To date, very little data is available on the extensive, familiar, serological screening of Trypanosoma cruzi from infected-index cases. As it is a parasite with possibility of mother-to-child foetal transmission, the study of the offspring of chronically infected women has a special relevance.
MethodsAn observational study using a capture-recapture method that evaluates the offspring serological status of women diagnosed with T. cruzi infection (positive serology) in the northern metropolitan area of Barcelona during 2005–2016.
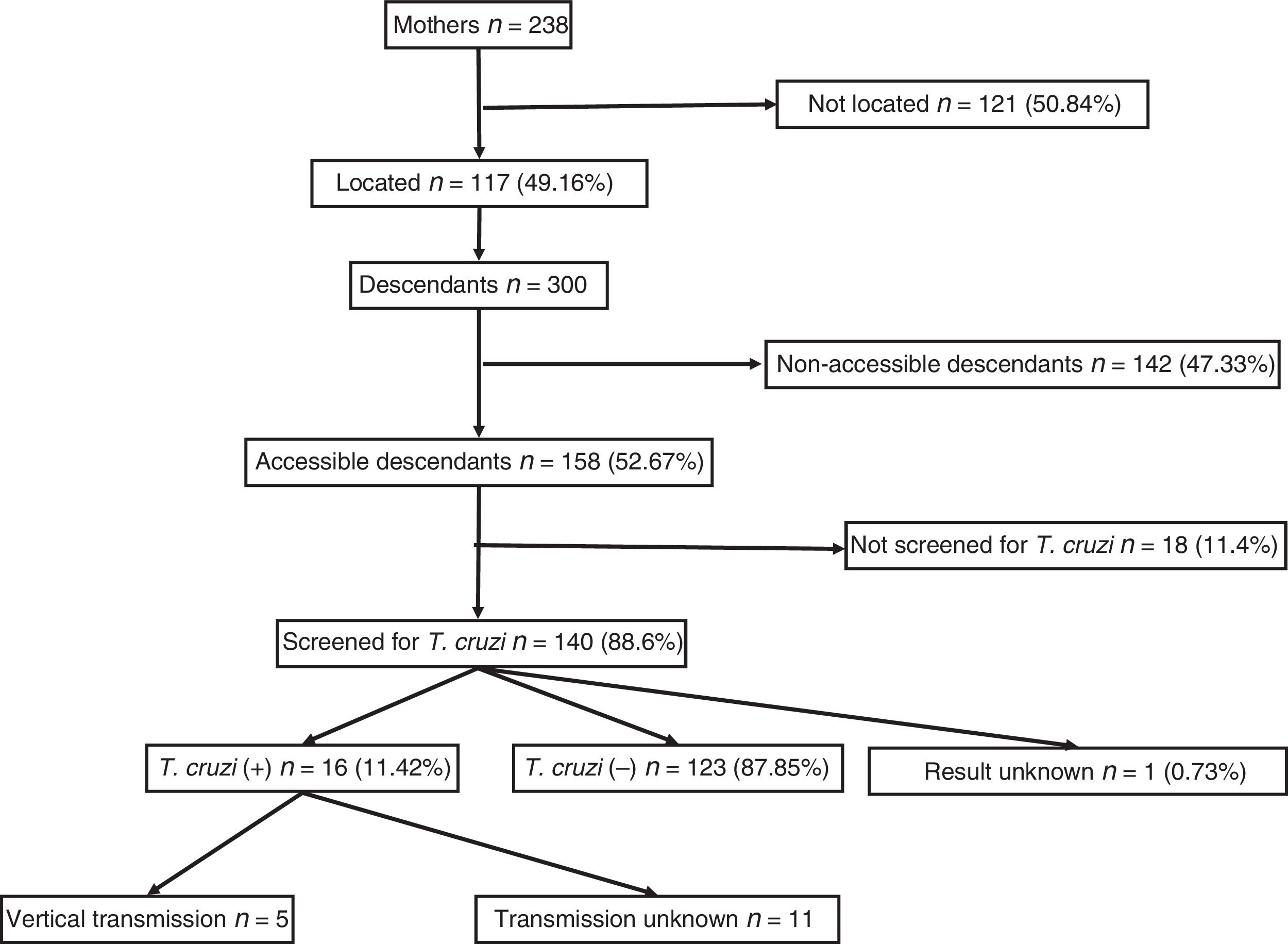
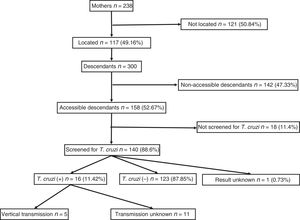
ResultsA total of 238 women with positive serology for T. cruzi were identified. Of these, 117 (49.2%) could be localised. Their offspring summarised 300 individuals, of which 192 (64%) had serology records, with 23 positive for T. cruzi (11.98%; CI95%: 8.1–17.3). Among the 53 children born within the study area, 5 (9.8%, CI95%: 4.2–20.9) cases of vertical transmission were recorded. All children born as of 2010 (the starting year of mother screening) had serological outputs.
ConclusionsOffspring of T. cruzi-seropositive women showed a high rate of seropositivity. The prevalence of vertical transmission is also remarkably high but comparable to that obtained in other European studies. The main source of loss was non-accessible women. It is reasonable to formally include extensive, familiar, serological assessment in Chagas screening guidelines. In order to avoid losses, any eventual screening should be implemented at the time of the maternal diagnosis.
Existen escasos datos sobre el cribado serológico extenso, familiar, de Trypanosoma cruzi a partir de un infectado-índice. Por tratarse de una parasitosis con posibilidad de transmisión materno-fetal, el estudio de la descendencia de mujeres crónicamente infectadas posee una especial relevancia.
MétodosEstudio observacional por método de captura-recaptura que valora el estado serológico en la descendencia de las mujeres diagnosticadas de infección por T. cruzi en el área metropolitana norte de Barcelona durante el periodo 2005-2016.
ResultadosSe identificaron 238 mujeres son serología positiva para T. cruzi. De ellas, se pudieron localizar 117 (49.2%) y sus 300 descendientes. Entre los descendientes, 192 (64%) tenían registro de serología, con 23 positivas para T. cruzi (11,98%; IC 95%: 8,1-17,3). Hubo 53 niños nacidos en el área de estudio, con 5 casos de transmisión vertical (9,8%; IC 95%: 4,2-20,9). Todos los nacidos a partir de la implementación del programa de cribado materno (en 2010) tenían registro serológico.
ConclusionesLa población de descendientes de mujeres con serología positiva para T. cruzi muestra una tasa elevada de seropositividad. La prevalencia de transmisión vertical es notablemente alta, pero comparable a la obtenida en otros estudios europeos. La principal fuente de pérdidas lo constituyen las mujeres ilocalizables. Es razonable incluir la determinación serológica familiar extensa en los protocolos de cribado de enfermedad de Chagas. A fin de evitar pérdidas, se debería implementar un eventual cribado en el momento del diagnóstico materno.
Chronic parasitic infestation by the protozoan Trypanosoma cruzi leads to so-called Chagas disease or American trypanosomiasis, a New World parasitic disease which is widespread within a geographic framework spanning from Texas to northern Argentina. Approximately 30% of those infected develop serious dysfunction in organs with parasitic infestation, especially the heart.1 This not only has significant repercussions for individuals but also represents a very high impact and economic burden on healthcare systems in Latin America. Although the primary route of contagion is vector–orne (triatomine bugs), the maternal–foetal route of contagion represents 5–% of new cases of contagion, according to various studies.2,3 Spain received a large number of immigrants from the so-called “Chaco focus” (Bolivia, Paraguay and northern Argentina) in the first decade of the 21st century. This led to the appearance of cases of Chagas disease. Programmes for serological screening of blood products and transplants were designed with relative speed (2005 and 2006).4,5 Subsequently, various regions of Spain defined and implemented protocols for serological detection of maternal T. cruzi infection in the first trimester of pregnancy (2010).6–8 Said documents specify how pregnant women and neonates with positive serology for T. cruzi should be managed. However, while they advise screening all prior descendants, they do not incorporate it into protocol. This weak recommendation is based more on expert opinion and common sense than on available data supplying evidence in this regard.9
The possibility of protocolising widespread screening of all children of women infected with T. cruzi, whether or not T. cruzi infection is detected during the first trimester of pregnancy, will depend on the means of the healthcare system, the rate of identified cases and, ultimately, the cost–benefit ratio of such screening. This study was intended to supply epidemiological and clinical data in this regard.
Patients and methodThis was an observational, retrospective study intended to determine and characterise T. cruzi serological status in descendants of women diagnosed with chronic T. cruzi infection in the northern Barcelona metropolitan area. This healthcare area comprised the municipalities of Santa Coloma de Gramenet, Badalona, El Masnou, Sant Adrià del Besòs and Montgat (totalling 404,023 inhabitants according to the 2015 register), where 13.8% of the population consisted of immigrants. According to data taken from municipal registers (2016), approximately 18,000 people (4.5%) were from countries with endemic Chagas disease. The most commonly affected population, Bolivian immigrants, consisted of 3606 people (0.9%).10
The study population was defined as all descendants of women with a known diagnosis of Chagas infection (T. cruzi+) and residing in the study area, regardless of whether or not said diagnosis derived from a specific screening programme (neonatal or blood and tissue bank). The population was identified by consulting the databases of a tertiary hospital (Hospital Universitari Germans Trias i Pujol, Badalona) and a regional international health unit (PROSICS Metropolitana Nord, Santa Coloma de Gramenet) as well as the database shared by the 22 primary care centres in the healthcare area. In addition, a capture–recapture strategy was employed in that the population was also identified by subsequently consulting the database of the shared laboratory. Once the database had been prepared, the women were contacted by telephone and their permission to proceed to an interview was obtained. At the end of the interview, they were offered the option of a serology study on their children who had no known result, with the prior express consent of the father or mother in the case of minor children. The study variables were: number of children, sex, age, birth in Catalonia or another place with a pre-natal screening programme in force (yes/no), current place of residence, whether or not T. cruzi serology had been performed (yes/no), serological result (positive/negative) and treatment of seropositive individuals (yes/no). In addition, children were categorised as: accessible (place of birth or current place of residence in Catalonia) or not accessible (place of birth or current residence outside Catalonia). All descendants of mothers with Chagas disease with no known serological status for T. cruzi were offered the option of performing a serology study, with the prior consent of their parents or guardians if they were minors.
To calculate the minimum sample size, the number of infected women (from endemic areas) with descendants in the study area was estimated at approximately 700 people, considering that there could have been around 1300 descendants (1.86 children per woman), for a confidence level of 95%, a precision of 3% and an estimated rate of positive cases for T. cruzi of 8% (297 individuals who were descendants of a mother with positive serology for T. cruzi).
The data were analysed using the programme Stata 10©, version 10.0 (Stata Corporation, Texas, United States). The chi-squared test was used to compare qualitative variables. A p value <0.05 was considered statistically significant.
ResultsA total of 238 women with descendants and positive serology for T. cruzi were identified. Of them, 117 (49.16%) could be located. The diagnosis of Chagas infection derived primarily from clinical or epidemiological suspicion, and just 18 (15.4%) of the women were identified from screening during pregnancy. No significant differences were found between the group of 117 mothers who could be located and the 121 mothers who could not be located with respect to age, number of children, proportion originating from Bolivia or proportion having asymptomatic infections.
They had a total of 300 descendants (mean: 2.5 children/woman). Of them, 156 were male (52%) and 144 were female (48%), with a mean age of 21.31 (±7.4) years. They had been born in the European Union in 53 cases (17.67%) and in South America in 247 cases (82.33%). Of those who had been born in South America, 238 had been born in Bolivia (96.36%). Those who were considered individuals accessible for serological screening totalled 158 (52.67%); those who were considered non-accessible due to permanent residence in South America numbered 142 (47.33%).
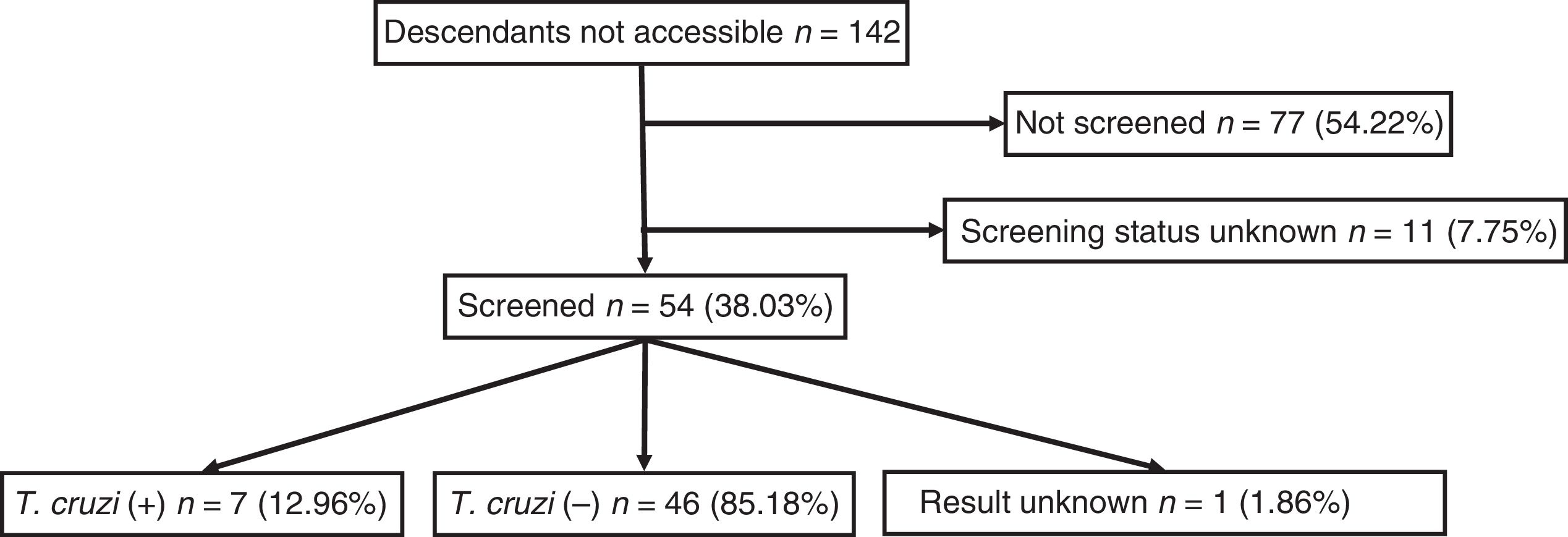
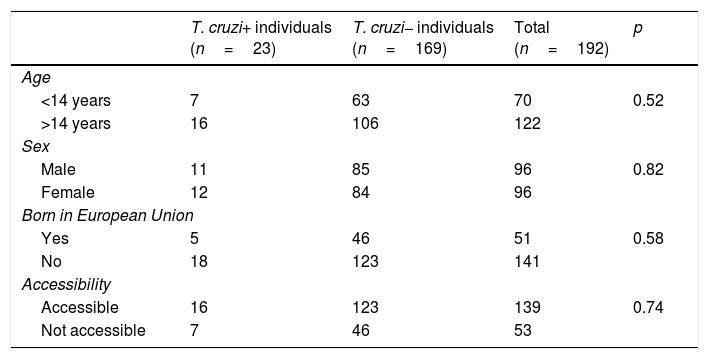
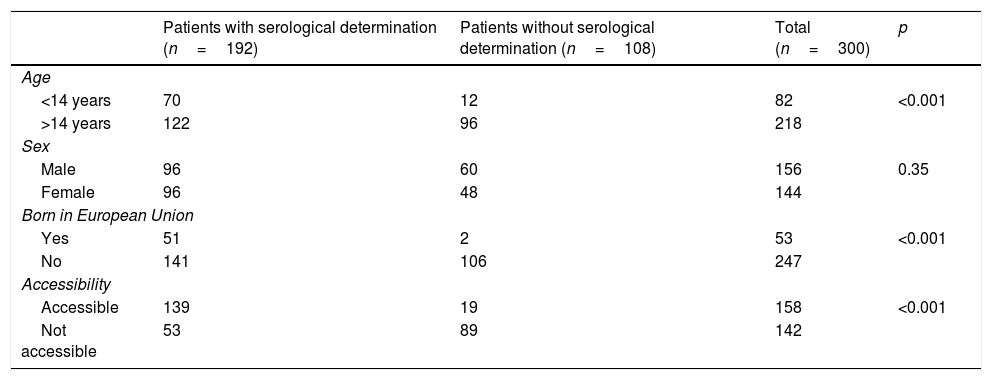
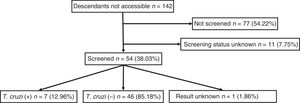
Out of all 300 descendants studied, 192 (64%) had serological results, and out of these, 23 were seropositive for T. cruzi (11.98%; 95% CI 8.1–17.3%). With respect to their accessibility: (a) of the 158 individuals considered accessible descendants, 140 (88.6%) had results, with 16 positives (11.42%), 123 negatives (87.85) and one unknown (0.7%) and (b) of the individuals considered non-accessible descendants, serology testing had been performed in South America on 54 (38.03%) individuals, with 7 positives (12.96%). Figs. 1 and 2 present the data for both groups. No significant relationship was found between having positive serology for T. cruzi and any social or health-related variable. However, there was a significant relationship between having a serology study and being under 14 years of age, having been born in the European Union and being accessible to diagnosis (in all cases p<0.001); said associations are shown in Tables 1 and 2.
Serological status in relation to various socio-demographic variables.
| T. cruzi+ individuals (n=23) | T. cruzi− individuals (n=169) | Total (n=192) | p | |
|---|---|---|---|---|
| Age | ||||
| <14 years | 7 | 63 | 70 | 0.52 |
| >14 years | 16 | 106 | 122 | |
| Sex | ||||
| Male | 11 | 85 | 96 | 0.82 |
| Female | 12 | 84 | 96 | |
| Born in European Union | ||||
| Yes | 5 | 46 | 51 | 0.58 |
| No | 18 | 123 | 141 | |
| Accessibility | ||||
| Accessible | 16 | 123 | 139 | 0.74 |
| Not accessible | 7 | 46 | 53 | |
T. cruzi+: positive serology; T. cruzi−: negative serology.
Serological determination in relation to various socio-demographic variables.
| Patients with serological determination (n=192) | Patients without serological determination (n=108) | Total (n=300) | p | |
|---|---|---|---|---|
| Age | ||||
| <14 years | 70 | 12 | 82 | <0.001 |
| >14 years | 122 | 96 | 218 | |
| Sex | ||||
| Male | 96 | 60 | 156 | 0.35 |
| Female | 96 | 48 | 144 | |
| Born in European Union | ||||
| Yes | 51 | 2 | 53 | <0.001 |
| No | 141 | 106 | 247 | |
| Accessibility | ||||
| Accessible | 139 | 19 | 158 | <0.001 |
| Not accessible | 53 | 89 | 142 | |
Of the 53 children born in Catalonia, 51 (96.23%) had results; 5 of them were seropositive for T. cruzi (with a vertical transmission rate of 9.8%; 95% CI: 4.2–20.9). Since 2010, when serological screening was implemented for all children born to a mother known to be infected, 34 births have been recorded; all had serological results at birth, although 4 cases (11.8%) were lost to the second determination at 9 months and 2 positive cases were recorded. Seven women gave birth to 8 children after having completed treatment for Chagas disease with benznidazole. All these children were properly screened and had negative serology for T. cruzi.
The 18 accessible descendants with no serological determination were actively sought by telephone. Through this process, 3 individuals were recovered; one of them was positive. Of the 23 T. cruzi+ descendants, 16 cases were accessible and, of them, 12 individuals (75%) had a treatment prescription: benznidazole for 6, nifurtimox for 2 and unknown in the remaining 4 cases. In 4 cases (25%), all contact was lost before treatment was started.
DiscussionThe year 2006 marked the height of one of the biggest migration waves from the Americas to Europe. Some sources estimate that, between 2000 and 2010, as many as 2,400,000 citizens of Bolivia, the country with the highest prevalence of Chagas infection in the world (approximately 18%),11 migrated abroad. Spain was the top European country to host said population, with around 190,000 registered immigrants applying for legal status, to whom must be added illegal immigrants.12 Inevitably, Chagas disease surfaced. Previously little known and largely ignored, it came to be considered a medical challenge as it affected developed countries.13 The first case of American trypanosomiasis transmitted by the maternal–child route was reported in 2006 and placed this disease on the radar of healthcare planners.14 In Catalonia, in January 2010, the “Protocol for screening of Chagas disease in pregnant women and their neonates”15 was drafted and implemented with notable efficacy in light of the evidence provided by articles supporting its favourable cost–effectiveness ratio.16 This protocol advises screening of other children having been born before their mother tested seropositive for T. cruzi, but is not explicit about the healthcare level of its implementation, nor does it provide for its reporting, follow-up or evaluation.
There are many references in the literature from endemic and non-endemic areas that assess, discuss and draw conclusions in favour of pre-natal serological T. cruzi screening in order to prevent maternal–foetal transmission of the parasite.17,18 This contrasts with the limited number of studies evaluating the serological status of children of infected mothers in general, none of which was conducted in the European Union. Recommendations by expert groups with clearer positions in favour of widespread family screening come from the World Health Organisation Latin America (from the so-called Technical Group IVa).19
In our case, although the approximate number of women of child-bearing age with seropositivity for T. cruzi in the study area was estimated at 700, just 238 were located; therefore, most likely, there is a large number of infected people with no diagnosis. This “iceberg effect” particular to infectious diseases with long asymptomatic periods had already been observed by other authors20; therefore, it should be concluded that a screening programme intended for infected women and their descendants should include community-based measures.21
In our study, the total Chagas infection prevalence rate (11.98%) in the population studied consists of the sum of maternal–child infections and vector-borne infections, originally transmitted in South America, and exceeds the rates specified in one of the few similar studies published (7.2%), conducted in Brazil.22 Therefore, the gross rate of vertical transmission (9.8%) derives solely from the study of children born in Catalonia. Compared to the figures considered typical in South America (4%-7%),22 our figure is also high, although its confidence interval (4.2–20.9) includes them and therefore it could derive from the limited number of cases. However, this is not the first European study showing rates around 10%, since other studies have specified rates of 7.3%3 and 13.8%,23 whether due to a better quality in determinations or to the presence in Europe of immigrants with parasitic lineages with a greater affinity for the placenta.24 Assuming that the pre-natal screening programme has a broad coverage in the area studied (85% in 2011 and >97% in 2015),25 the reported rate could be very close to the actual rate. A total of 82 of the descendants (27.3%) were children under 14 years of age, representing a substantial proportion which is important, since that is the population in which treatment is most effective and therefore determines in large part the cost–effectiveness ratio of a possible widespread screening programme.20
The primary source of losses was the inability to locate the index mother. The population studied is mobile and often changes residences, jobs, telephone numbers and healthcare providers. Therefore, the time of diagnosis with Chagas infection in a woman with children, if it is still wanted under the effects of the emotional impact that it entails, is the best time for counselling and screening all her prior descendants. Retrospective epidemiological research based on a capture–recapture method proved effective (139 of 158 serologies were located) and efficient (it required no specific economic resources), but only recovered 3 of the 18 accessible individuals with no known serology. Therefore, it cannot replace an immediate “family” determination upon maternal diagnosis performed at the same healthcare centre.19 It should be noted that in 25% of descendants with positive serology, all contact was lost between diagnosis and the start of treatment. Treatment with benznidazole is key in the child population as it is highly effective in children under 12 years of age. Inevitably, the process of determining serology and, above all, getting medication (through Foreign Pharmacy) takes time; therefore, routes of communication with the mother must be ensured.
Various articles support a favourable cost–effectiveness ratio with respect to screening of pregnant women and newborns. The “Consensus document on approaching Chagas disease” (2015)26 published by primary care professionals proposes screening of all immigrants from continental South America, and prioritises screening in certain groups (immunosuppressed patients, patients with family histories, etc.). Taking an even stronger position in defence of universal screening based on origin, at least one model has been prepared with a similar population that considers indiscriminate serological screening for Chagas infection for all South American patients both economically viable and cost-effective, as it estimates a prevalence of seropositivity of 4.2% and a programme coverage of 80%.27 If that is accepted, then arguments against screening a population of descendants of mothers with Chagas disease, with a prevalence of nearly 12%, fall apart.28 Although this is neither a baseline study nor a study with economic objectives, the limited direct costs of serologies (€44.25 final price of serology with raw and recombinant antigens), plus the cost that could be attributed to medical visits (€140 for the first visit and another €18 for each subsequent visit), plus the cost that could be attributed to the purchase and transport of benznidazole (approximately €170 per patient) with a forecast of the costs due to the side effects of the medication, add up to an amount that is much lower than that associated with the costs of care for asymptomatic cases (€677.2; CI: 294.6–1060), not including the 30% of patients who experience progression to gastrointestinal disease (€16,475; 95% CI: 7711–39,111) or heart disease (€25,475; 95% CI: 13,105–67,999).29 The figure of €170 is consistent with or even somewhat lower than the estimate of direct costs for “family members” in a base case (€399) prepared by the Spanish Agency for Evaluation of Healthcare Technologies.29 Beyond estimates and calculations, it must not be forgotten that, on an ethical level, this disease may be transmitted to descendants, has an effective treatment in newborns, threatens serious long-term complications and may be detected in mothers with a simple, non-invasive and low-cost test, regardless of the incidence of maternal–child transmission in real life.30
The study's limitations included the fact that many women were lost to analysis as they could not be located. This means that there could be a selection bias in that women with symptomatic Chagas infection tended to remain more in contact with the healthcare system and therefore were over-represented. However, the majority of women who could not be located were listed as deregistered in the database of the Servei Català de la Salut, indicating relocation or return to their country of origin. These events are typically due to non-healthcare factors. With respect to the methodology used, it should be concluded that, although a retrospective study such as this one is simple and feasible, it could only recover some cases. Previous studies have encountered the same limitation.31
Ultimately, the population of descendants of women diagnosed with Chagas infection has a high prevalence of seropositivity for T. cruzi and justifies the existence of a widespread, systematic and protocol-bound screening programme that strengthens and complements epidemiological surveillance programmes for Chagas disease. Serological determination immediately after maternal diagnosis would be the best time to perform widespread family screening, with monitoring after results are received and treatment for Chagas disease is started.
Conflicts of interestThe authors neither believe nor declare that there are any real or potential conflicts of interest.
Please cite this article as: Alcántara Román A, Sallent LV, Pérez Quílez O, Roure Díez S, Moreno Millán N, Villanova Sanfeliu X, et al. Cribado ampliado de Trypanosoma cruzi en la descendencia de mujeres infectadas en la zona metropolitana norte de Barcelona, Cataluña (España), 2005–2016. Enferm Infecc Microbiol Clin. 2018;36:397–402.