influenzae (NTHi) is the leading cause of invasive H. influenzae disease in adults and is associated with high mortality. The goal of this study was to determine whether current antimicrobial treatments for H. influenzae infection in Spain are suitable based on their probability of achieving pharmacokinetic/pharmacodynamic (PK/PD) targets.
MethodsPharmacokinetic parameters for the antibiotics studied (amoxicillin, amoxicillin/clavulanic acid, ampicillin, cefotaxime, ceftriaxone, imipenem and ciprofloxacin) and susceptibility data for H. influenzae were obtained from literature. A Monte Carlo simulation was used to estimate the probability of target attainment (PTA), defined as the probability that at least a specific value of a PK/PD index is achieved at a certain MIC, and the cumulative fraction of response (CFR), defined as the expected population PTA for a specific drug dose and a specific microorganism population.
ResultsRegardless of dosing regimen, all antibiotics yielded CFR values of 100% or nearly 100% for all strains, including BL+, BL− and BLNAR, except amoxicillin and ampicillin for BL+. Thus, if an infection is caused by BL+ strains, treatment with amoxicillin and ampicillin has a high probability of failure (CFR≤8%). For standard doses of amoxicillin, amoxicillin/clavulanic acid and imipenem, PK/PD breakpoints were consistent with EUCAST clinical breakpoints. For the other antimicrobials, PK/PD breakpoints were higher than EUCAST clinical breakpoints.
ConclusionsOur study confirms by PK/PD analysis that, with the antimicrobials used as empirical treatment of invasive H. influenzae disease, a high probability of therapeutic success can be expected.
H. influenzae no tipable (NTHi) es la principal causa de enfermedad invasiva por H. influenzae en adultos en Europa, y frecuentemente está asociada a una alta mortalidad. El principal objetivo de nuestro estudio fue determinar si el tratamiento antibiótico actual es adecuado para tratar infecciones invasivas por H. influenzae en España, teniendo en cuenta la probabilidad de alcanzar el objetivo farmacocinético/farmacodinámico (PK/PD).
MétodosLos parámetros farmacocinéticos de los antibióticos (ampicilina, amoxicilina, amoxicilina/clavulanato, ceftriaxona, cefotaxima, imipenem y ciprofloxacino) y los datos de sensibilidad de H. influenzae se obtuvieron de la literatura. Mediante simulación de Montecarlo, se estimó la probabilidad de alcanzar el objetivo farmacodinámico (PTA) y la fracción de respuesta acumulada (CFR), ambas indicativas de la probabilidad de éxito del tratamiento.
ResultadosIndependientemente del régimen de dosificación, todos los antibióticos proporcionaron valores de CFR del 100% o cerca del 100% para todas las cepas, incluidas BL+, BL− y BLNAR, excepto amoxicilina y ampicilina para BL+. Si la infección se debe a cepas BL+, el tratamiento con amoxicilina y ampicilina tiene una baja probabilidad de éxito (CFR≤8%). Los puntos de corte PK/PD de la dosis estándar de amoxicilina, amoxicilina/clavulanato e imipenem concuerdan con los puntos de corte clínicos de EUCAST. Para el resto, los puntos de corte PK/PD son más altos que los puntos de corte EUCAST.
ConclusionesNuestro estudio ha demostrado, mediante análisis PK/PD, que los antibióticos utilizados para el tratamiento de la enfermedad invasiva de H. influenzae proporcionan una probabilidad de éxito elevada.
Being part of the microflora of the human upper respiratory tract, Haemophilus influenzae, a pleomorphic Gram-negative coccobacillus, may cause a wide range of infections, among which is severe invasive disease, including meningitis, septicemia and pneumonia.1,2H. influenzae is divided into capsulated (serotypes a–f) and non-capsulated strains. Non-capsulated strains are commonly referred to as non-typeable H. influenzae (NTHi).1,3 Among capsulated strains, serotype b (Hib) is known to be the most pathogenic. In the past, Hib was one of the most frequent organisms causing invasive infections in industrialized countries, mainly among healthy children less than 5 years of age due to their lack of T-cell independent immune response to polysaccharides. The widespread of conjugated Hib vaccination in national immunization programmes provided herd protection leading to a sharp reduction of infections caused by Hib3,4 and to a decrease in the prevalence of carriers, but there is no clear evidence of carriage or disease replacement by no-type b H. influenzae serotypes.1–3 At present, NTHi and/or non-Hib capsulated strains are the predominant serotype of invasive H. influenzae disease.2 In Europe, NTHi is the main cause of invasive H. influenzae disease in adults, who frequently presents underlying conditions, associated with a high mortality rate.1 In a previous study carried out in Spain,5 the incidence of invasive H. influenzae disease was 2.12/100,000, similar to that reported in USA and in Europe; and it increased with age (6.8/100,000 in patients≥65 years-old).
Invasive H. influenzae disease is commonly treated with β-lactam antibiotics, being aminopenicillins and cephalosporins the first choice of the treatment. However, the prevalence of many well documented resistance mechanisms in this pathogen, such as TEM-1 and ROB-1 β-lactamase production and ftsI gene encoding alterations in transpeptidase domain of penicillin-binding protein 3 (PBP-3), which may produce β-lactamase-negative ampicillin-resistant (BLNAR) strains,6,7 may limit the choice of a suitable agent for the treatment.6,8
When treating an infection, susceptibility patterns of the microorganism as well as patients’ characteristics determine the choice of the agent and the dosing regimen, which are the conditioning factors of the success of the therapy. Pharmacokinetic/pharmacodynamic (PK/PD) analysis combines information about the antibiotic time-course in the body and susceptibility of the pathogen against the antibiotic, employing minimum inhibitory concentration (MIC) as PD parameter, and provides the clinically relevant relationship between time and effect. Thus, the optimal agent and dosing regimen for each infectious process and patient may be chosen, enhancing the likelihood of the therapy success and minimizing adverse effects as well as the emergence of resistance.9
The main objective of this study was to determinate if the current antimicrobial treatments of invasive H. influenzae infections, including meningitis, in Spain (amoxicillin, amoxicillin/clavulanate, ampicillin, cefotaxime, ceftriaxone, imipenem and ciprofloxacin) are adequate based not only on the susceptibility patterns of Spanish isolates, but also on the probability of achieving the PK/PD targets.
MethodsThe study was performed by following three steps: (i) dosing regimen selection and acquisition of pharmacokinetic data of antimicrobials; (ii) microbiological data acquisition; and (iii) Monte Carlo simulation to estimate the probability of target attainment (PTA), defined as the probability that at least a specific value of a PK/PD index is achieved at a certain MIC, and to calculate the cumulative fraction of response (CFR), defined as the expected population PTA for a specific drug dose and a specific population of microorganisms.10 Breakpoints based on PK/PD were also calculated.
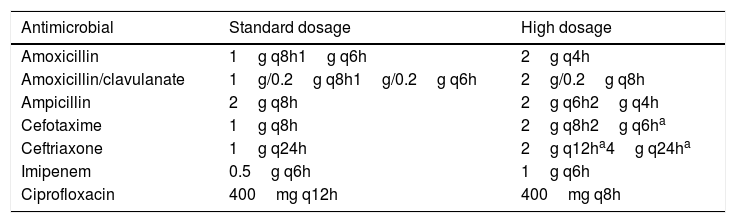
Dosing regimen selection and acquisition of pharmacokinetic dataIntravenous amoxicillin, amoxicillin/clavulanate, ampicillin, cefotaxime, ceftriaxone, imipenem and ciprofloxacin were studied. Standard and high dosing regimens (including doses for special situations such as meningitis) used for breakpoint decisions by the European Committee on Antimicrobial Susceptibility Testing (EUCAST)11 were selected (Table 1).
Selected antibiotics and dosing regimens.
For simulations, β-lactams in 0.5 hour infusions were considered, except imipenem (1 hour infusion). Pharmacokinetic parameters were obtained from previous studies. All parameters were expressed as means and standard deviation (Table 2).
Pharmacokinetic parameters and PK/PD targets of the antimicrobial studied.12-18
| V (L) | CL (mL/min) | t1/2 (h) | fu | PK/PD target | References | |
|---|---|---|---|---|---|---|
| Amoxicillin | 27.7±9.5 | 355.0±91.7 | – | 0.8 | ƒT>MIC≥50% | 12, 17 |
| Ampicillin | 23.6±5.8 | 250.3±39.3 | – | 0.8 | ƒT>MIC≥50% | 9, 13 |
| Cefotaxime | 16.6±2.2 | – | 1.1±0.3 | 0.6a | ƒT>MIC≥60% | 9, 14, 18, 19 |
| Ceftriaxone | 7.9±1.4 | 14.4±1.4 | – | 0.1b | ƒT>MIC≥60% | 9, 15, 18, 20 |
| Imipenem | 15.1±1.4 | 208.1±17.3 | – | 0.9 | ƒT>MIC≥40% | 15, 21, 22 |
| Ciprofloxacin | – | 516.6±87.8 | – | – | AUC24/MIC≥125 | 16, 22, 23 |
AUC24, area under the curve concentration–time over 24h; CL, total body clearance; ƒT>MIC, percentage of time that free drug concentration remains over de MIC; expressed as percentage of the dosing interval; fu, unbound fraction; t1/2, elimination half-life; V, volume of distribution.
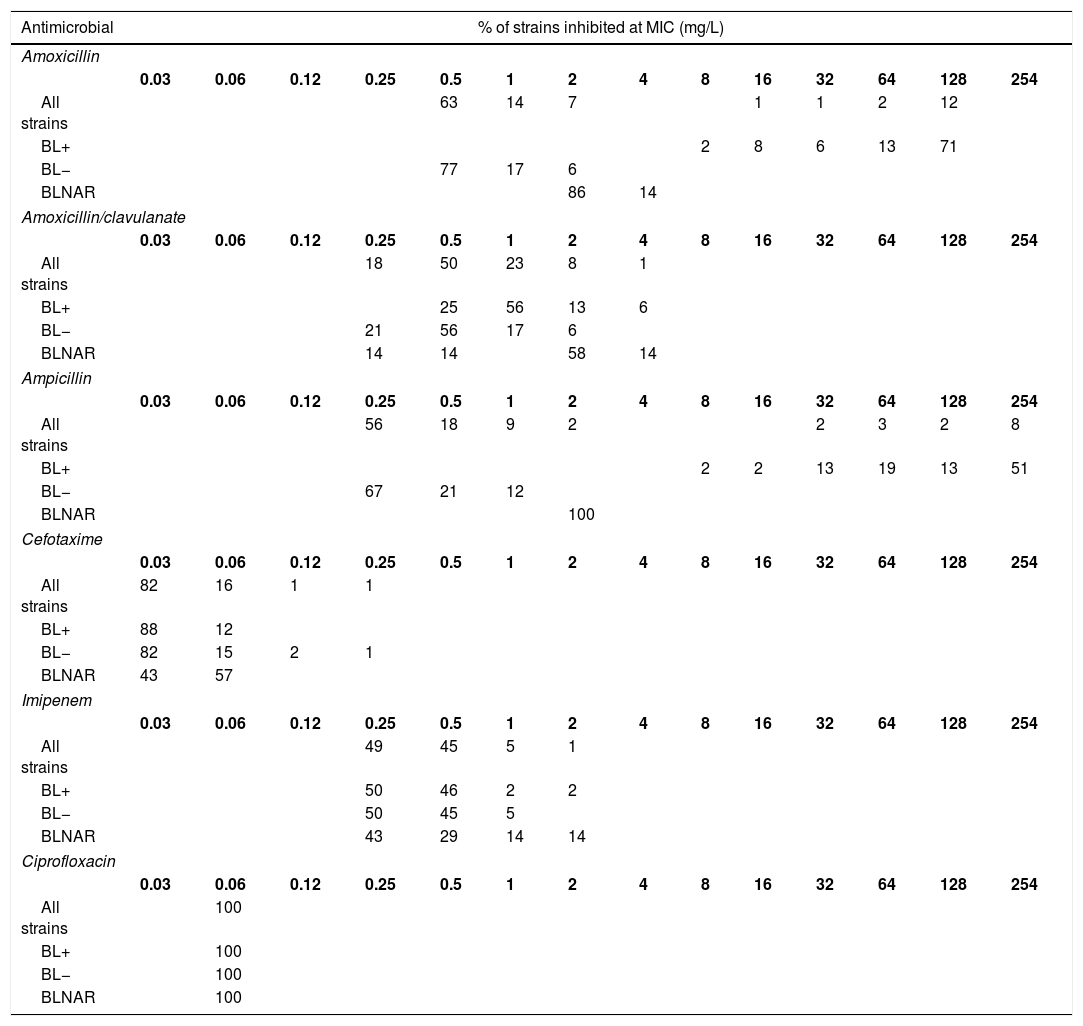
The MIC distribution data were obtained from the study by García-Cobos et al.6 among clinical isolates obtained from the active national surveillance programme for invasive H. influenzae infections in Spain (Table 3). Ceftriaxone was not tested and therefore, for this antibiotic we assumed the same MIC distribution than that of cefotaxime.5,19 We used separately MIC distributions of all strains, β-lactamase-positive (BL+), β-lactamase-negative (BL−), and β-lactamase-negative ampicillin resistant (BLNAR) strains according EUCAST definition (ampicillin MIC>1mg/L).
Activity of the studied antibiotics against invasive H. influenzae isolates. (6) All strains, n=307; β-lactamase negative strains (BL−), n=248; β-lactamase positive strains (BL+), n=52; β-lactamase negative ampicillin resistant strains (BLNAR), n=7.
| Antimicrobial | % of strains inhibited at MIC (mg/L) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amoxicillin | ||||||||||||||
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 254 | |
| All strains | 63 | 14 | 7 | 1 | 1 | 2 | 12 | |||||||
| BL+ | 2 | 8 | 6 | 13 | 71 | |||||||||
| BL− | 77 | 17 | 6 | |||||||||||
| BLNAR | 86 | 14 | ||||||||||||
| Amoxicillin/clavulanate | ||||||||||||||
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 254 | |
| All strains | 18 | 50 | 23 | 8 | 1 | |||||||||
| BL+ | 25 | 56 | 13 | 6 | ||||||||||
| BL− | 21 | 56 | 17 | 6 | ||||||||||
| BLNAR | 14 | 14 | 58 | 14 | ||||||||||
| Ampicillin | ||||||||||||||
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 254 | |
| All strains | 56 | 18 | 9 | 2 | 2 | 3 | 2 | 8 | ||||||
| BL+ | 2 | 2 | 13 | 19 | 13 | 51 | ||||||||
| BL− | 67 | 21 | 12 | |||||||||||
| BLNAR | 100 | |||||||||||||
| Cefotaxime | ||||||||||||||
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 254 | |
| All strains | 82 | 16 | 1 | 1 | ||||||||||
| BL+ | 88 | 12 | ||||||||||||
| BL− | 82 | 15 | 2 | 1 | ||||||||||
| BLNAR | 43 | 57 | ||||||||||||
| Imipenem | ||||||||||||||
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 254 | |
| All strains | 49 | 45 | 5 | 1 | ||||||||||
| BL+ | 50 | 46 | 2 | 2 | ||||||||||
| BL− | 50 | 45 | 5 | |||||||||||
| BLNAR | 43 | 29 | 14 | 14 | ||||||||||
| Ciprofloxacin | ||||||||||||||
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 254 | |
| All strains | 100 | |||||||||||||
| BL+ | 100 | |||||||||||||
| BL− | 100 | |||||||||||||
| BLNAR | 100 | |||||||||||||
The PTA, that is, the probabilities that the PK/PD indexes reach the defined target (Table 2), were estimated for every dosing regimen by means of 10,000 subject Monte Carlo simulations using Oracle® Crystal Ball Fusion Edition v.11.1.1.1.00 (Oracle USA Inc., Redwood City, CA). For β-lactam antibiotics, the PK/PD parameter best related to its activity is the percentage of time that free drug concentration remains over de MIC, expressed as percentage of the dosing interval (%fT>MIC).20,21 For the treatment of meningitis with cefotaxime and ceftriaxone, the cerebrospinal fluid to serum AUC ratio (AUCCSF/AUCserum) was used instead the unbound drug fraction in serum. On the other hand, for fluorquinolones the relation between the area under the curve concentration–time over 24h (AUC24) and the MIC (AUC24/MIC) shows the best correlation for its efficacy.22%fT>MIC and AUC24/MIC were calculated for over an MIC range of serial twofold dilutions from 0.03mg/L to 256mg/L. We assumed one-compartment pharmacokinetic models and according statistical criteria, a log-normal distribution for the pharmacokinetic parameters was used. AUC24/MIC was calculated as the relationship between daily dose (D) and total body clearance (CL) multiplied by the MIC value:
Following equations were used to calculate %fT>MIC:
where tinf (h) is the infusion time, t1 (h) corresponds to the time at which the drug concentration reaches the MIC during the infusion phase, t2 (h) corresponds to the post-infusion time at which the serum concentration equals the MIC and τ is the dosing interval.Assuming β-lactams show linear pharmacokinetics, t1 and t2 were calculated as follows:
where fCmin,ss and fCmax,ss are the minimum and maximum serum concentration of unbound drug (mg/L) at a steady state, respectively. Total body clearance, volume distribution (V), and unbound fraction (fu) were used to estimate fCmin,ss and fCmax,ss according to the following equations:The values of time in which concentration equals the MIC values were calculated and used to estimate fT>MIC (%) as follows:
where t1 and t2 corresponds to the time at which the drug concentration reaches the MIC in the ascendant and in the elimination phase of the plasma concentration–time curve, respectively.The treatment was considered successful if the PTA was ≥90%20,21 although PTA values between 80 and 90% were associated with moderate probability of success.23
Estimation of cumulative fraction of response (CFR)The CFR, understood as the expected probability of success of a dosing regimen against a specific population of microorganisms, is a useful parameter for guiding empiric therapy. It results from the total sum of the products of the PTA at a certain MIC times the frequency of isolates of microorganism exhibiting that MIC over the range of susceptibility, according to the following equation:
where i indicates the MIC category, PTAi is the PTA of each MIC category, and Fi is the fraction of microorganism population in each MIC category. As for PTA, a treatment was considered successful if the CFR value was equal to 90% or higher20,21 even though CFR values of 80–90% were associated with moderate probability of success.23PK/PD breakpointsWe calculated the PK/PD breakpoints for every dosing regimen of the antibiotics included in the study. PK/PD breakpoints were the highest MIC values at which PTA were ≥90%, as this is the accepted target attainment cut-off currently used when determining MIC breakpoints.24,25 A range from the lowest to the highest breakpoint is obtained for each antimicrobial agent, which depends on the dosing regimen. Afterwards, PK/PD breakpoints were compared with the EUCAST and the Clinical and Laboratory Standards Institute (CLSI) breakpoints.11,26
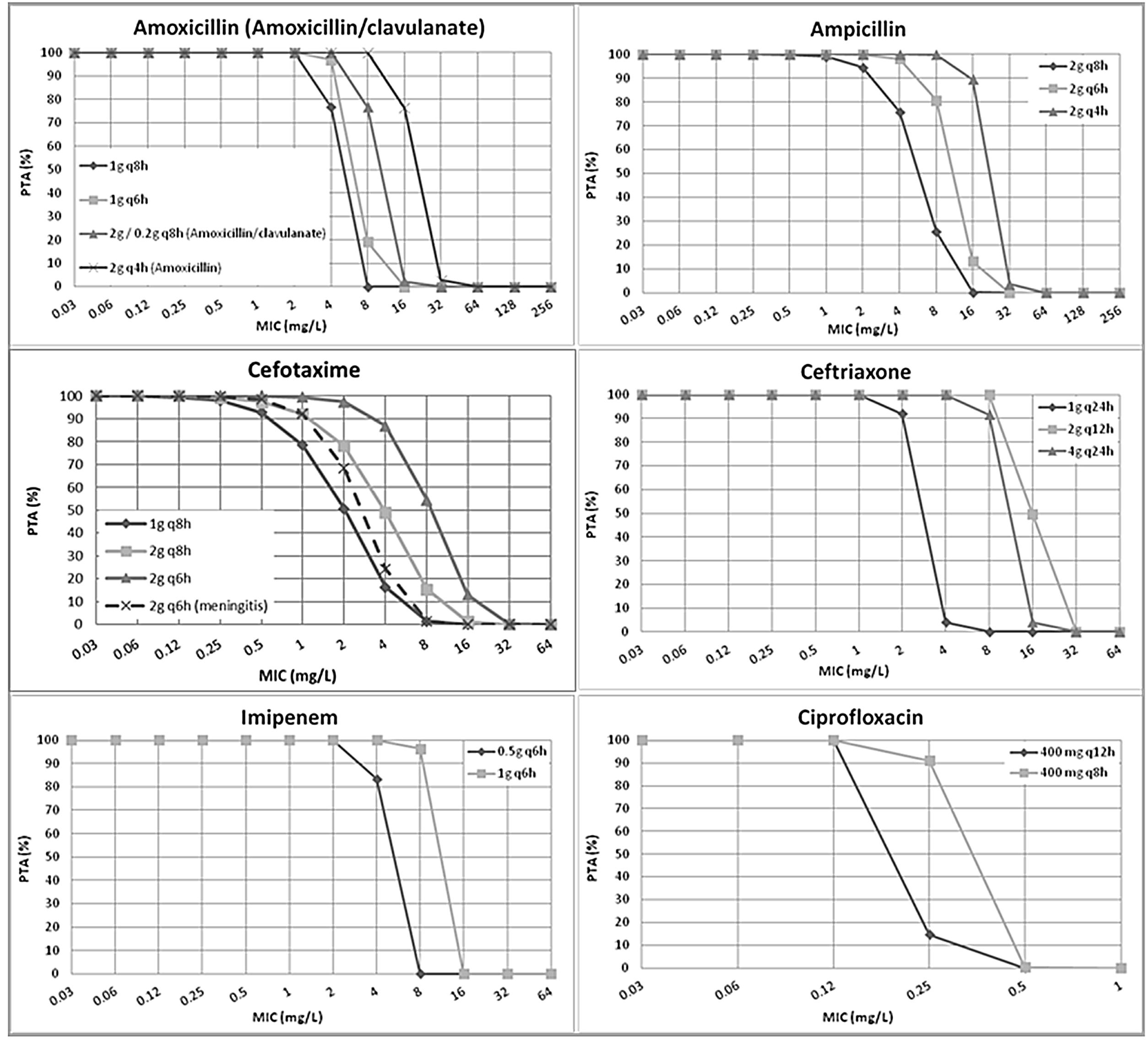
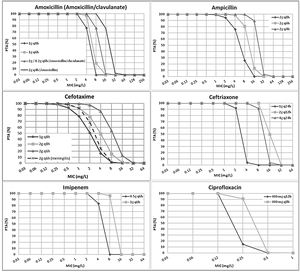
ResultsFig. 1 features the PTA values of amoxicillin (amoxicillin/clavulanate), ampicillin, cefotaxime, ceftriaxone, imipenem and ciprofloxacin for the studied dosing regimens. As expected, the highest PTA values were achieved with the highest doses. As shown in the figure, the calculated PTA values for amoxicillin and ampicillin were higher than 90% for MIC ≤2mg/L with the lowest dosages and for MIC ≤8mg/L with the highest dose level (2g q4h). High dosage of amoxicillin/clavulanate (2g/0.2g q8h) reached a PTA ≥90% for MIC values ≤4mg/L. With the standard dosage of cefotaxime (1g q8h) PTA ≥90% was obtained for MIC values ≤0.5mg/L; however, higher doses (2g q8h and 2g q6h) ensured PTA ≥90% for a MIC values of 1 and 2mg/L, respectively. PTA of cefotaxime used for the treatment of meningitis (2g q6h) is higher than 90% up to MIC of 1mg/L. Regarding ceftriaxone, PTA ≥90% was achieved for MIC ≤2mg/L with the standard dose (1g q24h), and for MIC ≤8mg/L with the higher doses (2g q12h and 4g q24h). Standard dosage of imipenem (0.5g q6h) ensured a PTA ≥90% for MIC values ≤2mg/L and the high dosage (1g q6h) for MIC values ≤8mg/L. Eventually, PTA ≥90% was obtained for MIC values ≤0.125mg/L and ≤0.25mg/L with the standard and high dosage of ciprofloxacin (400mg q12h and 400mg q8h), respectively.
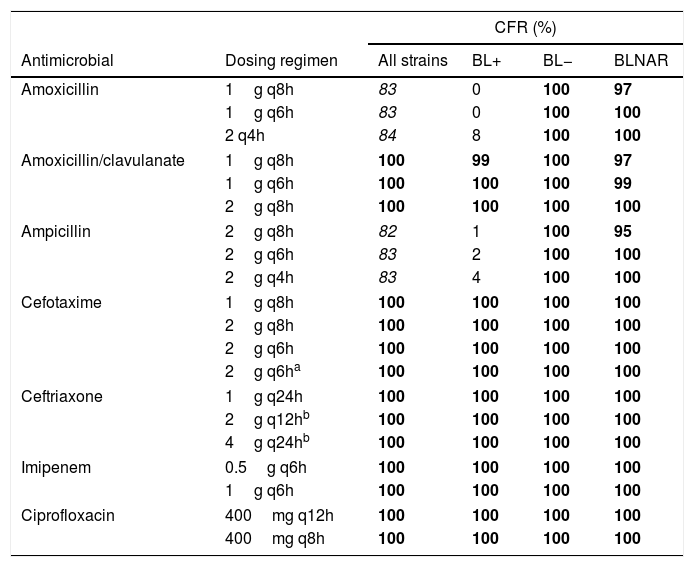
Table 4 shows the CFR values, for the different dosing regimens and groups of isolates. Regardless the dosing regimen, all antibiotics provided CFR values of 100% or near 100% for all strains, including BL+, BL− and BLNAR, with the exception of amoxicillin and ampicillin for BL+.
Cumulative fraction of response (CFR) of the different dosing regimens studied considering all isolates, β-lactamase-positive (BL+), β-lactamase-negative (BL−), and β-lactamase-negative ampicillin resistant (BLNAR) strains.
| CFR (%) | |||||
|---|---|---|---|---|---|
| Antimicrobial | Dosing regimen | All strains | BL+ | BL− | BLNAR |
| Amoxicillin | 1g q8h | 83 | 0 | 100 | 97 |
| 1g q6h | 83 | 0 | 100 | 100 | |
| 2 q4h | 84 | 8 | 100 | 100 | |
| Amoxicillin/clavulanate | 1g q8h | 100 | 99 | 100 | 97 |
| 1g q6h | 100 | 100 | 100 | 99 | |
| 2g q8h | 100 | 100 | 100 | 100 | |
| Ampicillin | 2g q8h | 82 | 1 | 100 | 95 |
| 2g q6h | 83 | 2 | 100 | 100 | |
| 2g q4h | 83 | 4 | 100 | 100 | |
| Cefotaxime | 1g q8h | 100 | 100 | 100 | 100 |
| 2g q8h | 100 | 100 | 100 | 100 | |
| 2g q6h | 100 | 100 | 100 | 100 | |
| 2g q6ha | 100 | 100 | 100 | 100 | |
| Ceftriaxone | 1g q24h | 100 | 100 | 100 | 100 |
| 2g q12hb | 100 | 100 | 100 | 100 | |
| 4g q24hb | 100 | 100 | 100 | 100 | |
| Imipenem | 0.5g q6h | 100 | 100 | 100 | 100 |
| 1g q6h | 100 | 100 | 100 | 100 | |
| Ciprofloxacin | 400mg q12h | 100 | 100 | 100 | 100 |
| 400mg q8h | 100 | 100 | 100 | 100 | |
Italic font indicates CFR≥80% but <90%. Font in bold indicates CFR≥90%.
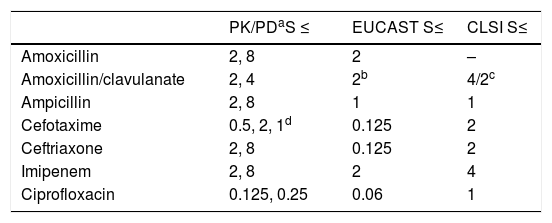
Table 5 shows the PK/PD breakpoints calculated for every antimicrobial agent, and the clinical breakpoints published by EUCAST and the CLSI. Contrary to clinical breakpoints, PK/PD breakpoints are regimen-dependent and species-independent. The PK/PD breakpoints of the standard dose of amoxicillin, amoxicillin/clavulanate and imipenem agree to the clinical breakpoints of EUCAST. For the other antimicrobials, the PK/PD breakpoints are higher than EUCAST breakpoints. Cephalosporin PK/PD breakpoints agree to those of CLSI, and ciprofloxacin PK/PD breakpoint is higher than that of EUCAST but lower than CLSI.
Comparison of the pharmacokinetic/pharmacodynamic (PK/PD) breakpoints, and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical and Laboratory Standards Institute (CLSI) clinical breakpoints. Breakpoints are expressed as mg/L.
In this study, we have evaluated by PK/PD analysis the adequacy of different dosing regimens of the antibiotics used to treat invasive H. influenzae disease; that is, the likelihood of success of the empirical therapy, considering the population MIC distribution of H. influenzae in Spain after the implantation of the conjugated Hib vaccination. This vaccine was implemented in Spain in 1997,27 and the data of the MIC distribution collected for this study corresponded to years 2004–2009.6 Unfortunately, more recent data are not available. It is important to take into account that after the implantation of vaccination programmes, there is a serotype displacement and therefore, changes in the antibiotic susceptibility profiles. According to our results, when treating H. influenzae infections with amoxicillin and ampicillin, the presence or the absence of β-lactamase production is a main determining factor for the success of the empirical treatment. With the other antimicrobial agents evaluated (third generation cephalosporins, carbapenems and quinolones), irrespective of the dosing regimen and resistance mechanism, high probability of successful clinical outcome is expected.
Ceftriaxone (2g q12h and 4g q24h) and cefotaxime (2g q6h) are used for the treatment of meningitis. For these infections, it is important to take into account the penetration of the antibiotic through the blood-cerebrospinal fluid/blood-brain barrier. To have a better estimation of the PTA and CFR with these two cephalosporins when used for meningitis, we have considered the AUCCSF/AUCserum ratio in the simulations. For ceftriaxone, unbound drug in serum is similar to the AUCCSF/AUCserum ratio, and therefore, the PTA and CFR are valid for all infections due to H. influenzae, including meningitis. However, in the case of cefotaxime, the AUCCSF/AUCserum ratio (0.2) is much lower than the unbound drug (0.6).28 Therefore, we calculated the probability of treatment success by using the AUCCSF/AUCserum ratio. According to that, the treatment with cefotaxime 2g q6h would cover meningitis due to H. influenzae with MIC values up to 1mg/L, and in empiric treatment, the probability of success is 100%, even in the presence of β-lactamases.
Changes in serotype distribution and modifying resistance mechanisms could lead to changes in the activity of the antibiotics frequently used for treating H. influenzae infections. Along with epidemiological studies, PK/PD analysis has also been demonstrated to be useful to assess changing of antimicrobial activity against clinical isolates, and also as a tool to evaluate the adequacy of the antimicrobial therapy after implantation of a vaccine, as complementary to the simply assessment of MIC values.20,29-31 Our work is based on epidemiologic and MIC values from a Spanish surveillance study of invasive H. influenzae infections6; that study revealed that NTHi were responsible for the majority of these infections; moreover the most common resistant mechanism among invasive infections was the reduced susceptibility to β-lactams due to PBP3 amino acid substitutions, followed by β-lactamase production. Regardless the mechanism of resistance to β-lactam antibiotics, the vast majority of the isolates were susceptible to amoxicillin/clavulanate, cefotaxime, ceftriaxone and imipenen, considering both the EUCAST11 and the CLSI clinical breakpoints.26 In this context, and according to our results based on the CFR values we calculated, high probability of treatment success (CFR ≥90%) is expected with amoxicillin/clavulanate, the two cephalosporins, imipenem and ciprofloxacin, all of them at the lowest dose level (standard doses) when used as empirical treatment (Table 4). With amoxicillin and ampicillin, even with the highest dosing regimens, the probability of empirical therapy success was moderate (80%≤CFR<90%). However, and as it is expected, if the infection is due to BL+ strains, which represent 16.6% of all isolates,6 the treatment with amoxicillin and ampicillin has high probability of failure (CFR≤8%).
Previous studies had already described that the presence of PBP3 mutations have only low-level resistance and may not show the phenotypes of ampicillin or amoxicillin/clavulanate resistance when tested by disc diffusion or microbroth dilution methods. Such isolates are classified as genetically BLNAR/BLPACR (gBLNAR/g/BLPACR), respectively.7,32–34 Even for BLNAR isolates (ampicillin MIC>1mg/L), our study reveals that all antibiotics, including ampicillin, provide a high probability of treatment success (CFR≥90%). However, due to the low number of BLNAR isolates, these results should be taken with caution.
Despite of the fact that antibiotic susceptibility testing is necessary for the selection of the appropriate agent and dosing regimen for the targeted treatment, it seems insufficient to consider only the MIC value, particularly when it is around the clinical breakpoint. This is why it has been frequently suggested to use PK/PD breakpoints to predict the susceptibility to antibiotics7 and PK/PD analysis has been proved to be a very useful tool to establish PK/PD breakpoints.7,9,24 The PK/PD breakpoints calculated in this study are similar to the EUCAST clinical breakpoints for all antibiotics at the standard doses, except the 3th generation cephalosporins and ciprofloxacin. For the standard dose of ceftriaxone and the high dose of cefotaxime, the PK/PD breakpoints agree with those of CLSI. Based on the low values of the clinical breakpoints proposed by EUCAST for cefotaxime, ceftriaxone and ciprofloxacin, these antimicrobials may be rejected by the clinicians to treat invasive H. influence infections. However, our study reveals sufficient exposure for MIC values higher than the clinical breakpoints by EUCAST. Discrepancies between clinical and PK/PD breakpoints are not infrequent,35 and result in diverging susceptibility estimates. In previous studies discrepancies between breakpoints defined by EUCAST and the CLSI and PK/PD breakpoints were also detected against both Gram-positive and Gram-negative bacteria.24,36 Discrepancies in the breakpoints may justify success of antimicrobial treatments although isolates had been categorized as non-susceptible. For instance, in a previous study, a patient with pneumonia due to NTHi infection responded to a therapy with high dosage of cefotaxime (2g q8h) although the isolate, with a MIC value of 1mg/L, was categorized as resistant according to EUCAST clinical breakpoints.8 However, according to the PK/PD breakpoints calculated in our study for the dose of 2g q8h, a strain with MIC of 1mg/L would be considered as susceptible (PTA >90%, Fig. 1). Differences in breakpoints present problems for clinical practice, epidemiologists and microbiologists trying to compare results from different geographical regions and time periods.37 The recent increase in NTHi and non-Hib capsulated strains and the reducing ampicillin/cephalosporins susceptibility due to mutations in PBP3 make further efforts to continuous monitoring of invasive H. influenzae infections and also to harmonize breakpoints.38
In conclusion, our study confirms, by PK/PD analysis, that with the current treatments for invasive H. influenzae disease (amoxicillin/clavulanate, 3rd generation cephalosporins, imipenem and ciprofloxacin), used as empiric therapy, high probability of therapy success can be expected. Additionally, we confirm that PK/PD studies are a very useful tool to follow the potential effect of MIC changes on the therapeutic efficacy of antimicrobial treatments, and hence, to select the more adequate dosing regimens.
Conflict of interestNo conflicts of interest to report.
This work was supported by the University of the Basque Country UPV/EHU (GIU17/32), Spain. The authors would like to thank Silvia-García-Cobos and José Campos from the Antibiotic Laboratory of Centro Nacional de Microbiología, Instituto de Salud Carlos III (Majadahonda, Madrid, Spain), for providing MIC distribution from the surveillance study of invasive H. influenzae in Spain.