Genital herpes is a sexually transmitted disease caused by herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) belonging to the alphaherpesvirus family, that includes the varicella zoster virus. HSV infection continues to be the most common cause of vulvar ulcers among the sexually active population. Its incidence increases every year. This review summarises the microbiology of the virus, pathogenesis and infection in genitalia, clinical manifestations and correct identification, the different laboratory diagnostic methods, and choice of the correct treatment according to the first infection, recurrence or special cases. Finally, the cost of routine herpes simplex virus infection is analysed.
El herpes genital es una enfermedad de transmisión sexual causada por los virus herpes simplex tipo 1 (VHS-1) y tipo 2 (VHS-2), pertenecientes, junto al virus varicela zoster, a la familia alfaherpesviridae. La lesión por VHS continúa siendo la causa más frecuente de úlcera vulvar entre la población sexualmente activa, y su incidencia aumenta cada año. En esta revisión resumiremos la microbiología del virus, la patogenia y la infección en genitales, las manifestaciones clínicas para su correcta identificación, las diferentes técnicas diagnósticas de laboratorio y la elección del correcto tratamiento según sea primera infección, recurrencia o casos especiales. Finalmente, se discute un análisis de costes de la enfermedad por VHS.
Genital herpes is a sexually transmitted disease caused by herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2), which belong to the herpesviridae family of DNA viruses.
The herpes viruses have a well-defined structure, being composed of an icosahedral capsid surrounded by a tegument containing 15–20 proteins. The tegument is then in direct contact with the envelope, which is made up of numerous glycoproteins. The genome is a single linear double-stranded DNA molecule 152–155kbp in size. These two viruses share up to 40% homology in their genome structure, reaching up to 83% in the coding regions, which largely explains their antigenic cross-reactivity.1–3
The herpesviridae family is divided into three subfamilies: alpha, beta and gamma (based on biological and genomic similarities). The alphaherpesviruses include HSV-1, HSV-2 and varicella zoster virus (VZV). Humans are the only known reservoirs.
HSV-1 is a large, neurotropic virus which causes mainly oral infections ranging from minor lesions, such as cold sores, to severe lesions, such as meningoencephalitis. HSV-2 is very similar, but it causes anogenital infections or neonatal herpes. In recent years, these data have varied as a consequence of oral sex practices, with HSV-2 manifesting in labial lesions and an increase in the prevalence of HSV-1 in anogenital infections. In the light of these changes, we need to break with the traditional assumptions that HSV-2 means genital herpes and that HSV-1 is limited to orolabial infections with non-sexual transmission.
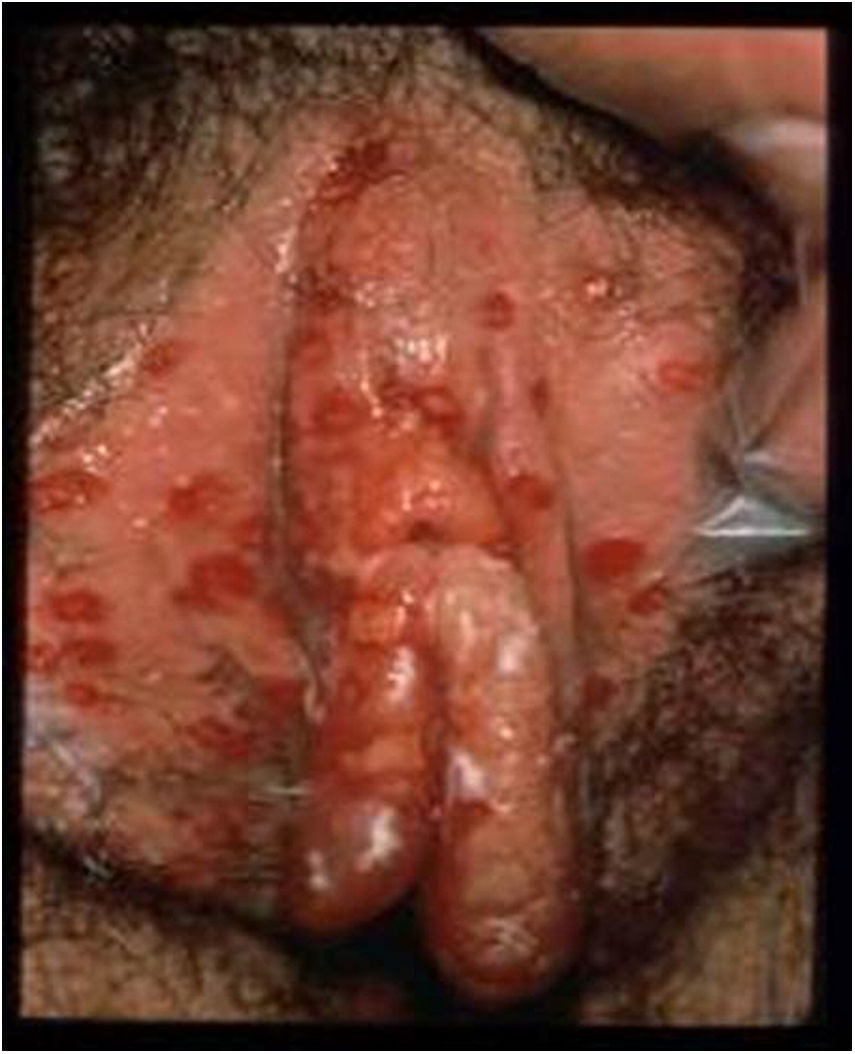
HSV lesions continue to be the most common cause of vulva ulcers in the sexually active population (Fig. 1). A continuous increase in HSV infection has been detected in recent years, due in part to sociocultural changes and risky sexual practices. The majority of these infections are asymptomatic, which favours transmission.4 Recent studies in sexually active people aged from 14 to 49 in the United States indicate that the prevalence of HSV-1 is 47.8%, while that of HSV-2 is 11.9%; both prevalences increase with age, and are higher in women than in men.5,6
Ulcerative vulvar lesions. Adapted from Garland and Steben.13
WHO data estimate that, worldwide, 11% of the population aged from 15 to 49 is infected with HSV-2.7 Meanwhile, we have to consider the potential for morbidity and mortality in the newborn due to the risk of transmission during pregnancy, and as a cofactor in the transmission of HIV.8
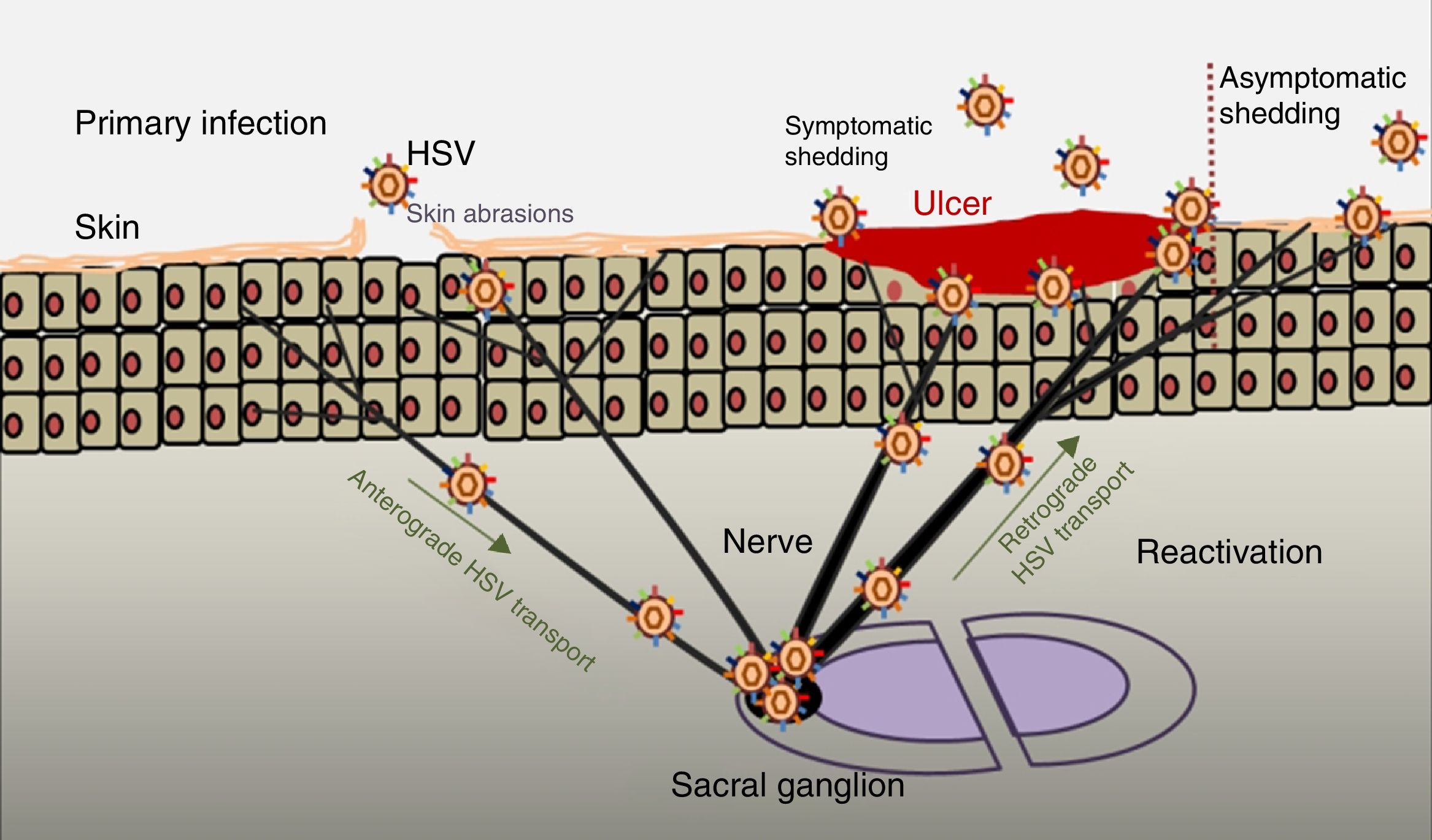
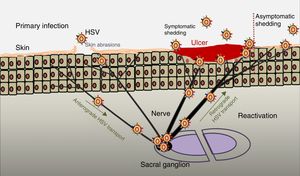
PathogenesisThe infectious cycle of the virus starts after contact with the oral or genital mucosa, through abrasions or micro-cracks in the host's skin (Fig. 2). Replication begins in the epidermis and penetrates sensory nerve endings in the skin. Through the axon of these nerves it is transported to the nucleus of the neurons in the sensory ganglia in the spinal cord (spinal or trigeminal). HSV can replicate within sensory ganglion neurons or remain dormant for months and even years, and reactivate spontaneously or in response to various stimuli (solar radiation, stress, fever, certain medications, acute diseases or immunosuppressive processes). This ability of the latent virus to reactivate is defined as recurrent HSV disease. When the virus is reactivated, it descends through the sensory nerve to the surface of the initially infected dermatomes (orolabial or vulva). Replication continues in the epidermal cells and can cause asymptomatic excretion in oral or genital secretions, or obvious clinical recurrence, resulting in vesicles and inflammation of local lymph nodes.9,10
Primary infection and reactivation. Adapted from Jaishankar and Shukla.43
The clinical signs and symptoms of genital herpes vary, depending on the type of HSV, gender, age, immune status of the patient and previous exposure to the virus. Episodes in patients with no evidence of previous HSV infection (primary infection) may be more severe on average than in patients with previous infection (non-primary first-episode genital herpes). However, more commonly, we find asymptomatic primary infections, meaning that most people with either genital or oral herpes are unaware of their infection status.11
In the case of primary genital herpes we find that 7–50% of cases are caused by HSV-1 infection, while 50–93% are due to HSV-2. These proportions vary according to geographical location and socioeconomic status.9 The incubation period is about 4 days (it can range from 2 to 12 days). In most cases, clinically visible lesions are preceded by a prodromal phase (2–24h before the lesions appear), which can manifest with fever, malaise, headache, myalgia, stinging or itchiness in the anal/genital area, abnormal vaginal discharge and pain in legs, buttocks or genitals. In immunocompetent women, lesions (blisters that can progress to ulcers) develop in areas of infection (vulva, cervix, vagina, perineum and/or urethra),12,13 causing urethritis and/or painful inguinal lymphadenopathy. The most common complications derive from extragenital skin lesions, central nervous system involvement and opportunistic fungal infections. Less frequently, sacral radiculomyelitis with urinary retention, transverse myelitis and neuralgia may occur. Complications are more common in women than in men.9,11
Both local and systemic symptoms are less severe and resolve more quickly in non-primary first-episode genital herpes than in primary infections.
The average duration of viral shedding is 12 days in primary disease and 7 days in non-primary disease.
Recurrent genital herpes is one of the main problems of this disease, as it depends on the type of virus, the intensity of the first episode and also the host. Most cases of recurrence occur in the first year after primary infection (80–90% for HSV-2 and 20% for HSV-1). There is a great deal of variation in the number of flare-ups, the severity and the natural course of recurrences.14,15
Skin manifestations tend to be in the same area of the first episode and are sometimes nonspecific and barely perceptible, such as fissures and cracks, which can lead to mistaken diagnosis. Occasionally, there may be no lesions, making it difficult to diagnose the recurrences. Systemic symptoms are rare and less severe, although they can be more painful and prolonged in women. The recurrence rate increases during pregnancy, but the course and duration are similar.14
DiagnosisA clinical diagnosis is enough to start early empirical treatment and this improves the symptoms and shortens their duration. However, the diagnosis should always be confirmed with laboratory tests in order to deliver a prognosis and choose the optimal treatment.
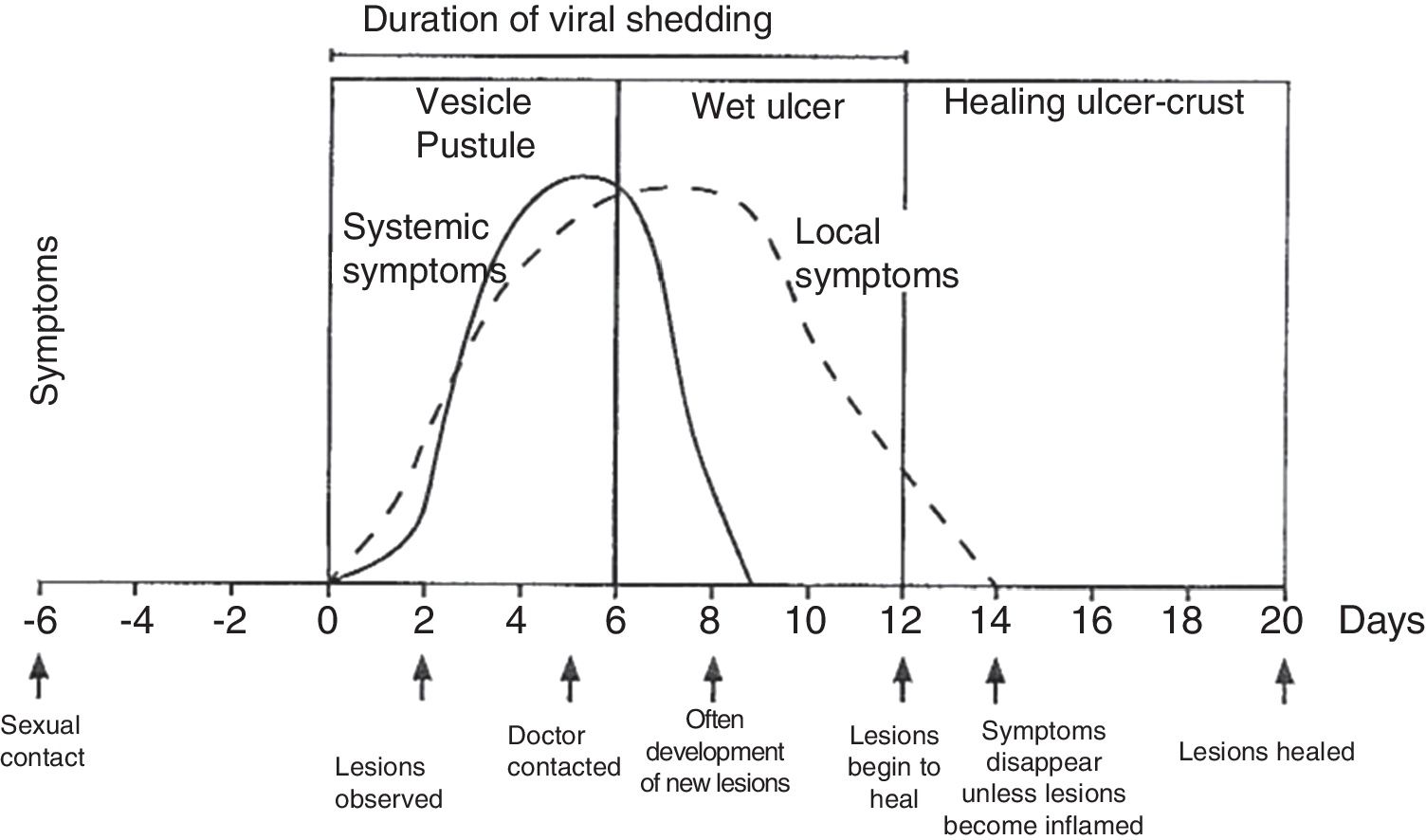
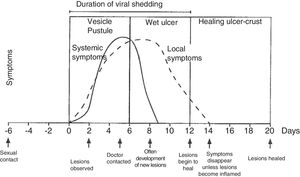
Physical examination involves external and internal examination of genitals, and checking for lymphadenopathy (hard, mobile, bilateral and very painful) and location of the virus in other areas (mouth and eyes), along with the patient's medical history. The initial lesion is one or more grouped vesicles on an erythematous base. These vesicles subsequently open up and lead to shallow ulcerations. In the area around the labia and rectum the vesicles often burst before they are noticed, and crusts may appear (Fig. 3). In primary genital infection, the pain tends to last 10 days and the lesions heal in 2–3 weeks. They are less painful and of limited duration.
Course of symptoms and clinical manifestations in herpetic diseases. Adapted from Garland and Steben.13
The most commonly used diagnostic techniques are:
- -
Tzanck smear, from an unbroken vesicle. Cheap and quick tool, but requires experienced staff and is limited to this type of lesion.15,16
- -
Viral culture: although isolation in cell culture is relatively simple and fast compared to other viruses, the yield is higher in the presence of vesicles (80%) and when the samples are taken in the first two days after they appear, and lower in the crust phase or in recurrences (25–50%). It is a sensitive and specific, but lengthy, method. The characteristic cytopathic effects tend to appear within 12–48h.17,18
- -
Direct/indirect immunofluorescence techniques: fast, cheap techniques, many of them available fully automated or semi-automated, with a sensitivity-specificity of 85–99%.19–23
- -
Type-specific serology for specific anti-HSV IgG antibodies: relevant in patients with a history of undiagnosed atypical genital lesion, suspicion of partner infection with infected patient and pregnant women at risk of transmission to the newborn. These are also fast, inexpensive techniques, many of which are fully automated or semi-automated. However, the limitation is that the antibody for the viral glycoprotein is detected from 6 to 8 weeks and approximately 5% of the patients may have undetectable levels. They have high sensitivity (93–96%) and specificity (80–98%),24 but their use is not recommended in asymptomatic patients, given the high rate of false positives in the low-risk population.
- -
A negative serology result may indicate both the absence of previous contact with HSV and absence of immune response, either due to initial phase of infection (primary infection) or alterations in the patient's immune system. In contrast, the presence of total antibodies is evidence of herpetic infection. A negative IgM indicates a non-active infection at the time of analysis, while the presence of IgM shows an evolving infection (not always a primary infection, as in some recurrences significant amounts of IgM may be detected).25,26
- -
PCR: use of PCR in clinical practice is booming thanks to the multiplex PCR technique that enables the simultaneous detection of the main organisms present in infectious ulcers of sexual origin (Treponema pallidum, Haemophilus ducreyi, Chlamydia trachomatis L serovar, which causes lymphogranuloma venereum, and HSV 1–2). This technique has a high sensitivity and specificity (both close to 100%), it is fast, its cost is decreasing and, combined with the fact that it is automated, it enables access for an increasing number of laboratories. Mainly because of its high sensitivity (even in cases with low viral load) it is useful for detecting viral shedding in asymptomatic patients and diagnosing lesions which were negative in the culture. The biggest drawback is the cost and the requirement for laboratories and specialised staff. Commercial kits are now available for the simultaneous determination of several STIs or exclusively herpes simplex infection which can differentiate between HSV types 1 and 2, something that a culture cannot do. For all these reasons, PCR has progressively displaced performing a culture as the diagnostic method for HSV infection.27,28
The most commonly used molecular targets are conserved sequences of the genes encoding the surface glycoproteins gD and gB, or the enzymatic proteins DNA polymerase and thymidine kinase, with differences in their sequence which enable discrimination in the detection of HSV-1 and HSV-2, such as those approved by the FDA for the detection of HSV in genital lesions: IsoAmp HSV Assay (BioHelix Corporation), Multi-Code-RTx Herpes Simplex Virus 1 & 2 Kit (EraGen Biosciences Inc.) and BD ProbeTec Herpes Simplex Viruses HSV 1 & 2 (HSV QxAssays).29,30
TreatmentThe WHO recommends “empirical” or “syndromic” treatment, as the results of the diagnostic tests are not available at the time of the first consultation. Such early treatment can lead to faster healing, relief of symptoms and a reduced risk of transmission. It is recommended in patients with genital ulcers suggestive of HSV infection, but has the disadvantage of potentially being inappropriate treatment. It is therefore essential to follow up patients to assess their treatment response and diagnostic tests results and to reassess treatment decisions, if necessary.
In the first episode of genital herpes, antivirals should be used for the first five days from onset of the episode, or during the formation of new lesions. The treatment of choice is oral antivirals, aciclovir, valaciclovir and famciclovir, which reduce the severity and duration of the episode (level of evidence Ib, A).31 Topical treatments are not recommended, as they are less effective than oral treatments and can generate resistance (IV, C). The only indication for the use of intravenous therapy is when the patient cannot ingest or tolerate oral treatment. The recommended regimens for 5–10 days are as follows:
- •
Aciclovir 400mg three times a day, or aciclovir 200mg five times a day.
- •
Famciclovir 250mg three times a day.
- •
Valaciclovir 500mg twice a day.
As treatment support measures, saline baths and the use of analgesics are recommended.
In cases of recurrent genital herpes, the choice of treatment is made according to the severity of the symptoms and the time of recurrence.
There is a range of treatment regimens, including the following:
- -
Short therapy (Ib, A):
- •
Aciclovir 800mg three times a day for two days.
- •
Famciclovir 1g two tablets in a single day.
- •
Valaciclovir 500mg twice a day for three days.
- •
- -
Five-day treatment:
- •
Aciclovir 400mg three times a day for 3–5 days, or aciclovir 200mg five times a day.
- •
Valaciclovir 500mg twice a day.
- •
Famciclovir 125mg twice a day.
- •
In special situations, such as HIV-positive patients, there are no clinical trials for the duration and treatment, so some clinicians opt for 10 days of treatment with twice the dose of any of the above oral treatments (IV, C). In pregnant women, treatment regimens and treatment management vary according to the trimester of virus acquisition.31,32
Suppressive therapy is recommended in patients with more than six episodes per year, and can reduce flare-ups by up to 70–80%.31 This treatment regimen aims to reduce the number of recurrences and the risk of transmission to sexual partners. The recommended doses are:
- -
Aciclovir 400mg twice a day for five days.
- -
Valaciclovir 500mg a day (if fewer than 10 recurrences/year).
- -
Valaciclovir 1g a day (more than 10 recurrences/year).
Recent studies indicate that topical microbicides, such as the 1% tenofovir or 3% SPL7013 gels, may be a promising option, having achieved a 51% reduction in the risk of HSV-2 infection.33,34 Another alternative is helicase-primase inhibitors, such as ASP2151, for the treatment of recurrent genital herpes.35,36
Therapeutic vaccines are another potential strategy for the management of HSV-positive patients, but few of the earlier trials obtained optimal results.32 New trials focused on the immune response of the host, through the use of HSV peptides (HerpV), have achieved promising results in phase I and are now in phase I/IIa.37–40
Prophylactic vaccines have been under continuous development and improvement for years, prominent among which are two containing the HSV-2 glycoprotein D subunit, with a demonstrated efficacy of 73–74% among women seronegative for both types of HSV.41
Analysis of health service costs related to HSV diseaseA recently published study42 calculated the costs associated with HSV infection in hospital accident and emergency units. In the period 2006–2013 the cost amounted to 543 million dollars, going from 45 million dollars in 2006 to 91 million in 2013, primarily due to a 24% increase in the number of patients. The study emphasises the need for continuous prevention and patient sex education to avoid new cases.
Level of evidenceFor more information and details about levels of evidence and grades of recommendation see: http://www.iusti.org/regions/Europe/pdf/2013/Levels_of_Evidence.pdf
Conflicts of interestThe author declares that there are no conflicts of interest regarding the research, authorship, and/or publication of this literature review.
The author would like to thank Ms Morilla for revising and commenting on this literature review.
Please cite this article as: Parra-Sánchez M. Úlceras genitales por virus herpes simplex. Enferm Infecc Microbiol Clin. 2019;37:260–264.