The immunosuppressive treatment that recipients receive from a solid organ transplantation hinders the defensive response to infection. Its transmission from the donor can cause dysfunction or loss of the graft and even death of the recipient if proper preventive measures are not established. This potential risk should be thoroughly evaluated to minimise the risk of infection transmission from donor to recipient, especially with organ transplantation from donors with infections, without increasing graft dysfunction and morbidity and mortality in the recipient. This document aims to review current knowledge about infection screening in potential donors and offer clinical and microbiological recommendations about the use of organs from donors with infection based on available scientific evidence.
El tratamiento inmunosupresor que recibe el receptor de un trasplante de órgano sólido dificulta la respuesta defensiva frente a la infección. La transmisión de la misma desde un donante puede provocar la disfunción o pérdida del injerto e, incluso, la muerte del receptor si no se establecen las medidas preventivas oportunas. Este riesgo potencial debe ser evaluado minuciosamente para minimizar el riesgo de transmisión de infección del donante al receptor, especialmente con el trasplante de órganos de donantes con infecciones, sin aumentar la disfunción del injerto y la morbimortalidad en el receptor. Este documento pretende revisar los conocimientos actuales sobre la detección sistemática de infecciones en los donantes potenciales y ofrecer recomendaciones clínicas y microbiológicas acerca del uso de órganos procedentes de donantes con infección basadas en la evidencia científica disponible.
Infectious complications remain the main cause of morbidity and mortality after organ transplantation. Many of these complications have an exogenous origin that includes those caused by pathogens transmitted by the transplanted organ and by substances that are exposed to the organ before or during its implantation (e.g. preservation fluids). The transmission of donor-derived infections in solid organ transplantation (SOT) recipients is a rare complication, the incidence of which ranges from less than 1% to 1.7% but is associated with significant morbidity and mortality. Strict evaluation of latent and active infections in the donor is essential to optimise transplantation results and serves to avoid the accidental use of unfit organs or initiate preventive and/or therapeutic measures in a streamlined way after performance of the procedure.
The need to review a previous document named “Criterios de selección del donante respecto a la transmisión de infecciones” has arisen because of changes regarding the treatment of certain infections such as hepatitis C virus (HCV) or multidrug-resistant bacteria, the increasing geographic mobility of the population, which brings about imported pathologies. In addition, the appearance and development of new diagnostic techniques such as the detection of nucleic acids by polymerase chain reaction. Conversely, the new document is not only aimed at facilitating decision-making regarding the donor's suitability to accept the donation but also at offering monitoring, prophylaxis and/or treatment guidelines for the recipient to ensure transplantation success rates.
As for the transmission of the donor's infection to the recipient, other factors should also be taken into account, such as assuming that the risk of transmission will never be “zero”. There are time limitations from the moment of evaluation of a donor and proceeding to the transplantation as information exchange between laboratories and the professionals ultimately in charge of the procedure must be fast, efficient and safe.
Finally, the evidence to recommend different interventions in this field is limited and is usually based on communications of cases and cohort studies. In any case, local epidemiology should always be considered before making any decision about the risk of transmission of an infectious disease.
Thus, several professionals with experience in the field of infection and organ donation have developed this consensus document sponsored by the Grupo de Estudio de la Infección en Trasplante [Transplant Infection Study Group] (GESITRA), Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [Spanish Society of Infectious Diseases and Clinical Microbiology] (SEIMC) and Organización Nacional de Trasplantes [National Transplant Organization] (ONT).
The target populations of this document are organ donors and their recipients. The document is addressed to all professionals involved in the donation and transplantation process, especially those who have to make decisions about the donor's suitability, such as transplant coordinators, the ONT staff (as ONT staff advises other professionals on several occasions regarding the viability of the donation, by taking into account all the background information). This includes the professionals working in transplantation teams (the ones who ultimately decide on the use of the organ and are in charge of the selection of a suitable recipient by considering the characteristics of both the donor and recipient).
Here we demonstrate a consensus from an infection transmission perspective from donor to recipient in order to evaluate the available evidence and propose recommendations on the following key sections:
- 1.
What information should be collected regarding the medical history of the potential solid organ donor?
- 2.
Does the prior administration of vaccines contraindicate donation?
- 3.
What infections should be forcefully ruled out in order to assess the suitability of a donor of solid organs?
- 4.
What chronic or latent infections should be screened to assess the risk of transmission?
- 5.
Should hidden infections in the donor be ruled out?
- 6.
What clinical situations should be assessed for the donation of a solid organ?
- 7.
How important is the place of origin of the donor?
A systematic review of the literature has been conducted to evaluate the potential transmission of any infection from a donor to a recipient of a solid organ transplantation and the measures to prevent it. The necessary data were identified by search in PubMed and the search terms used in each section were specified to answer the target question. The search criteria included articles in English or Spanish in which humans had participated without a time limit.
The Notify project database (www.notifylibrary.org), an initiative of the World Health Organization, was also consulted. Experts from across the globe collaborate to share educational information on documented adverse outcomes, associated with the clinical use of human organs, blood, tissues and cells.
Each question included, if applicable, first, the assessment of the risk of transmission of the infection according to Alliance-O (Annex 1, supplementary material) and, secondly, the list of recommendations and grading of their strength and quality according to the table in annex 2, supplementary material. The document has been written in accordance with the Appraisal of Guidelines Research and Evaluation (AGREE II) recommendations. The authors met on one occasion to discuss the final recomannmendations. The coordinators and authors agreed on the content and the conclusions. The consensus was sent to the members of GESITRA-SEIMC and the ONT, the Donation and Transplantation Network and the Comisión de trasplantes de Consejo Interterritorial de Trasplantes del Sistema Nacional de Salud (Transplantation Committee of the Inter-Territorial Transplantation Council of the National Health System) for independent peer review and institutional adoption.
Recommendations- 1.
What information should be collected regarding the medical history of the potential solid organ donor?
- •
Recommendations
- a.
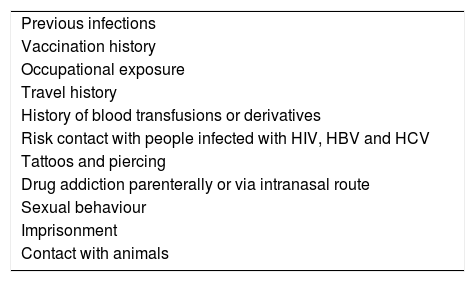
All potential donors of solid organs should be screened concerning their medical and social history along with a physical examination. AIII. Table 1.
Table 1.Data to be collected in the donor's medical history.
Previous infections Vaccination history Occupational exposure Travel history History of blood transfusions or derivatives Risk contact with people infected with HIV, HBV and HCV Tattoos and piercing Drug addiction parenterally or via intranasal route Sexual behaviour Imprisonment Contact with animals
- a.
- •
- 2.
Does the prior administration of vaccines contraindicate donation?
- •
Transmission risk
- a.
Prior administration of inactivated vaccines in the potential donor does not pose a risk to the recipient. RL5.
- b.
The administration of live virus vaccines in the potential donor more than 30 days before the donation does not pose a risk to the recipient. RL4.
- c.
The administration of live virus vaccines in the potential donor within 30 days prior to the donation may pose a risk to the recipient. RL2–3.
- a.
- •
Recommendations
- a.
Prior administration of inactivated vaccines in the potential donor does not contraindicate the donation. CIII.
- b.
A donor's organs can be accepted for transplantation if such a donor received a live virus vaccine (Table 2) in case it has been administered more than 30 days before the donation. CIII.
- c.
The organs of people who have been administered live virus vaccines within 30 days prior to donation can be accepted for transplantation in case the recipient has confirmed immunity (natural or acquired) against the vaccine virus. CIII.
- d.
Donors vaccinated with live virus vaccines within 30 days prior to donation can only be accepted for non-immune recipients if the health conditions of the recipient are extremely sever and upon signing of the informed consent form. CIII.
- a.
- •
- 3.
What infections should be forcefully ruled out in order to assess the suitability of a donor of solid organs?
- 3.1.
What should be done in relation to an HIV-positive donor?
- •
Transmission risk
- a.
The risk of transmission of HIV infection is well documented. RL1–2.
- a.
- •
Recommendations
- a.
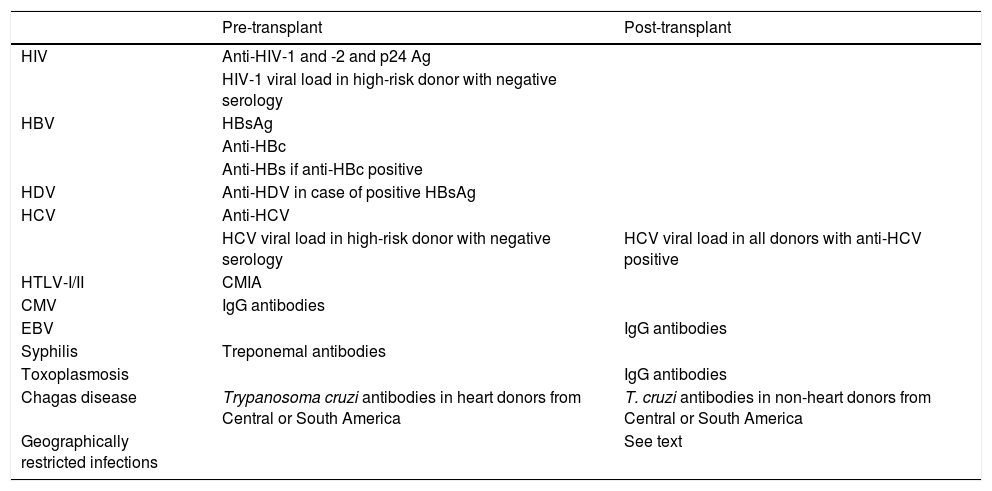
HIV infection in all donors should be ruled out (Table 3) using chemiluminescent immunoassay (CLIA) techniques that include simultaneous detection of antibodies (anti-HIV-1 and anti HIV-2) and HIV-1 p24 antigen. AI.
Table 3.Recommended study in the donor.
Pre-transplant Post-transplant HIV Anti-HIV-1 and -2 and p24 Ag HIV-1 viral load in high-risk donor with negative serology HBV HBsAg Anti-HBc Anti-HBs if anti-HBc positive HDV Anti-HDV in case of positive HBsAg HCV Anti-HCV HCV viral load in high-risk donor with negative serology HCV viral load in all donors with anti-HCV positive HTLV-I/II CMIA CMV IgG antibodies EBV IgG antibodies Syphilis Treponemal antibodies Toxoplasmosis IgG antibodies Chagas disease Trypanosoma cruzi antibodies in heart donors from Central or South America T. cruzi antibodies in non-heart donors from Central or South America Geographically restricted infections See text CMIA: chemiluminescent microparticle immunoassay.
- b.
For potential high-risk donors with negative serology, the detection of nucleic acids would be indicated to reduce the window period. AII.
- c.
The organs of a donor with HIV infection will not be accepted for a seronegative recipient. AII.
- d.
The organs of a donor with HIV infection could be considered as suitable for a seropositive recipient. BII.
- a.
- •
- 3.2.
What should be done in relation to an HBV-positive donor?
- •
Transmission risk
- a.
The risk of transmission is well documented in donors with positive HBV surface antigen (HbsAg) or positive viral load. RL1–2.
- b.
The transmission of HBV infection from donors with positive core antigen (anti-HBc) antibodies and reactive surface antigen (anti-HBs) antibodies (>10IU/L) is exceptional. RL3.
- c.
The risk of donor transmission with isolated anti-HBc positive will depend on the immunological status of the recipient and the type of transplanted organ. RL3.
- a.
- •
Recommendations
- a.
For hepatitis B screening, HBs Ag and anti-HBc should be determined by CLIA techniques. AI.
- b.
Transplantation from an HBsAg-positive donor to an HBsAg-negative and anti-HBs-negative recipient is not recommended except in cases of emergency. AII.
- c.
Transplantation from an HBsAg-positive donor to an HBsAg-positive recipient or with anti-HBs >10IU/ml can be performed. BII.
- d.
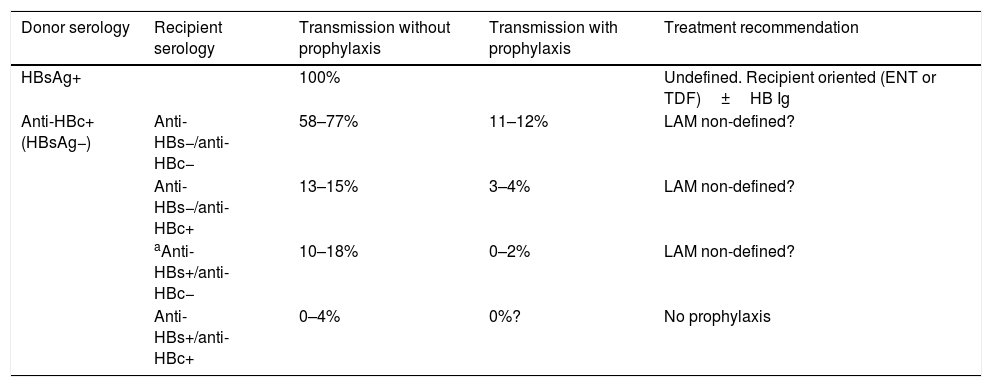
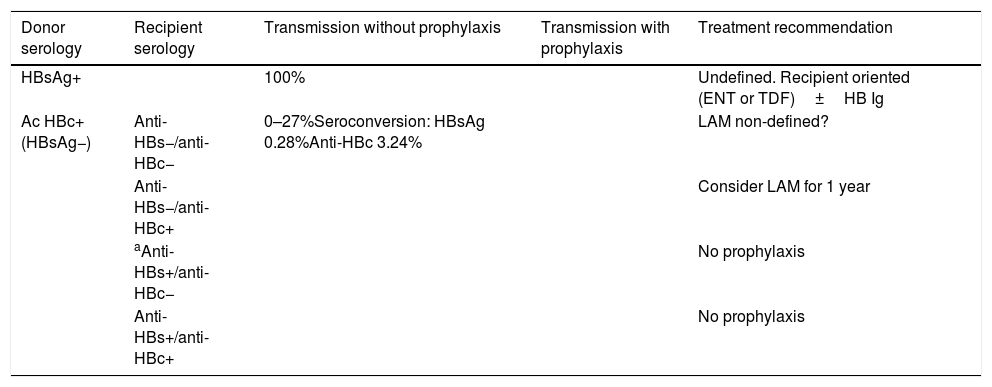
A transplantation from an anti-HBc-positive and HBsAg-negative donor can be performed pursuant to the recommendations in Tables 4 and 5. BII.
Table 4.Summary of the transmission data of anti-HBc+ donors in liver transplantation.
Donor serology Recipient serology Transmission without prophylaxis Transmission with prophylaxis Treatment recommendation HBsAg+ 100% Undefined. Recipient oriented (ENT or TDF)±HB Ig Anti-HBc+(HBsAg−) Anti-HBs−/anti-HBc− 58–77% 11–12% LAM non-defined? Anti-HBs−/anti-HBc+ 13–15% 3–4% LAM non-defined? aAnti-HBs+/anti-HBc− 10–18% 0–2% LAM non-defined? Anti-HBs+/anti-HBc+ 0–4% 0%? No prophylaxis Table 5.Summary of the transmission data of anti-HBc+ donors in kidney transplantation. Other organs (heart, lung) could be assimilated in terms of risk, given the scarcity of data.
Donor serology Recipient serology Transmission without prophylaxis Transmission with prophylaxis Treatment recommendation HBsAg+ 100% Undefined. Recipient oriented (ENT or TDF)±HB Ig Ac HBc+(HBsAg−) Anti-HBs−/anti-HBc− 0–27%Seroconversion: HBsAg 0.28%Anti-HBc 3.24% LAM non-defined? Anti-HBs−/anti-HBc+ Consider LAM for 1 year aAnti-HBs+/anti-HBc− No prophylaxis Anti-HBs+/anti-HBc+ No prophylaxis
- a.
- •
- 3.3.
What should be done in relation to an HCV-positive donor?
The information in this section is an excerpt from the consensus document promoted by the ONT that can be accessed on the following website: http://www.ont.es/infesp/DocumentosDeConsenso/Documento%20Consenso%20Valoración%20Donantes%20Virus%20C_ABRIL2019.pdf
It is worth mentioning that rapid advances in this field and ongoing studies could modify these recommendations in the forthcoming months.
- •
Transmission risk
- a.
The transmission of infection from an anti-HCV+ non-viraemic donor is exceptional.
- b.
Anti-HCV+ viraemic donors transmit HCV infection to almost all patients, regardless of the transplanted organ. RL1–3.
- a.
- •
Recommendations (Table 6)
- a.
HCV serological screening should be performed in all donors based on the detection of HCV antibodies (anti-HCV) using CLIA techniques. AII.
- b.
HCV RNA screening should be performed to rule out viraemia in all anti-HCV+ donors during the donation process. CIII.
- c.
In potential high-risk donors with negative serology, HCV-RNA detection would be indicated to reduce the window period. AII.
- d.
The organs of an anti-HCV+ non-viraemic donor (after effective treatment or spontaneous clearance) may be used in anti-HCV positive recipients without restrictions. CIII.
- e.
The organs of an anti-HCV+ non-viraemic donor (after effective treatment or spontaneous clearance) may be used in anti-HCV negative recipients that accept the risk after informed consent and undergo close monitoring and treatment in case of infection. CIII.
- f.
Donation of organs from an anti-HCV+ viraemic donor can be performed in HCV viraemic recipients who receive early or post-exposure treatment. CIII.
- g.
Donation of organs from anti-HCV+ viraemic donors can be performed in an anti-HCV negative recipient who agrees to the risk after informed consent and undergoes post-exposure treatment. CIII.
- h.
In the case of liver transplantation of an anti-HCV+ donor, the liver fibrosis stage should be established by elastography or biopsy. BII.
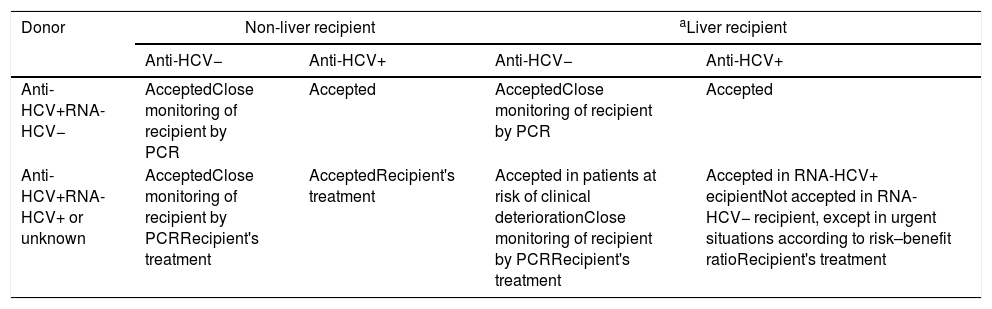
Table 6.Recommendations regarding transplantation of organs from donors with HCV infection.
Donor Non-liver recipient aLiver recipient Anti-HCV− Anti-HCV+ Anti-HCV− Anti-HCV+ Anti-HCV+RNA-HCV− AcceptedClose monitoring of recipient by PCR Accepted AcceptedClose monitoring of recipient by PCR Accepted Anti-HCV+RNA-HCV+ or unknown AcceptedClose monitoring of recipient by PCRRecipient's treatment AcceptedRecipient's treatment Accepted in patients at risk of clinical deteriorationClose monitoring of recipient by PCRRecipient's treatment Accepted in RNA-HCV+ ecipientNot accepted in RNA-HCV− recipient, except in urgent situations according to risk–benefit ratioRecipient's treatment - a.
- •
- 3.4.
What should be done in relation to a high-risk donor?
- •
Transmission risk
- a.
Transmission of HIV, HBV or HCV infection from a high-risk donor to a recipient is well documented. RL1–3.
- a.
- •
Recommendations
- a.
In high-risk donors, testing for nucleic acids is recommended in the case of negative serology for HIV-1/2 and/or HCV. AII.
- b.
Donation can be accepted if there is prior acceptance by the recipient upon signing the informed consent form and in case of emergency. BII.
- a.
- •
- 3.1.
- 4.
What latent infections should be screened in order to assess the risk of transmission?
- 4.1.
What decision should be made in relation to a donor with latent syphilis?
- •
Transmission risk
- a.
There is a risk of transmission of Treponema pallidum from donors with positive serology. RL3.
- a.
- •
Recommendations
- a.
It is recommended to routinely perform serologic tests for T. pallidum in both the recipient and donor. AII.
- b.
Treatment for syphilis of undetermined evolution should be administered in recipients from donors who have been tested positive for T. pallidum (positive treponemal test accompanied or not by positive reaginic test). AIII.
- a.
- •
- 4.2.
What decision should be made in relation to a donor with latent tuberculosis?
- •
Transmission risk
- a.
There is a risk of tuberculosis transmission from donors with latent tuberculosis infection (LTBI), particularly in lung transplant recipients. RL3.
- a.
- •
Recommendations
- a.
LTBI screening in living donors is recommended by performing PPD and/or interferon-γ release assay (IGRA); in case they are positive, the presence of active tuberculosis disease should be systematically ruled out before the transplant. AII.
- b.
The administration of chemoprophylaxis in living donors with untreated LTBI, AII, and ideally, deferring transplantation for at least 2 months is recommended. AIII.
- c.
Although LTBI screening in deceased donors has practical difficulties, the previous history of untreated tuberculosis, the epidemiological risk profile (countries of origin with high incidence) and/or the presence of residual lesions in chest X-Rays should be considered on a case-by-case basis. AIII. It is also possible to consider the performance of IGRA, although the experience in this regard is limited. CIII.
- d.
The administration of chemoprophylaxis in recipients of organs from donors (living or deceased) with documented LTBI (or high suspicion) that have not been previously treated or with insufficient information is recommended. AIII.
- e.
The administration of chemoprophylaxis in recipients of organs from donors (living or deceased) with a prior history of properly treated LTBI is not recommended. AIII.
- a.
- •
- 4.3.
What decision should be made in relation to a donor with CMV infection?
- •
Transmission risk
- a.
There is a high risk of CMV transmission from seropositive donors to seronegative recipients (D+/R−) with potentially serious consequences. RL3.
- a.
- •
Recommendations
- a.
It is recommended to routinely perform serologic tests for CMV in both the recipient and donor. AII.
- b.
The application of specific prevention strategies is recommended in recipients with D+/R− serological status for CMV. AI.
- a.
- •
- 4.4.
What decision should be made in relation to a donor with Epstein–Barr virus (EBV) infection?
- •
Transmission risk
- a.
There is a high risk of EBV transmission from seropositive donors to seronegative recipients (D+/R−) with potentially serious consequences. RL3.
- a.
- •
Recommendations
- a.
Systematic monitoring of EBV viraemia by viral load detection is recommended in recipients with D+/R− serological status for EBV. BII.
- a.
- •
- 4.5.
Should infection by other herpesvirus, parvovirus B19, BK virus or hepatitis E virus (HEV) be ruled out?
- •
Transmission risk
- a.
The risk of transmission from donors with chronic herpes simplex virus (HSV) infection, varicella-zoster virus (VZV), human herpes virus type 6 (VHH-6), 7 (VHH-7) and 8 (VHH-8), parvovirus B19 and BK virus is considered of low probability and/or with little clinical impact. RL5.
- b.
Although HEV transmission by liver graft has been described with severe ramifications, there is not enough information to systematically assess this risk. RL4.
- a.
- •
Recommendations
- a.
It is not recommended to routinely perform the serologic tests for these viruses in the donor. AIII.
- a.
- •
- 4.6.
What decision should be made in relation to a donor with Toxoplasma gondii infection?
- •
Transmission risk
- a.
There is a risk of T. gondii transmission in (D+/R−) with potential clinical implications, particularly in heart transplant recipients. RL3.
- a.
- •
Recommendations
- a.
It is recommended to routinely perform serologic tests for T. gondii in both recipient and donor, especially in heart transplant recipients. AII.
- b.
In heart transplant recipients with D+/R− serological status, T. gondii treatment should be administered at full doses during the first three months after transplantation. AIII.
- a.
- •
- 4.1.
- 5.
Should hidden infections in the donor be ruled out?
- 5.1.
Is it necessary to rule out bacteremia in the donor?
- •
Transmission risk
- a.
The risk of transmission of bacteraemia from donor to recipient is due to the identified microorganism and its susceptibility to antibiotics. RL2–3.
- a.
- •
Recommendations
- a.
Blood donor cultures should be obtained routinely at the time of donation. AII.
- b.
All the necessary organisational measures must be taken to ensure that the information on the result of the blood culture collected in the centre, where the donor is located, arrives to the centre where the recipient is located in the shortest time possible and with the highest quality (in case these centres are different). AII.
- c.
The organs of a donor with bacteraemia can be safely used for transplantation if the following conditions are met. AII.
- i.
absence of signs of sepsis in the donor
- ii.
if the donor has been treated with an effective antibiotic, at least for 24–48h
- iii.
prompt transmission of information on blood culture isolation to the centre where the recipient is located
- iv.
continuity of an effective antibiotic treatment in the recipient for 7–14 days (depending on the microorganism pathogenicity and the characteristics of the antimicrobial treatment)
- i.
- a.
- •
- 5.2.
Should urinary tract infection (UTI) in the donor be ruled out?
- •
Transmission risk
- a.
The presence of a positive urine culture in the donor represents a risk of transmission in kidney transplant recipients. RL3.
- a.
- •
Recommendations
- a.
The systematic performance of urine culture in a SOT donor other than kidney transplant is not recommended. AIII.
- b.
The presence of a positive urine culture in a SOT donor other than kidney transplant is not deemed a contraindication for the transplant. AIII.
- c.
The presence of a positive urine culture (including candiduria without candidemia) in a deceased kidney transplant donor is not considered a contraindication for transplantation, as long as it corresponds to a mild urinary tract infection or asymptomatic bacteriuria. The recipient must receive antibiotic treatment according to the donor's urine culture antibiogram for at least 10 days. AII.
- a.
- •
- 5.3.
Should respiratory infection in the donor be ruled out?
- •
Transmission risk
- a.
The risk of transmission of infection from donor to recipient is well documented in lung transplantation. Its consequence will depend on the identified microorganism and its susceptibility to antibiotics. RL2–3.
- a.
- •
Recommendations
- a.
A bronchial aspirate should be performed in the donor and in the lung transplant recipient at the time of the procedure. AII.
- b.
The lung donor with an active bacterial infection should receive antibiotic treatment before the donation of the organ (preferably for more than 48h). Treatment should continue in the recipient. AII.
- c.
In the case of lung transplantation, colonisation by microorganisms with low therapeutic reserves (Klebsiella pneumoniae or Acinetobacter baumanii resistant to carbapenems, extremely resistant (XDR) Pseudomonas aeruginosa, Burkholderia cenocepacia, Mycobacterium abscessus) should be considered a relative contraindication. The lungs should be used based on the urgency of the transplant and post-transplant therapeutic possibilities. AIII.
- d.
Donor organs with positive respiratory secretion cultures, including microorganisms with different antibiotic resistance patterns, can be considered for transplantation. Recipients should be monitored. AIII.
- a.
- •
- 5.4.
Should bile duct infection in the donor be ruled out?
- •
Transmission risk
- a.
The risk of transmission of a donor with positive bile duct culture is unknown. RL4.
- a.
- •
Recommendations
- a.
Systematic culture of donor bile in liver transplantation is not recommended. AII.
- a.
- •
- 5.5.
Should preservation fluid-related infection be ruled out?
- •
Transmission risk
- a.
The transmission of preservation fluid-related infection is well documented. Its impact will depend on the identified microorganism and its susceptibility to antibiotics. RL2–3.
- a.
- •
Recommendations
- a.
Although there is no ambiguous evidence that systematic culture of preservation fluids should be a routine practice in organ transplantation, the panel recommends its practice. CIII.
- b.
A positive culture of preservation fluid for potentially pathogenic bacteria would require the administration of proper antimicrobial treatment in the recipient for not less than two weeks. BIII.
- c.
In the presence of Candida spp. in the preservation fluid it is advisable to obtain blood cultures, urine culture and drainage and fungal biomarkers in the recipient, as well as assess the start of antifungal treatment. In these cases, a baseline Doppler ultrasonography should be performed due to the risk of vascular involvement by Candida spp. BIII.
- d.
When the result of the preservation fluid culture is positive for low virulence bacteria (negative staphylococcus plasmocoagulase, Corynebacterium spp, etc.) antibiotic treatment in the recipient does not seem to be necessary. BII.
- a.
- •
- 5.1.
- 6.
What clinical situations should be assessed for the donation of a solid organ?
- 6.1.
What course of action should be undertaken in the case of a potential donor with active tuberculosis?
- •
Transmission risk
- a.
Transmission from a donor infected with active tuberculosis has been documented. RL1.
- a.
- •
Recommendations
- a.
It is recommended to contraindicate solid organ transplantation in cases of active tuberculosis and in cases of justified suspicion. AII.
- a.
- •
- 6.2.
What should be done in relation to a potential donor with pneumonia?
- •
Transmission risk
- a.
Acute pneumonia without systemic dissemination does not constitute a contraindication for transplantation (RL5) except for both single and double lung transplant. RL1–3.
- a.
- •
Recommendations
- a.
Donors with pneumonia should receive effective antibiotic treatment before the organ removal (preferably for more than 48h) and present haemodynamic stability. AII.
- b.
Treatment should be continued in the recipient for a period of 7–14 days. AII.
- a.
- •
- 6.3.
What should be done in the case of a potential donor with influenza?
- •
Transmission risk
- a.
Donors with influenza virus infection can transmit the infection to the recipient. RL1–3.
- a.
- •
Recommendations
- a.
The deceased subjects with suspected or confirmation of influenza virus infection, whether they have received antiviral treatment or not, can be considered as SOT donors provided that the recipient is treated prophylactically with neuraminidase inhibitors. BIII.
- b.
Deceased subjects with suspected or confirmed influenza virus infection should be ruled out as lung or bowel donors. AIII.
- c.
Transplantation from a living donor with influenza should be postponed until the infection is resolved. AIII.
- a.
- •
- 6.4.
What should be done in the case of a potential donor with pyelonephritis?
- •
Transmission risk
- a.
Acute pyelonephritis without systemic dissemination does not constitute a contraindication for transplantation (RL5) except for transplantation of any of the two kidneys. RL1–3.
- a.
- •
Recommendations
- a.
The potential donor with acute pyelonephritis should receive effective antibiotic treatment before removal of the organ (preferably for more than 24–48h) and present haemodynamic stability. AII.
- b.
Treatment should be continued in the recipient for a period of 7–14 days. AII.
- c.
Acute pyelonephritis or renal abscesses at the time of the donor's death are considered a contraindication for kidney transplantation. AIII.
- d.
Any type of urinary tract infection (high or low) in a living donor is considered an indication to delay the transplant until it has been resolved. AIII.
- a.
- •
- 6.5.
What should be done in the case of a potential donor with meningitis?
- •
Transmission risk
- a.
Acute meningitis without systemic dissemination does not constitute a contraindication for transplantation. RL2–3.
- a.
- •
Recommendations
- a.
The donor with bacterial meningitis should receive effective antibiotic treatment before the organ removal (preferably for more than 24–48h) and present haemodynamic stability. Treatment should be continued in the recipient for a period of 7–14 days. AII.
- a.
- •
- 6.6.
What should be done in the case of a potential donor with encephalitis?
- •
Transmission risk
- a.
The transmission of viral encephalitis (West Nile virus, lymphocytic choriomeningitis, rabies, etc.) from donor to recipient with a fatal outcome has been documented. RL1.
- a.
- •
Recommendations
- a.
Organs from donors with encephalitis without etiologic diagnosis should not be used for transplantation due to the high transmission risk of infection in the recipient. AII.
- b.
Organs from donors with encephalitis of known aetiology (herpes simplex virus) will be assessed individually. CIII.
- a.
- •
- 6.7.
What should be done in the case of a potential donor with a prion disease?
- •
Transmission risk
- a.
The transmission of diseases caused by prions from donor to recipient is well documented. RL1.
- a.
- •
Recommendations
- a.
The organs of donors diagnosed with prion diseases should not be used for transplantation. AII.
- a.
- •
- 6.8.
What should be done in the case of a potential donor with endocarditis?
- •
Transmission risk
- a.
Endocarditis does not constitute a contraindication for transplantation, except for heart transplantation. RL1–3.
- a.
- •
Recommendations
- a.
In the patient with endocarditis, heart donation is contraindicated. AII.
- b.
Patients with endocarditis can be accepted as donors of other organs if they have received proper antibiotic treatment prior to donation (preferably a minimum of 48h), if they have been tested negative for bacteraemia and there is no evidence of embolic phenomena that have damaged the organs to be transplanted. Targeted antibiotic treatment should be continued in the recipient. AII.
- a.
- •
- 6.9.
What should be done in the case of a potential donor with other localised infections: cholecystitis, cholangitis, arthritis, osteomyelitis, cellulitis, abscesses, etc.?
- •
Transmission risk
- a.
There is transmission risk if the infected organ is transplanted. RL1.
- a.
- •
Recommendations
- a.
The organ affected by the infection should not be transplanted. AII.
- b.
The donor with localised bacterial infection must have received adequate treatment prior to donation (preferably a minimum of 24–48h). Targeted antibiotic treatment should be continued in the recipient. AII.
- a.
- •
- 6.10.
What should be done in the case of a potential donor with septic shock?
- •
Transmission risk
- a.
The donor suffering from septic shock of unknown origin can transmit the infection to the recipient. RL1.
- a.
- •
Recommendations
- a.
Septic shock of unknown origin and even its well-founded suspicion should, in principle, contraindicate the use of organs for transplantation. AIII.
- b.
If the septic shock origin is fungal or related to tuberculosis, the use of organs for transplantation should be contraindicated. AII.
- a.
- •
- 6.11.
What should be done in the case of a potential donor with disseminated fungal infection?
- •
Transmission risk
- a.
The donor with disseminated fungal infection has a high risk of infection transmission. RL1–2.
- a.
- •
Recommendations
- a.
It is recommended to rule out the existence of an invasive mycosis in donors with CNS or pulmonary pathology whose origin is not known, especially if they present risk factors such as immunosuppressed donors, prolonged stay in ICU, prolonged mechanical ventilation or drowning victims. BIII.
- b.
Patients with disseminated mycosis or CNS mycosis should not be accepted as donors. In cases of extreme need, transplantation can be assessed if the donor has received prior treatment and microbiological eradication has been documented. BIII.
- a.
- •
- 6.12.
What should be done in the case of a potential donor with localised fungal infection?
- •
Transmission risk
- a.
Donors with localised fungal infection present a risk of transmission of the infection to the recipient. RL1–2.
- a.
- •
Recommendations
- a.
In donors with focal lung lesions, a histopathological and microbiological study of the biopsy specimen should be performed. Transplantation of an organ with fungal infection is not recommended, except in situations of extreme urgency and prior documentation of microbiological eradication. BIII.
- b.
Organ transplantation of patients with cryptococcal meningitis is not recommended, except in conditions of extreme urgency. BIII. In donors with pulmonary or extraneural cryptococcosis, lumbar puncture with cryptococcal antigen should be performed, as well as CSF cultures, blood cultures, urine cultures and serum cryptococcal antigen. BII.
- a.
- •
- 6.13.
What should be done in the case of a potential donor with fever of unknown origin?
- •
Transmission risk
- a.
There are no data on the risk of infection transmission in donors suffering from fever of unknown origin (FUO). RL4.
- a.
- •
Recommendations
- a.
Screening of potential donors includes comprehensive medical history and social behaviour, as well as thorough physical examination. It is necessary to perform laboratory analysis, microbiological and radiological tests according to the patient's clinical condition and personal history. AII.
- b.
In addition to the usual tests, specific serologic tests are recommended based on the donor's medical history and clinical suspicion. AII.
- c.
The possibility of an autopsy should be considered in all donors who died with fever in order to diagnose a hidden infection. AII.
- d.
In the event that the subjects have died from a suspected or confirmed infection of unknown origin, informed consent is required by the recipient assuming the risk of transmission of an infection. AIII.
- a.
- •
- 6.14.
What should be done in the case of a potential donor colonised by multidrug resistant microorganisms?
- •
Transmission risk
- a.
There is insufficient data to determine the risk of transmission of infection from a donor colonised by multidrug resistant bacteria to a recipient. RL4.
- a.
- •
Recommendations
- a.
The use of organs from patients with active systemic infection by multidrug resistant bacteria is not recommended. BIII.
- b.
There is no evidence about the benefit of systematically conducting colonisation investigation by Staphylococcus aureus or Enterococcus R to vancomycin in the donor, since it is not clear what course of action should be taken with the recipient, and if the establishment of empirical antibiotic treatment has any benefit to avoid related infections that may arise during the postoperative period. If colonisation is documented there is no contraindication for the use of these organs. BIII.
- c.
It is recommended to perform a rectal exudate to search for multidrug resistant gram-negative bacteria (carriers of extended-spectrum beta lactamases and carbapenemases). If positive, the use of donor organs is not contraindicated. It is not known if the administration of antibiotic prophylaxis to recipients of organs from donors colonised by these microorganisms has any impact on the prevention of infections arising thereof. However, it is important to have the epidemiological history recorded in the medical history in order to adjust the empirical antibiotic treatment in case of suspected infection immediately after the transplantation period. BIII.
- a.
- •
- 6.1.
- 7.
How important is the place of origin of the donor?
Table 7 summarises the approach to infection screening according to the geographical area of origin of the donor.
- 7.1.
What course of action should be undertaken in cases of suspected malaria?
- •
Transmission risk
- a.
RL2. If donor dies of malaria: RL1.
- a.
- •
Recommendations
- a.
It is recommended to screen all immigrant donors or travellers to endemic areas (tropical and subtropical areas, especially sub-Saharan Africa) in the last 3 years by smear and thick peripheral blood drop and detection of antigens by rapid immunochromatography techniques (RDT-malaria). II.
- b.
It is recommended to performe Plasmodium PCR in a deferred way to detect low or mixed parasitaemia. AIII.
- c.
Malaria in the donor is not considered an absolute contraindication for the use of the organs (unless the patient has died of malaria). AIII.
- d.
In the case of a donor with malaria, treatment should be initiated early in the recipient. BIII.
- a.
- •
- 7.2.
What course of action should be undertaken in cases of suspected Trypanosoma cruzi infection?
- •
Transmission risk
- a.
RL2. If donor has acute Chagas disease or if donated organ is the heart/intestine: RL1.
- a.
- •
Recommendations
- a.
The donor should be screened by serology if there are risk factors for T. cruzi (donor residing in endemic area of Latin America, except the Caribbean, even years before, who has received a transfusion in endemic area or son of a mother born in endemic area). II.
- b.
The use of organs from donors with acute infection is contraindicated and the use of heart/intestine from donors with chronic T. cruzi infection is contraindicated. AIII.
- c.
The use of other organs such as liver and kidney (not heart/intestine) from donors with chronic T. cruzi infection can be assessed after adequate informed consent and proper post-transplant monitoring. BII.
- d.
In the case of an infected living donor, specific trypanocidal treatment before donation could reduce parasitic load and transmission. AIII.
- e.
Routine treatment/prophylaxis with benznidazole in recipients of organs from donors with positive T. cruzi serology is not recommended, but close monitoring (clinical and parasitological) is recommended. AIII.
- f.
Early specific anti-parasitic treatment is recommended in case of recipients affected by acute donor-derived infection with positive T. cruzi serology. AII.
- a.
- •
- 7.3.
What course of action should be undertaken in cases of suspected Strongyloides spp. infection?
- •
Transmission risk: RL2.
- •
Recommendations
- a.
A targeted screening (serology and stool analysis) will be carried out in donors with risk factors (stays in tropical and subtropical areas, even if that happened years before). II.
- b.
If the organs of a seropositive donor for Strongyloides sp. are accepted, ivermectin treatment of the recipient and close clinical monitoring should be considered in the post-transplant period. AII.
- a.
- •
- 7.4.
What course of action should be undertaken in cases of suspected Schistosoma spp. infection?
- •
Transmission risk: RL2.
- •
Recommendations
- a.
Screening with serology is recommended in donors with risk factors (stays in tropical and subtropical areas, especially in sub-Saharan Africa, even if that took place many years before donation). AIII.
- b.
The organs of a donor with positive serology could be used, but correct treatment with praziquantel should be performed on the infected living donor. AII.
- a.
- •
- 7.5.
What course of action should be undertaken in cases of suspected Clonorchis spp/Opistorchis spp. infection?
- •
Transmission risk: RL2.
- •
Recommendations
- a.
Screening is recommended for donors from risk areas, especially if there is peripheral eosinophilia, by studying faeces to visualise the parasite eggs. AIII.
- b.
Infection with these trematodes would not be an absolute contraindication for transplantation, but specific treatment with praziquantel should be administered to donors and recipients. AII.
- a.
- •
- 7.6.
What course of action should be undertaken in cases of suspected filarial infection?
- •
Transmission risk: RL4.
- •
Recommendations
- a.
There are no specific recommendations for screening donors from endemic areas. AIII.
- a.
- •
- 7.7.
What course of action should be undertaken in cases of suspected Coccidioides spp infection?
- •
Transmission risk: RL2.
- •
Recommendations
- a.
Screening by serological techniques is recommended in donors staying in or travelling to endemic areas. AII.
- b.
In Spanish reference centres, the available serological technique is immunodiffusion (IgG and IgM). AII.
- c.
The use of molecular techniques has been useful in transplants with clinical suspicion of reactivation and negative serology but there is no experience in donor screening. CIII.
- d.
In the case of donors who have lived in endemic areas and especially if they have a history of past infection or suggestive radiological changes, it is recommended to start prophylaxis in the recipient with post-transplant fluconazole pending serological results. BII.
- e.
If these are positive, it is mandatory to rule out active disease. AII. In its absence, prophylaxis with fluconazole, itraconazole or posaconazole should be maintained for at least 6 months and with quarterly serological monitoring during the first year and annually thereafter. BII.
- a.
- •
- 7.8.
What course of action should be undertaken in cases of suspected Histoplasma capsulatum infection?
- •
Transmission risk: RL2.
- •
Recommendations
- a.
In non-endemic areas, it is important to conduct a correct medical history in donors who have resided in or travelled to endemic areas and screen donors by serology, especially those with a clinical history and/or suggestive chest X-ray. II.
- b.
Immunodiffusion is the technique available in Spanish reference centres. II. The result should not rule the indication for the transplant. II.
- c.
Itraconazole is recommended to recipients of organs from seropositive donors for at least 3–6 months during the period of maximum immunosuppression. BIII.
- d.
Although posaconazole has been proven effective in the treatment of histoplasmosis, there is no experience on its use in prophylaxis. CIII.
- a.
- •
- 7.9.
What course of action should be undertaken in cases of suspected Paracoccidioides brasiliensis infection?
- •
Transmission risk: RL5.
- •
Recommendations
- a.
Given the low frequency of paracoccidioidomycosis in the post-transplant period and the low utility of serological markers, which are usually negative in this type of patients, there is no special recommendation for follow-up of this disease in transplant patients. CIII.
- a.
- •
- 7.10.
What course of action should be undertaken in cases of suspected Blastomyces dermatitidis infection?
- •
Transmission risk: RL5.
- •
Recommendations
- a.
Specific measures for the recipient or donor are not recommended, given the low prevalence of blastomycosis in transplants and the low profitability of antigen and/or antibody detection techniques. CIII.
- a.
- •
- 7.11.
What course of action should be undertaken in cases of suspected Penicillium marneffei infection?
- •
Transmission risk: RL4.
- •
Recommendations
- a.
No specific measures are recommended for the control of this infection in recipients or donors. CIII.
- a.
- •
- 7.12.
What course of action should be undertaken in cases of suspected human T-lymphotropic virus 1 (HTLV-1) infection?
- •
Transmission risk: RL1.
- •
Recommendations
- a.
Universal screening with serology in all donors through automated, approved tests that are efficient, fast with an adequate cost. AII.
- b.
Screening is especially indicated in: (a) donors from or who have lived in endemic areas of HTLV-1 infection; (b) donors who are children of mothers born or residing in endemic area; (c) donors, especially women, whose partners have resided in endemic areas. BII.
- c.
In the case of seropositive donor and seronegative recipient, reject the organ. AII.
- d.
In the case of seropositive donor and seropositive recipient for HTLV-1, assess acceptance of the organ, by considering potential lower risks of associated disease development in already infected subjects. BII.
- a.
- •
- 7.13.
What course of action should be undertaken in cases of suspected rabies virus infection?
- •
Transmission risk: RL1.
- •
Recommendations
- a.
Draw a history, as detailed as possible, of donor travels, exposures or accidents that occurred during these trips, such as bites, wounds, and a history of previous travel vaccinations. AII.
- b.
Donors with fever and an unexplained CNS event should be evaluated to rule out meningoencephalitis. AIII.
- c.
Reject the donor with unknown encephalitis data. II.
- d.
In case of transplant transmission, identify and perform early immunisation of the other recipients. AIII.
- a.
- •
- 7.14.
What course of action should be undertaken in relation to a donor with suspected West Nile Virus (WNV) infection?
- •
Transmission risk: RL1.
- •
Recommendations
- a.
Screening should be based on the donor's epidemiological background (stay in areas where there are cases of WNV transmission to humans in the previous 28 days) since most infections are asymptomatic. BII.
- b.
It is recommended to evaluate PCR screening in those donors with epidemiological risk and/or compatible symptoms, in case of:
- i.
Stay, travel or blood product transfusions during activity periods in areas with active WNV transmission (May to November in the northern hemisphere). BII.
- ii.
History of febrile syndrome with or without neurological symptoms during stay in areas of active WNV transmission. BII.
- iii.
Donors with fever and encephalopathy at the time of donation and epidemiological history of potential exposure to WNV. AII.
- iv.
History of diagnosis of WNV infection. AII.
- i.
- c.
If viraemia or documented WNV infection is detected within the previous 28 days, organ donation should be ruled out. II.
- d.
If screening is not possible and there are epidemiological risk factors or a medical history within the previous 28 days, organ donation should be rejected. BII.
- a.
- •
- 7.15.
What course of action should be undertaken in cases of suspected Dengue virus infection?
- •
Transmission risk: RL1.
- •
Recommendations
- a.
Adequate donor screening in case of epidemiological risk factors within 28 days prior to transplant. BIII.
- b.
For screening, NS1 antigen detection, PCR and NS1 IgM antibody detection are recommended. AII.
- c.
In the case of a donor with acute dengue infection (NS1 antigen and/or positive PCR), donation should be ruled out. AIII.
- d.
If the donor has positive IgM serology as the only screening marker, the risk–benefit ratio associated with the transplant should be assessed, given the difficulties of interpretation about the time of infection, and inform the recipient about the possible effects. CIII.
- a.
- •
- 7.16.
What course of action should be undertaken in cases of suspected lymphocytic choriomeningitis virus infection?
- •
Transmission risk: RL1.
- •
Recommendations
- a.
The sensitivity of the tests available for diagnosis is not appropriate for routine donor screening.
- b.
Draw an epidemiological history of the exposure or contact of the donor with rodents and evaluation of clinical symptoms. BIII.
- c.
In case of high suspicion of LCMV infection (previous exposure and compatible symptoms), donor should be excluded. AII.
- a.
- •
- 7.17.
What course of action should be undertaken in cases of suspected Chikungunya virus infection?
- •
Transmission risk: RL1.
- •
Recommendations
- a.
Perform donor screening of tissues (BII) and organs (BIII) if any of the following situations exist in the previous 28 days: stay in areas affected by the epidemic, previous Chikungunya virus (CHIKV) infection or signs and symptoms of active infection at the time of donation. BII.
- b.
PCR (RT-PCR) should be used as a screening technique (blood and tissues). AII.
- c.
Donors with positive PCR should be excluded from organ and tissue donation. BII.
- d.
Donation should be refused in those cases with a history of previous CHIKV infection in the previous 28 days. BIII.
- e.
People without active infection and epidemiological risk history may be donors if molecular tests have been carried out to rule out the infection. BIII.
- a.
- •
- 7.18.
What course of action should be undertaken in cases of suspected Zika virus infection?
- •
Transmission risk: RL1.
- •
Recommendations
- a.
Microbiological screening for the donor, given the possible risk of transmission in certain epidemiological contexts. BIII.
- i.
Within 28 days prior to:
- 1.
Travel or residence in areas with Zika virus transmission (ZIKV),
- 2.
Transfusions of blood products,
- 3.
Presence of related symptoms.
- 1.
- ii.
Within six months prior to:
- 1.
Unprotected sex with people who live or have recently been in areas with ZIKV transmission.
- 1.
- i.
- b.
Microbiological screening, if available, by PCR in blood and urine. BIII, in people with epidemiological risk factors.
- c.
If PCR positive, it is recommended to refuse the donation. AIII.
- d.
In case of documented infection, do not accept organs or tissues for transplantation until 6 months after resolution of symptoms. BIII.
- e.
In the case of negative PCR, but epidemiological risk factors in the previous 28 days, consider donation after risk assessment and benefit of the potential risk of infection derived from the donor and informed consent in the following situations. CIII.
- f.
If it is not possible to perform the screening and in case of the epidemiological factors abovementioned, it is recommended to:
- i.
In asymptomatic donors, consider donation after risk assessment and benefit of the potential risk of donor-derived infection and informed consent. CIII.
- ii.
In symptomatic donors, whose symptoms cannot be explained by alternative diagnosis, it is recommended to refuse donation. BIII.
- i.
- a.
- •
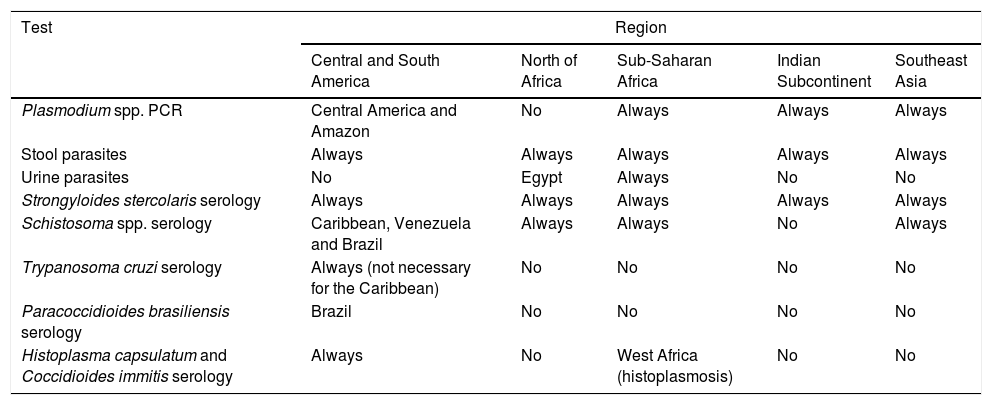
Table 7.Screening recommendations for donor-derived infections with geographic restriction according to their geographical origin.
Test Region Central and South America North of Africa Sub-Saharan Africa Indian Subcontinent Southeast Asia Plasmodium spp. PCR Central America and Amazon No Always Always Always Stool parasites Always Always Always Always Always Urine parasites No Egypt Always No No Strongyloides stercolaris serology Always Always Always Always Always Schistosoma spp. serology Caribbean, Venezuela and Brazil Always Always No Always Trypanosoma cruzi serology Always (not necessary for the Caribbean) No No No No Paracoccidioides brasiliensis serology Brazil No No No No Histoplasma capsulatum and Coccidioides immitis serology Always No West Africa (histoplasmosis) No No - 7.1.
The authors declare that there are no conflicts of interest.
This work was supported by GESITRA/SEIMC, ONT and ‘Plan Nacional de I+D+I’ and Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias 12/02269 and Proyecto Integrado de Excelencia 13/00045), Subdirección General de Redes y Centros de Investigacion Cooperativa, Spanish Ministry of Economy and Competitiveness, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016), co-financed by the European Development Regional FundA way to achieve Europe.
The complete consensus statement is available as Appendix in supplementary material.