Pneumonia continues to be one of the most frequent infectious syndromes and a relevant cause of death and health resources utilization. The OPENIN (“Optimización de procesos clínicos para el diagnóstico y tratamiento de infecciones”) Group is composed of Infectious Diseases specialists and Microbiologists and aims at generating recommendations that can contribute to improve the approach to processes with high impact on the health system. Such task relies on a critical review of the available scientific evidence. The first Group meeting (held in October 2023) aimed at answering the following questions: Can we optimize the syndromic and microbiological diagnosis of pneumonia? Is it feasible to safely shorten the length of antibiotic therapy? And, is there any role for the immunomodulatory strategies based on the adjuvant use of steroids, macrolides or immunoglobulins? The present review summarizes the literature reviewed for that meeting and offers a series of expert recommendations.
La neumonía es uno de los síndromes infecciosos más frecuentes y una importante causa de mortalidad y de consumo de recursos. Constituido por expertos en Enfermedades Infecciosas y Microbiología, el Grupo OPENIN (“Optimización de procesos clínicos para el diagnóstico y tratamiento de infecciones”) tiene por objeto la generación de recomendaciones que contribuyan a mejorar el abordaje de procesos con elevado impacto sobre el sistema sanitario a partir de una revisión crítica de la evidencia científica disponible. La primera reunión del Grupo (celebrada en Octubre de 2023) trató de contestar las siguientes preguntas: ¿Cómo podemos optimizar el diagnóstico sindrómico y etiológico de la neumonía? ¿Es posible reducir de forma segura la duración del tratamiento antibiótico? Y ¿tienen un papel las estrategias de inmunomodulación basadas en el tratamiento adyuvante con esteroides, macrólidos o inmunoglobulinas? La presente revisión sintetiza la literatura revisada para aquella ocasión y ofrece una serie de recomendaciones de expertos.
Community-acquired pneumonia (CAP) is the third leading cause of death in industrialised countries and the leading infectious cause, with an estimated three million attributable deaths annually worldwide. The incidence of hospitalisation due to pneumococcal CAP in Spain from 2016 to 2020 was 2.91 episodes per 10,000 population, with a fatality rate of 8.47%.1 In a retrospective study carried out from 2017 to 2019 on more than 7,000 cases of CAP of any aetiology, the average duration of hospital stay was 5.4 days, with a cost per episode of 3,955 euros.2 Although the clinical guidelines developed by American and European scientific societies are widely implemented in routine practice, the generation of new evidence (not always exempt from contradictory results) means continuous updating is necessary in the management of both CAP and hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP).
The OPENIN Group was set up with the aim of contributing to improving the clinical approach to infections with a high incidence and impact on the healthcare system. The Group began as a forum, with the intention that it would become a fixture, for the purposes of promoting the exchange of opinions and discussion between experts to produce recommendations based on the best available scientific evidence. It is made up of specialists in infectious diseases and microbiology from various centres across Spain and it held its first meeting on 26 October 2023 in Madrid. The Group's first activity, given its mortality burden and the associated use of resources, was focused on pneumonia in its various forms: CAP (with or without hospital admission criteria), HAP and VAP. The particular features of these conditions in the immunosuppressed patient were left out of the main initiative. The Executive Committee of the OPENIN Group had previously agreed on the following three questions: (1) How can we optimise both syndromic- and aetiology-based diagnosis?; (2) Is it possible to reduce the duration of antibiotic therapy without negatively impacting patient prognosis?; and (3) Is there a role for strategies which aim to modulate the immune response in patients with pneumonia? Three speakers were then selected to carry out a narrative review of the literature (based on a bibliographic search in PubMed without pre-established restrictions regarding publication date, type of article or language), and the conclusions were presented at said meeting. After several iterative rounds of discussion, the speakers and the Executive Committee developed a series of recommendations, which were circulated among the other members in a public consultation and review process. This document was finally submitted for the consideration of the Group as a whole before being sent for publication. The speakers selected in this first edition were the first three authors of the manuscript, while the members of the Executive Committee hold the remaining lead authorships. In line with the eminently deliberative nature of the OPENIN Group, this document does not contain a systematic review of the literature nor does it offer a formal grading of the expert opinions issued.
Can we improve the diagnostic process in patients with pneumonia?Community-acquired pneumoniaRapid microbiological diagnosis is essential for adequate and targeted treatment of CAP with criteria for admission. Early identification of the causative pathogen is crucial to guide antibiotic therapy, prevent the emergence of resistance and reduce the potential adverse effects associated with the use of antibiotics.3 However, in up to 30–50% of CAP episodes requiring admission, no aetiological diagnosis is reached, so treatment is frequently based on an exclusively empirical approach.4,5 Molecular syndromic panels for lower respiratory tract infections could contribute to improving the rate of microbiological diagnosis, although their effect on clinical outcomes and prognosis is yet to be established.5
Recommendations for the aetiological diagnosis of CAP differ depending on the healthcare setting, clinical severity, and the presence of comorbidities (with special attention to immunosuppressed patients). In community settings (primary care, outpatient clinics, accident and emergency departments and residential care), antibiotic treatment is often prescribed without the availability of diagnostic tests. In these circumstances, point-of-care tests can be useful to differentiate between infections of bacterial and viral origin, facilitating the appropriate use of antimicrobials in the community.3,6 Although rapid diagnostic techniques (RDT) based on the detection of antigens (influenza virus, respiratory syncytial virus [RSV] or human metapneumovirus) have a high specificity (over 80%), they all have suboptimal sensitivity (from 49% to 84%).6 The determination of C-reactive protein and procalcitonin (PCT) levels has been shown to be useful in reducing antibiotic prescribing in various healthcare settings, including primary care.5,7 PCT levels <0.1 μg/l are suggestive of viral infection, while the likelihood of bacterial infection increases with levels ≥0.25 μl.7 However, the 2019 update of the clinical practice guidelines of the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) (ATS/IDSA) does not recommend the use of PCT in decision-making for antimicrobial treatment in patients clinically suspected of having CAP and with radiological evidence of CAP. It is considered that the range of sensitivity of the levels of this biomarker for the diagnosis of bacterial infection is too wide (from 38% to 91%) to replace clinical criteria.3 The most recent (2023) European and Latin American guidelines (European Respiratory Society [ERS], European Society of Intensive Care Medicine [ESICM], European Society of Clinical Microbiology and Infectious Diseases [ESCMID] and Asociación Latinoamericana de Tórax [ALAT] [Latin American Thoracic Association]) consider the monitoring of PCT levels to determine the duration of treatment in severe CAP, although they do not refer to the potential utility of this biomarker when deciding whether or not to use it.8
In patients arriving in Accident and Emergency with suspected CAP, the utility of routine microbiological testing to rationalise the use of antibiotics and improve clinical outcomes has not been clearly established. The positivity rate of blood cultures in patients requiring admission is low (4–16%), and the diagnostic yield of sputum cultures does not exceed 50%.9,10 However, in adults with severe CAP and in immunosuppressed patients, it is recommended to obtain blood cultures at the time of diagnosis and perform a Gram stain and culture of respiratory secretions before starting treatment. A Gram stain and culture should also be performed in patients with suspected methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa infection (for example, history of infection with these organisms or exposure to antibiotics in the previous 90 days).3,9,10 In this scenario, screening for colonisation status in nasal exudate by culture or polymerase chain reaction (PCR) has an excellent negative predictive value (NPV) for ruling out MRSA pneumonia; in a meta-analysis based on 22 studies, the NPV was estimated at 98.1% for CAP and healthcare-related forms of pneumonia and 94.8% for VAP.11 RDT based on Streptococcus pneumoniae and Legionella pneumophila urinary antigen testing are simple, non-invasive, rapid tests, which are not affected by previous administration of antibiotics; data from a meta-analysis showed a sensitivity and specificity of 70% and 83%, respectively.6 It is important to highlight that most of the commercial tests available for L. pneumophila only detect infections caused by serogroup 1, which theoretically limits the sensitivity of this RDT. Therefore, in adults with severe CAP, culture for Legionella spp in selective media is recommended, or the use of nucleic acid amplification techniques (NAAT) from respiratory samples.3,12 Despite their potential utility, the latest ATS/IDSA guidelines do not routinely recommend Legionella urinary antigen testing, except in the case of severe CAP and in patients at epidemiological risk (such as in the context of an outbreak),3 or if the condition is accompanied by severe headache, diarrhoea, hyponatraemia, elevated creatine phosphokinase or recent travel. Indications for S.pneumoniae urinary antigen testing include intensive care unit (ICU) admission, failure of outpatient antibiotic therapy, alcoholism, pleural effusion, leucopenia, chronic liver disease and splenectomy.12,13
In recent years, NAAT have emerged as useful tools for the diagnosis of respiratory pathogens, particularly viruses, but also for the detection of difficult-to-culture bacteria and some resistance genes. These molecular assays should be used for the detection of influenza viruses during the community circulation season and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).3,5,6 The wide availability of commercial respiratory syndromic panels nowadays has reduced the diagnostic response time. These tests are little affected by the previous administration of antibiotics.5 The benefits of these panels include their high sensitivity, specificity and NPV, although they should not replace conventional cultures and antimicrobial susceptibility tests. Their limitations include that it is not possible to detect agents not included in the panel, the lack of complete information about susceptibility to antibiotics, the cost, the occasional lack of correlation with culture results, and the possibility of false positive results due to the detection of nucleic acids of non-viable pathogens.11,12,14 Moreover, the diagnostic yield reported in the literature for these molecular platforms should be interpreted with some caution, as in many studies the reference technique is not clearly defined and nor is the quality of the samples analysed detailed. In fact, the ATS/IDSA guidelines do not recommend performing NAAT for bacterial pathogens, due to the difficulty in determining the patient profile that could benefit the most from their use.3,15
Hospital-acquired pneumonia and ventilator-associated pneumoniaHAP and VAP represent a major health problem, as both conditions are associated with considerable morbidity and mortality rates. HAP is defined as pneumonia which develops in patients who have been hospitalised for more than 48 h. Within the spectrum of HAP, the term VAP is reserved for patients admitted to the ICU and subjected to mechanical ventilation for at least 48 h.16 In the previous ATS/IDSA guidelines, published in 2007, healthcare-related pneumonia was considered a distinct category from CAP, as it develops in patients who are not hospitalised but in whom there are risk factors for colonisation by multidrug-resistant microorganisms (for example, admission in the previous 90 days, coming from residential care or a medium-term care home, or receiving chemotherapy, haemodialysis or parenteral antibiotic therapy). However, they also recognised the existence of a certain overlap with CAP in certain patient profiles.13 In fact, the 2019 guidelines recommend abandoning this distinction and putting the emphasis on local epidemiology and the presence of well-established risk factors.3
According to data published in the latest report from the Estudio de Prevalencia de Infecciones Asociadas a la Atención Sanitaria y Uso de Antimicrobianos en Hospitales de Atención Aguda en España (EPINE) [Study of prevalence of healthcare-related infections and use of antimicrobials in acute care hospitals in Spain] corresponding to 2022, P. aeruginosa (18.2%) and S. aureus (12.2%) were the main pathogens identified in HAP, followed by Klebsiella pneumoniae (6.9%) and Escherichia coli (6.7%). These data are similar to those provided by the Estudio Nacional de Vigilancia de Infección Nosocomial en Servicios de Medicina Intensiva (ENVIN) [Spanish nosocomial infection surveillance study in intensive medicine departments]-Hospitals in Europe Link for Infection Control through Surveillance (HELICS) (ENVIN-HELICS), the latest available update of which, corresponding to 2023, shows that P.aeruginosa (20.7%) and K.pneumoniae (10.2%) were also the most frequently isolated microorganisms in patients with VAP.17 HAP can also be viral, with a predominance of SARS-CoV-2, influenza, RSV and rhinovirus.18 Although both the early start and the adequacy of empirical antibiotic therapy are crucial, RDT can play a role in establishing the aetiological diagnosis.16 It is recommended to perform a Gram stain with semiquantitative culture on respiratory tract samples of adequate quality, prioritising non-invasive procedures (sputum or endotracheal aspirate in intubated patients) over invasive procedures (bronchoalveolar lavage [BAL] or protected bronchial brushing).19 Blood cultures should also be obtained, with testing for Legionella and pneumococcal urinary antigens and screening for MRSA colonisation in the nasopharyngeal swab.19 There are several commercial platforms for respiratory pathogens (respiratory viruses, bacteria and fungi) and for the main resistance genes of the most common bacteria. These NAAT are based on PCR, reverse transcription PCR and microarray assays, and enable an aetiological diagnosis to be established within a few hours.20–22 As with all microbiological techniques, molecular methods are not exempt from the aforementioned limitations in terms of sensitivity, specificity, reproducibility and possibility of inconsistent results between different platforms. There are therefore challenges involved in the implementation of these molecular RDT in routine practice and they should not be considered as a replacement for conventional methods, but rather be used to complement them.
Implementation of imaging techniques in the diagnosis of pneumoniaChest X-ray remains the standard technique in the initial assessment of a patient with suspected pneumonia.3,19 However, the use of computed tomography (CT) has seen an increase in recent years. In certain clinical or epidemiological contexts, as the pandemic caused by SARS-CoV-2 has shown, chest CT can be useful for establishing the bacterial, viral or fungal origin of the condition.23 Moreover, it provides greater information about the location and extent of the infiltrate, its pattern and severity. CT also plays an important role in the diagnosis of complications and in the monitoring of conditions caused by multidrug-resistant pathogens or fungi.24
Chest X-ray is the most widely used imaging method for the diagnosis of CAP thanks to its availability and low cost. However, the sensitivity of this technique may be compromised in some immunocompetent patients who show radiological evidence of pneumonia on CT but not on conventional X-ray (as occasionally occurs in older adults). In HAP, chest X-ray may also have suboptimal sensitivity in bedridden patients with significant comorbidities or in critically ill patients with severity criteria. In these scenarios, obtaining quality images in chest X-rays can be a challenge in clinical practice. Moreover, the rapid development of symptoms in hospital-acquired infections may lead to a delay in the demonstration of radiological changes, especially in immunosuppressed patients.25 In the context of HAP, chest CT has greater sensitivity and facilitates early identification in patients who are severely ill. In fact, CT is known to be capable of revealing findings suggestive of pneumonia up to five days earlier than conventional radiography, in addition to aiding the differential diagnosis based on the different patterns (for example, alveolar, necrotising, interstitial pneumonia, bronchopneumonia). However, attributing aetiology based exclusively on radiology can have limitations, as a certain microorganism may produce different radiological patterns (which can also change depending on the patient's immunological status).24
Lung ultrasound has emerged as an alternative diagnostic tool as it provides real-time images and avoids radiation exposure. Several studies have evaluated its accuracy for the diagnosis of pneumonia. A systematic review and meta-analysis, which included 16 studies with a total of 2,378 patients, reported a sensitivity of 94% and a specificity of 96%.26 Furthermore, lung ultrasound has a higher diagnostic yield than chest X-ray and an accuracy comparable to chest CT. One of its advantages is that it can be carried out at the patient's bedside and the results are available immediately. Additionally, serial examinations may be performed to monitor disease progression and response to treatment. Although lung ultrasound is highly accurate in diagnosing pneumonia,26 it has limitations when it comes to identifying the specific cause. Its performance can also be limited by the patient's physique or the presence of underlying lung disease, such as emphysema, which can make it difficult to obtain clear and precise images. Furthermore, it requires specialised training, a factor that may limit the widespread application of this diagnostic tool.27
Can we reduce the duration of treatment in patients with pneumonia?Lower respiratory tract infections, both of community and nosocomial origin, are one of the main reasons for antibiotic prescribing in hospitals. Therefore, these conditions have become one of the priority objectives of antimicrobial stewardship programmes (ASP), because small reductions in the duration of their treatment mean a large decrease in the overall use of antibiotics. The main available evidence on the efficacy and safety of strategies to reduce the duration of antimicrobial treatment in CAP and HAP are reviewed below, along with the value monitoring PCT levels for this purpose may have. Situations that are not usually included in clinical studies and in which there is general agreement when recommending the use of prolonged treatment regimens have been excluded, such as lung abscess, pleural empyema, necrotising pneumonia, cystic fibrosis, bacteraemic pneumonia due to S. aureus or immunosuppressed patients.
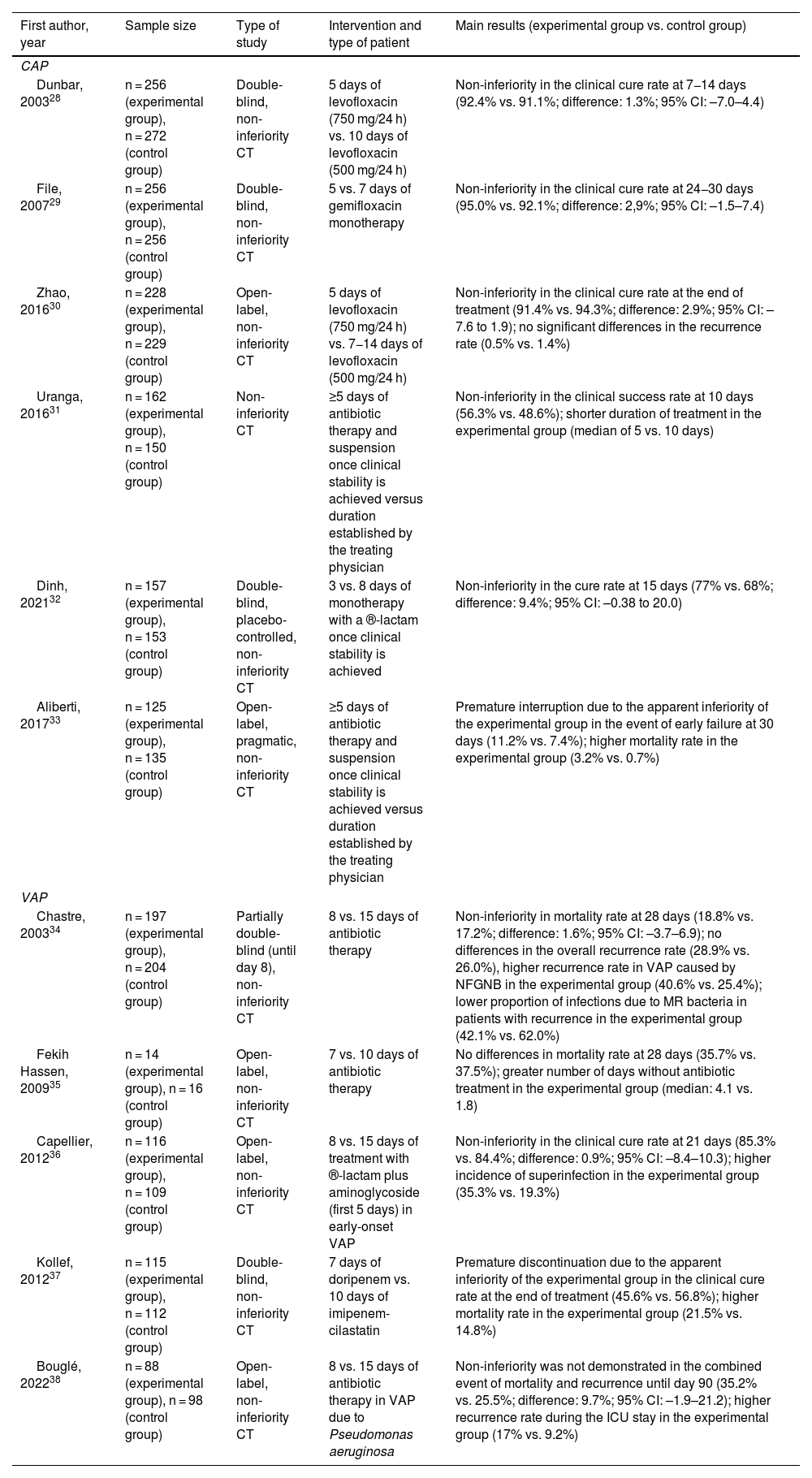
Community-acquired pneumoniaCurrently, there is evidence that supports the use of short-term treatments in patients with CAP or VAP who reach clinical stability early. Multiple randomised clinical trials (CT) have evaluated the efficacy and safety of reducing the duration of antibiotic treatment (Table 1).28–38 Most of them have concluded that short regimens (3–5 days for uncomplicated CAP without ICU admission criteria and 7–8 days for VAP) are at least non-inferior compared to longer ones,28–36 although negative results have also been reported.37,38 In one of these non-inferiority trials, carried out in four Spanish centres, Uranga et al.31 demonstrated that the administration of antibiotic therapy for five days in patients with CAP who had a temperature ≤37.8°C for 48 h and who did not have more than one sign of instability is comparable to longer courses, with comparable clinical response rates between the experimental group and the control group at 10 days (56.3% and 48.6%) and 30 days (91.9% and 88.6%), respectively. Another more recent CT showed that, in patients who achieve clinical stability after three days of therapy with ®-lactams, the strategy of discontinuing treatment at that time met the pre-established criterion of non-inferiority with respect to the alternative of continuing it for a total of eight days (clinical cure rates at 15 days of 68% and 77%, respectively).32 Some well-designed observational studies, whose results were adjusted using propensity scores, reported comparable efficacy between treatments lasting seven days or less compared to longer courses.39
Summary of the most relevant clinical trials to have evaluated the efficacy and safety of short courses of antibiotics (compared to long courses) in adult patients with CAP and VAP.
| First author, year | Sample size | Type of study | Intervention and type of patient | Main results (experimental group vs. control group) |
|---|---|---|---|---|
| CAP | ||||
| Dunbar, 200328 | n = 256 (experimental group), n = 272 (control group) | Double-blind, non-inferiority CT | 5 days of levofloxacin (750 mg/24 h) vs. 10 days of levofloxacin (500 mg/24 h) | Non-inferiority in the clinical cure rate at 7−14 days (92.4% vs. 91.1%; difference: 1.3%; 95% CI: –7.0–4.4) |
| File, 200729 | n = 256 (experimental group), n = 256 (control group) | Double-blind, non-inferiority CT | 5 vs. 7 days of gemifloxacin monotherapy | Non-inferiority in the clinical cure rate at 24−30 days (95.0% vs. 92.1%; difference: 2,9%; 95% CI: –1.5–7.4) |
| Zhao, 201630 | n = 228 (experimental group), n = 229 (control group) | Open-label, non-inferiority CT | 5 days of levofloxacin (750 mg/24 h) vs. 7−14 days of levofloxacin (500 mg/24 h) | Non-inferiority in the clinical cure rate at the end of treatment (91.4% vs. 94.3%; difference: 2.9%; 95% CI: –7.6 to 1.9); no significant differences in the recurrence rate (0.5% vs. 1.4%) |
| Uranga, 201631 | n = 162 (experimental group), n = 150 (control group) | Non-inferiority CT | ≥5 days of antibiotic therapy and suspension once clinical stability is achieved versus duration established by the treating physician | Non-inferiority in the clinical success rate at 10 days (56.3% vs. 48.6%); shorter duration of treatment in the experimental group (median of 5 vs. 10 days) |
| Dinh, 202132 | n = 157 (experimental group), n = 153 (control group) | Double-blind, placebo-controlled, non-inferiority CT | 3 vs. 8 days of monotherapy with a ®-lactam once clinical stability is achieved | Non-inferiority in the cure rate at 15 days (77% vs. 68%; difference: 9.4%; 95% CI: –0.38 to 20.0) |
| Aliberti, 201733 | n = 125 (experimental group), n = 135 (control group) | Open-label, pragmatic, non-inferiority CT | ≥5 days of antibiotic therapy and suspension once clinical stability is achieved versus duration established by the treating physician | Premature interruption due to the apparent inferiority of the experimental group in the event of early failure at 30 days (11.2% vs. 7.4%); higher mortality rate in the experimental group (3.2% vs. 0.7%) |
| VAP | ||||
| Chastre, 200334 | n = 197 (experimental group), n = 204 (control group) | Partially double-blind (until day 8), non-inferiority CT | 8 vs. 15 days of antibiotic therapy | Non-inferiority in mortality rate at 28 days (18.8% vs. 17.2%; difference: 1.6%; 95% CI: –3.7–6.9); no differences in the overall recurrence rate (28.9% vs. 26.0%), higher recurrence rate in VAP caused by NFGNB in the experimental group (40.6% vs. 25.4%); lower proportion of infections due to MR bacteria in patients with recurrence in the experimental group (42.1% vs. 62.0%) |
| Fekih Hassen, 200935 | n = 14 (experimental group), n = 16 (control group) | Open-label, non-inferiority CT | 7 vs. 10 days of antibiotic therapy | No differences in mortality rate at 28 days (35.7% vs. 37.5%); greater number of days without antibiotic treatment in the experimental group (median: 4.1 vs. 1.8) |
| Capellier, 201236 | n = 116 (experimental group), n = 109 (control group) | Open-label, non-inferiority CT | 8 vs. 15 days of treatment with ®-lactam plus aminoglycoside (first 5 days) in early-onset VAP | Non-inferiority in the clinical cure rate at 21 days (85.3% vs. 84.4%; difference: 0.9%; 95% CI: –8.4–10.3); higher incidence of superinfection in the experimental group (35.3% vs. 19.3%) |
| Kollef, 201237 | n = 115 (experimental group), n = 112 (control group) | Double-blind, non-inferiority CT | 7 days of doripenem vs. 10 days of imipenem-cilastatin | Premature discontinuation due to the apparent inferiority of the experimental group in the clinical cure rate at the end of treatment (45.6% vs. 56.8%); higher mortality rate in the experimental group (21.5% vs. 14.8%) |
| Bouglé, 202238 | n = 88 (experimental group), n = 98 (control group) | Open-label, non-inferiority CT | 8 vs. 15 days of antibiotic therapy in VAP due to Pseudomonas aeruginosa | Non-inferiority was not demonstrated in the combined event of mortality and recurrence until day 90 (35.2% vs. 25.5%; difference: 9.7%; 95% CI: –1.9–21.2); higher recurrence rate during the ICU stay in the experimental group (17% vs. 9.2%) |
NFGNB: non-fermenting Gram-negative bacilli; CT: clinical trial; 95% CI: 95% confidence interval; MR: multiresistant; CAP: community-acquired pneumonia; VAP: ventilator-associated pneumonia; IQR: interquartile range; ICU: intensive care unit.
Most studies comparing the effectiveness of short courses of treatment versus long courses in HAP have focused on the specific context of VAP. As part of a CT twenty years ago, Chastre et al.34 compared a strategy of eight days of treatment versus 15 days in patients with VAP, finding no significant differences in mortality rates between the two arms at 28 days. They also found no differences in mortality rates when analysing the subgroups of patients with VAP caused by non-fermenting gram-negative bacilli (NFGNB) and MRSA. When interpreting these results, however, we have to bear in mind that this trial only randomised patients who had received adequate empirical treatment. A meta-analysis40 and a systematic review,41 including four and six CT that compared short courses (7–8 days) versus longer courses (10–15 days), respectively, also found no differences in terms of mortality rates. However, a higher incidence of infections due to resistant microorganisms was found in association with the longer treatments.41
This evidence has led to the latest updates of both the European (ERS/ESICM/ESCMID) and the ATS/IDSA guidelines recommending a treatment duration for HAP and VAP of 7–8 days.19,42
Treatment duration in Pseudomonas aeruginosa pneumoniaTreatment for pneumonia caused by P. aeruginosa has traditionally been longer than for other aetiologies. For example, in a comparative CT between meropenem and ceftolozane/tazobactam in HAP and VAP in which the duration of the regimen was decided by the treating physician, the median number of days of treatment was 7.7 in cases caused by Enterobacterales compared to the more than 12 days administered in patients with P. aeruginosa infection.43 Longer courses in this indication have classically been justified by the results derived from a sensitivity study in the CT by Chastre et al.34 focused on one of its secondary endpoints, in which patients with VAP due to NFGNB assigned to the eight-day arm had a higher risk of superinfection and recurrence (40.6% vs. 25.4% in the control group). However, it has been suggested that these results may be explained by a possible evaluation bias, as there was a longer time period from the end of treatment to the assessment of recurrence in the short treatment group. Additionally, the fact that the trial was open-label meant a greater likelihood of misinterpretation of symptoms attributed to recurrence in patients who received shorter courses.44 In fact, neither the ERS/ESICM/ESCMID nor the ATS/IDSA guidelines establish different regimens for cases caused by P. aeruginosa in their general recommendation to limit the duration of treatment for HAP and VAP to 7–8 days.19,42 However, some authors have criticised these recommendations by citing a series of methodological inconsistencies in the meta-analysis on which the ATS/IDSA guidelines are based; starting from the same CT, Albin et al.45 draw a different conclusion from this evidence, in which short courses of treatment would be associated with a higher risk of recurrence of VAP due to NFGNB. The response provided by the authors of the guidelines suggests that this issue has not yet been definitively resolved.44
Role of procalcitonin in the duration of pneumonia treatmentIn addition to the diagnostic role already mentioned, PCT monitoring has been proposed as a useful biomarker for monitoring serious bacterial infections. In a CT conducted in critically ill patients (60% of whom had pneumonia) who were randomised to discontinuation of antibiotic therapy according to changes in PCT in relative terms (decrease ≥80% with respect to the maximum level) or absolute terms (≤0.5 μg/l) or according to usual clinical practice, a significant reduction in the median duration of treatment was demonstrated in the experimental group (5 days) compared to the control (7 days).46 A subsequent meta-analysis based on 6,708 patients (from 26 CT) with other types of respiratory tract infection in addition to pneumonia also revealed a shorter duration of antibiotic therapy associated with the use of PCT as a blood infection marker (mean of 5.7 versus 8.1 days) and a reduction in the incidence of related adverse events.47 The results of these studies, however, must be interpreted with caution, as the duration of treatment in patients in whom PCT was not monitored to guide discontinuation was much longer than that recommended in clinical practice guidelines. The determination of PCT levels is not currently considered necessary in patients who are clinically stable after 48−72 hours of starting antibiotic therapy and who would not therefore require the course to be prolonged beyond of the 5 days recommended for CAP and 7–8 days for HAP and VAP.4,19 As previously noted, the ERS/ESICM/ESCMID/ALAT guidelines state that PCT levels can be used in combination with clinical judgement to determine the duration of treatment in severe CAP. The utility of PCT is also not clear in patients who have already achieved clinical stability, in whom the expected duration of treatment is 5–7 days.8
Can we modulate the host immune response in patients with pneumonia?The theoretical advantages derived from the use of immunomodulatory therapies in severe pneumonia are based on the accumulated knowledge about the role of the host-pathogen interaction in the pathogenesis of sepsis.48 Molecules released by microorganisms and from inside damaged cells (molecular patterns associated with pathogens and damage respectively) are capable of inducing a harmful inflammatory response by acting as ligands for various receptors involved in the innate response. Among these are Toll-like receptors (TLR), nucleotide oligomerisation domain (NOD)-like receptors and C-type mannose receptors. The consequent release of cytokines (including interleukin [IL]-1®, IL-6 and IL-8) generates tissue damage, which in the case of pneumonia can evolve into acute respiratory distress syndrome, and eventually leads to a situation of multiple organ failure. The hyperinflammatory state induced by this deregulated response is also accompanied by a situation of immunoparalysis and immune cell exhaustion.48 The clinical relevance of this process has been widely demonstrated in SARS-CoV-2 infection, in which the use of dexamethasone and IL-1® and IL-6 blocking agents has been effective in reducing mortality in the context of CT in patients with pneumonia and elevated inflammatory markers. This benefit contrasts strikingly with previous evidence in patients with influenza, which, although of poorer quality, suggested a negative effect in terms of mortality and risk of superinfection associated with the administration of adjuvant steroids.
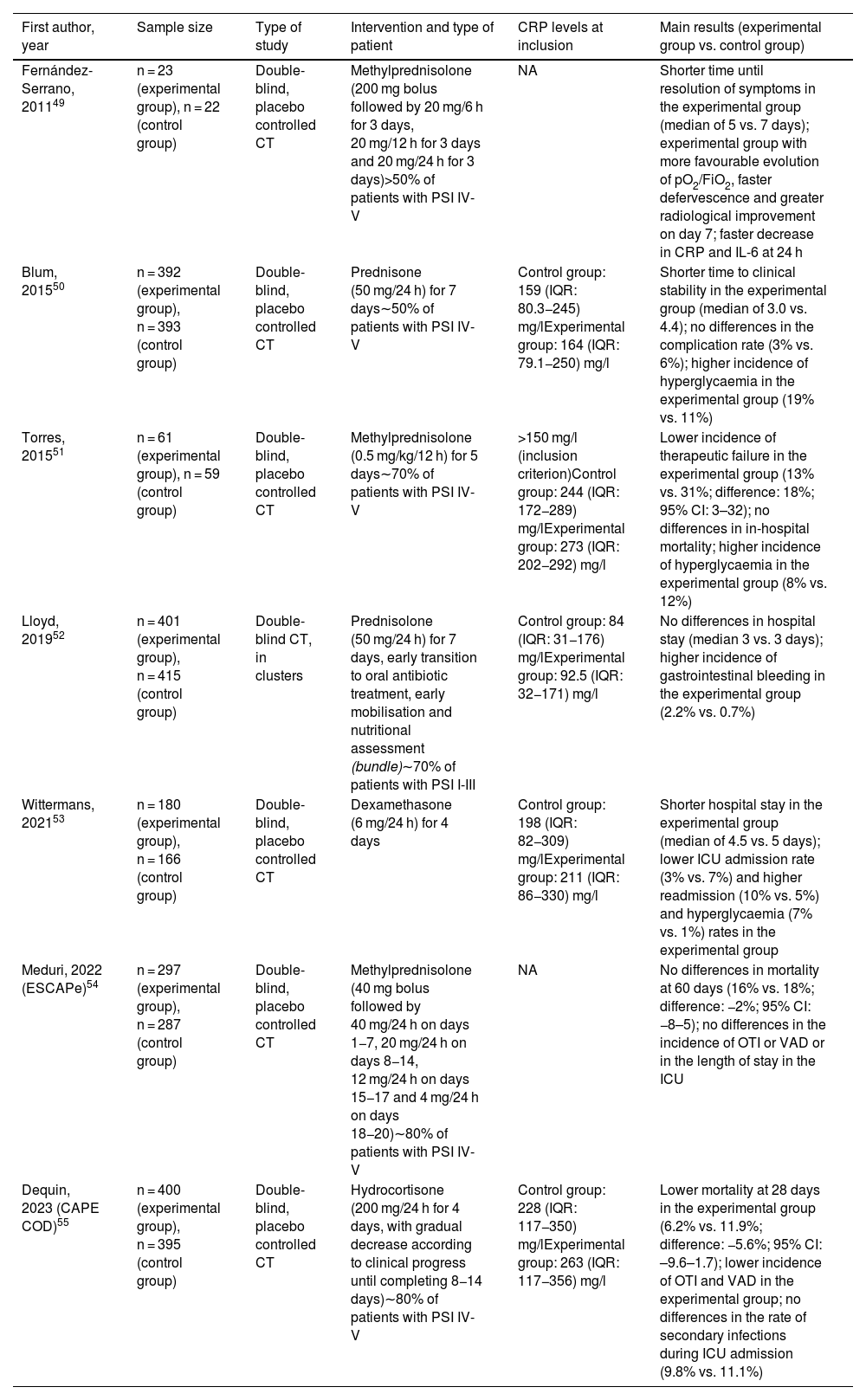
Steroid treatment in community-acquired pneumoniaSeveral intervention studies have evaluated the role of steroids in CAP (Table 2).49–55 For example, in 2015 Torres et al.51 reported the results of a CT in which patients with severe forms and high levels of C-reactive protein (>15 mg/dl) were randomised in the first 36 h of admission to receive methylprednisolone (0.5 mg/kg every 12 h) or placebo for five days. The therapeutic failure rate was significantly reduced in the experimental group compared to the control group, with no differences in in-hospital mortality. A meta-analysis based on 10 CT published up to January 2018 (with a total of 665 patients) confirmed that adjuvant treatment with steroids was associated with a reduction in all-cause mortality, the incidence of septic shock and the need for mechanical ventilation, as well as lower levels of C-reactive protein throughout follow-up. This benefit seemed more evident with the use of low or intermediate doses (≤86 mg of prednisone or equivalent) for more than five days.56 It should be noted, however, that all these studies had a small sample size, so the quality of this evidence was debatable. In fact, neither the ATS/IDSA guidelines for CAP published in 2007 nor those for HAP/VAP in 2016 made express mention of treatment with steroids (with the exception of patients with septic shock and adrenal insufficiency).13,19
Summary of the CT to have evaluated the role of steroids as immunomodulatory treatment in adult patients with CAP.
| First author, year | Sample size | Type of study | Intervention and type of patient | CRP levels at inclusion | Main results (experimental group vs. control group) |
|---|---|---|---|---|---|
| Fernández-Serrano, 201149 | n = 23 (experimental group), n = 22 (control group) | Double-blind, placebo controlled CT | Methylprednisolone (200 mg bolus followed by 20 mg/6 h for 3 days, 20 mg/12 h for 3 days and 20 mg/24 h for 3 days)>50% of patients with PSI IV-V | NA | Shorter time until resolution of symptoms in the experimental group (median of 5 vs. 7 days); experimental group with more favourable evolution of pO2/FiO2, faster defervescence and greater radiological improvement on day 7; faster decrease in CRP and IL-6 at 24 h |
| Blum, 201550 | n = 392 (experimental group), n = 393 (control group) | Double-blind, placebo controlled CT | Prednisone (50 mg/24 h) for 7 days∼50% of patients with PSI IV-V | Control group: 159 (IQR: 80.3−245) mg/lExperimental group: 164 (IQR: 79.1−250) mg/l | Shorter time to clinical stability in the experimental group (median of 3.0 vs. 4.4); no differences in the complication rate (3% vs. 6%); higher incidence of hyperglycaemia in the experimental group (19% vs. 11%) |
| Torres, 201551 | n = 61 (experimental group), n = 59 (control group) | Double-blind, placebo controlled CT | Methylprednisolone (0.5 mg/kg/12 h) for 5 days∼70% of patients with PSI IV-V | >150 mg/l (inclusion criterion)Control group: 244 (IQR: 172−289) mg/lExperimental group: 273 (IQR: 202−292) mg/l | Lower incidence of therapeutic failure in the experimental group (13% vs. 31%; difference: 18%; 95% CI: 3–32); no differences in in-hospital mortality; higher incidence of hyperglycaemia in the experimental group (8% vs. 12%) |
| Lloyd, 201952 | n = 401 (experimental group), n = 415 (control group) | Double-blind CT, in clusters | Prednisolone (50 mg/24 h) for 7 days, early transition to oral antibiotic treatment, early mobilisation and nutritional assessment (bundle)∼70% of patients with PSI I-III | Control group: 84 (IQR: 31−176) mg/lExperimental group: 92.5 (IQR: 32−171) mg/l | No differences in hospital stay (median 3 vs. 3 days); higher incidence of gastrointestinal bleeding in the experimental group (2.2% vs. 0.7%) |
| Wittermans, 202153 | n = 180 (experimental group), n = 166 (control group) | Double-blind, placebo controlled CT | Dexamethasone (6 mg/24 h) for 4 days | Control group: 198 (IQR: 82−309) mg/lExperimental group: 211 (IQR: 86−330) mg/l | Shorter hospital stay in the experimental group (median of 4.5 vs. 5 days); lower ICU admission rate (3% vs. 7%) and higher readmission (10% vs. 5%) and hyperglycaemia (7% vs. 1%) rates in the experimental group |
| Meduri, 2022 (ESCAPe)54 | n = 297 (experimental group), n = 287 (control group) | Double-blind, placebo controlled CT | Methylprednisolone (40 mg bolus followed by 40 mg/24 h on days 1−7, 20 mg/24 h on days 8−14, 12 mg/24 h on days 15−17 and 4 mg/24 h on days 18−20)∼80% of patients with PSI IV-V | NA | No differences in mortality at 60 days (16% vs. 18%; difference: −2%; 95% CI: −8–5); no differences in the incidence of OTI or VAD or in the length of stay in the ICU |
| Dequin, 2023 (CAPE COD)55 | n = 400 (experimental group), n = 395 (control group) | Double-blind, placebo controlled CT | Hydrocortisone (200 mg/24 h for 4 days, with gradual decrease according to clinical progress until completing 8−14 days)∼80% of patients with PSI IV-V | Control group: 228 (IQR: 117−350) mg/lExperimental group: 263 (IQR: 117−356) mg/l | Lower mortality at 28 days in the experimental group (6.2% vs. 11.9%; difference: −5.6%; 95% CI: –9.6–1.7); lower incidence of OTI and VAD in the experimental group; no differences in the rate of secondary infections during ICU admission (9.8% vs. 11.1%) |
95% CI: 95% confidence interval; CRP: C-reactive protein; CT: clinical trial; ICU: intensive care unit; IL-6: interleukin-6; IQR: interquartile range; NA: not available; OTI: orotracheal intubation; PSI: Pneumonia Severity Index; VAD: vasoactive drug.
The publication in the last year of two multicentre CT with a large number of patients may have contributed to clarifying the role of this therapeutic intervention.54,55 In the ESCAPe study, Meduri et al.54 randomised 586 patients with severe CAP and need for ICU admission to receive methylprednisolone (40 mg daily for 7 days with subsequent progressive tapering) or placebo. They were unable to detect significant differences in the primary endpoint of all-cause mortality at 60 days, either in the total population (16% versus 18% in the experimental and control groups respectively) or in any of the subgroups analysed. In contrast, Dequin et al.55 carried out a CT in 31 French hospitals (CAPE COD study) in which 800 participants, also with severe CAP and criteria for admission to the ICU, received hydrocortisone (200 mg daily for 4–7 days with progressive reduction until day 14) or placebo. An interim analysis revealed a lower 28-day mortality rate in the steroid-treated group (6.2% vs. 11.9%), as well as a reduced need for intubation and support with vasoactive drugs (secondary endpoints). Pre-specified subgroup analysis suggests that this benefit was greater in patients with elevated C-reactive protein levels (>15 mg/dl). How to reconcile these contradictory results despite the apparent similarity in the intervention analysed and the inclusion criteria? There are several methodological differences that should be pointed out. The ESCAPe trial was conducted at the United States Veterans Administration and, therefore, recruited almost exclusively men with a mean age close to 70. The CAPE COD study, by contrast, included one-third women, and the average age of participants was 67. Unlike the American trial, the diagnosis of influenza was an exclusion criterion in the CAPE COD study in view of the already cited literature suggesting a harmful effect associated with steroids. The most important difference is probably the window for starting steroid treatment from the time of recruitment, which was 24 h in the French study versus 96 h in the American study. There were also sizeable differences in the effective duration of steroid therapy, with medians of five and 20 days, respectively. The inclusion of these two CT in a recent meta-analysis (based on 3,367 patients) confirmed the findings of previous reviews, with lower 30-day mortality rates in patients treated with steroids and no evidence of an increased risk of adverse events. This meta-analysis also revealed significant differences depending on the dose of steroid administered (lower mortality rate with cumulative doses ≥400 mg of prednisone or equivalent compared to lower doses).57 In any event, the latest ATS/IDSA guidelines expressly discourage the routine use of steroids in patients with CAP with or without severity criteria (conditional and strong recommendation, respectively), although it should be noted that at the time of writing the results of the CAPE COD and ESCAPe trials had not yet been reported.3 The latest ERS/ESICM/ESCMID guidelines for management of severe CAP published in 2023 do take into account the findings of the ESCAPe trial, although they restrict the recommendation of the use of corticosteroids to patients with septic shock in the absence of poorly controlled diabetes, as long as the infection is not viral (flu or SARS-CoV-2) or caused by MRSA (conditional recommendation with low level of evidence). The suggested regimen is 0.5 mg/kg of methylprednisolone every 12 h for five days (which in a 70-kg patient would be approximately equivalent to a total cumulative dose of 430 mg of prednisone).8
Immunomodulatory role of macrolidesAside from their antibiotic role, macrolides exert a wide range of anti-inflammatory effects and modulate the immune response in vitro.58 They decrease the release of pathogen-associated molecular patterns and the expression of TLR in macrophages, reducing alveolar damage induced by the recruitment of inflammatory cells and the production of cytokines and chemokines. They are capable of enhancing phagocytosis and intracellular killing of bacteria, and inhibit bacterial quorum sensing signals and biofilm formation. They also stimulate the apoptosis of neutrophils and guide the differentiation of monocytes towards an M2 (tolerogenic) phenotype, actions that limit the excessive inflammatory response and facilitate tissue repair after infection.59 There is some evidence about the biological translation of these findings in vitro. For example, the use of regimens containing a macrolide has been associated with lower concentrations of IL-6 and tumour necrosis factor−α in the BAL of patients with CAP and poor clinical response after 72 h of antibiotic therapy.58
Numerous observational and intervention studies have tried to establish the potential benefit associated with the use of macrolides in CAP, with results that are not always conclusive. A large RCT that enrolled 560 patients revealed a non-significant trend towards a higher likelihood of clinical stability after seven days of treatment with a ®-lactam combined with clarithromycin compared to the ®-lactam alone.59 In a meta-analysis of 47 studies (contributing more than 58,000 patients), the use of a macrolide was associated with a lower 30-day mortality rate and a higher likelihood of resolution. The most frequently identified pathogens were S. pneumoniae (24.9% of cases) and K. pneumoniae (12.9%), which suggests that the effect of the macrolide is not completely explained by its broader spectrum compared to ®-lactams thanks to its activity against intracellular microorganisms.60 In a recently published CT (ACCESS study), 278 adult patients with CAP and systemic inflammatory response syndrome, a Sequential Organ Failure Assessment (SOFA) score ≥2 and PCT levels ≥0.25 ng/mL were randomised to receive a ®-lactam in combination with oral clarithromycin or placebo. The chances of achieving the primary endpoint (clinical improvement and decrease in SOFA and PCT levels) after 72 h of treatment were significantly greater in the group that received clarithromycin (67.9% vs. 38.3%). These patients also had lower serum levels of IL-10 and IL-18/IL-10 ratio, which suggests a protective effect of the macrolide against the immunoparalysis phenomenon induced by the pneumonia.61
However, the presumed benefit of combining a macrolide with the ®-lactam treatment is not so evident when the comparator is a quinolone. In fact, a meta-analysis based on 18 CT showed that patients treated with a respiratory fluoroquinolone as monotherapy (mainly levofloxacin and moxifloxacin) had a greater likelihood of clinical cure and microbiological eradication than those who received a ®-lactam in combination with a macrolide, with no differences in mortality rates or the incidence of adverse events.62 In line with the inconclusive nature of this evidence, the ATS/IDSA guidelines do not prioritise one or other type of regimen when selecting empirical treatment for CAP, with or without hospital admission criteria.3 The ERS/ESICM/ESCMID/ALAT guidelines for management of severe CAP do prioritise the combination of ®-lactam plus a macrolide (administered for 3–5 days) over the alternative combination of ®-lactam plus a fluoroquinolone. This recommendation is based on a meta-analysis carried out for the preparation of the guidelines which revealed a lower mortality rate in patients in whom the ®-lactam was administered in combination with a macrolide compared to a fluoroquinolone (19.4% vs. 26.8%, respectively). It should be noted, however, that the document does not take a position regarding the use of fluoroquinolones in monotherapy.8
Immunomodulatory role of intravenous immunoglobulinsSeveral studies have demonstrated the negative impact on the outcome of CAP of decreased serum immunoglobulin G (IgG) levels at the time of diagnosis in immunocompetent patients. For example, in a study carried out in three Spanish centres, the presence of IgG levels ≤680 mg/dl was an independent predictive factor of the need for ICU admission.63 In addition to their direct contribution to the elimination of microorganisms (through processes such as opsonophagocytosis, antibody-dependent cell-mediated cytotoxicity or activation of complement through the classic pathway), immunoglobulins play an immunomodulatory role and neutralise bacterial toxins. For this reason, the administration of intravenous polyclonal immunoglobulin preparations at high doses is recommended (usually 0.5−1 g/kg) in patients with toxic shock due to Gram-positive cocci, although the quality of the evidence supporting this recommendation is low.
Based on this evidence, it has been postulated that the administration of polyclonal immunoglobulins could be useful as immunomodulatory therapy in severe CAP, even in the absence of underlying hypogammaglobulinaemia. However, a Japanese multicentre retrospective study showed no difference in mortality rates or ICU outcome in immunocompetent patients with CAP and septic shock who received up to 5 g of immunoglobulins daily for three days compared to a propensity score-matched control group.64 The use of a preparation enriched in IgM ("Trimodulin") at doses of 42 mg/kg/day for five days was also not associated with a greater number of days free of invasive mechanical ventilation or support with vasoactive drugs in a phase 2 CT carried out in critically ill patients with severe CAP.65 However, a post hoc analysis of this study suggested a more rapid normalisation of neutrophil counts and acute phase reactants in the Trimodulin arm, as well as a possible clinical benefit limited to the subgroup of patients with low IgM levels and elevated C-reactive protein at the time of recruitment.66
Ten recommendations from OPENIN group experts- A
Diagnosis
- 1
We do not consider that microbiological studies are indicated in patients with low-risk CAP treated on an outpatient basis.
- 2
We recommend obtaining blood cultures, Gram staining and culture of respiratory tract samples (preferably good quality sputum or endotracheal aspirate) and detection of pneumococcus and L. pneumophila urinary antigens in patients with CAP with severity criteria requiring hospital admission. In these cases we also recommend obtaining nasopharyngeal exudate to detect colonisation by S. aureus and rule out infection by community respiratory viruses (influenza virus, SARS-CoV-2, RSV).
- 3
We do not consider that there is sufficient evidence to recommend the routine use of NAAT-based respiratory syndromic panels in patients with CAP. Their use in patients with VAP may be recommended, especially for the early detection of resistance mechanisms or certain microorganisms in critically ill or immunosuppressed patients.
- 4
In patients with HAP or VAP, we recommend performing a Gram stain with semiquantitative culture on respiratory tract samples (prioritising sputum or endotracheal aspirate over BAL or protected bronchial brushing), as well as obtaining blood cultures and testing for pneumococcus and L. pneumophila urinary antigens.
- 1
- B
Treatment
- 1
We recommend that the duration of antibiotic therapy in patients with CAP without the need for ICU admission and with a favourable clinical course be 5 days. We do not consider that there is currently sufficient evidence to apply this short regimen to forms with severity criteria.
- 2
We recommend that the duration of antibiotic therapy in patients with VAP (including cases caused by P. aeruginosa) with a favourable clinical course be 7–8 days. The persistence of the causative microorganism in follow-up samples of the lower respiratory tract obtained after 4–5 days would require prolongation of treatment.
- 3
We do not recommend basing the decision to start antibiotic therapy (or its duration) on the determination of PCT levels in patients with CAP, HAP or VAP.
- 1
- C
Host immune response modulation
- 1
We recommend the administration of corticosteroids in patients with CAP and septic shock, as well as in those who require admission to the ICU, provided that an influenza aetiology has been ruled out. Although there is no clear evidence regarding the most appropriate regimen, we suggest the use of methylprednisolone at a dose of 0.5 mg/kg every 12 h. This adjuvant treatment should be started early (first 24 h) and maintained for no more than 5–7 days.
- 2
We consider that combining a macrolide with treatment with a ®-lactam agent is preferable to combining a fluoroquinolone or the use of ®-lactam alone in patients with severe CAP (particularly of pneumococcal aetiology) with admission criteria.
- 3
We do not consider that there is evidence to support adjuvant treatment with parenteral immunoglobulins for immunomodulatory purposes in patients with CAP without hypogammaglobulinaemia.
- 1
The authors declare that they have no conflicts of interest.
The OPENIN Group meeting had the financial help of MSD España. The funding source did not participate in the preparation of this manuscript, its contents or the decision to submit it for publication. Mario Fernández Ruiz has a “Miguel Servet” contract (CP18/00073) from the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation.
Ana Fernández Cruz (Hospital Universitario Puerta de Hierro-Majadahonda, Madrid). Ana Ventura (Hospital de Gandía, Valencia). Belén Loeches (Hospital Universitario La Paz, Madrid). Carlos Dueñas (Hospital Universitario Río Hortega, Valladolid). Cristina Tomás (Hospital General Universitario, Murcia). David Navarro (Hospital Clínico Universitario, Valencia). Rosa Oltra (Hospital Clínico Universitario, Valencia). Elena Resino-Foz (Hospital Universitario Rey Juan Carlos, Móstoles, Madrid). Elisa García Vázquez (Hospital Universitario Virgen de la Arrixaca, Murcia). Enrique Míguez (Complexo Hospitalario Universitario de A Coruña, A Coruña). Esperanza Merino (Hospital General Universitario Doctor Balmis, Alicante). Francisco Braojos (Hospital Clínico San Carlos, Madrid). Francisco Javier Martínez (Hospital Universitario Juan Ramón Jiménez, Huelva). Francisco López-Medrano Pérez (Hospital Universitario 12 de Octubre, Madrid). Isabel Machuca (Hospital Universitario Reina Sofía, Córdoba). Javier Cobo (Hospital Universitario Ramón y Cajal, Madrid). Joaquín López Contreras (Hospital de la Santa Creu i Sant Pau, Barcelona). José María Reguera (Hospital Regional Universitario, Málaga). Juan Diego Ruiz Mesa (Hospital Regional Universitario, Málaga). Juan Tiraboschi (Hospital Universitari de Bellvitge, Hospitalet de Llobregat, Barcelona). Lucy Abella (Hospital Universitario Nuestra Señora de La Candelaria, Tenerife). Mar Masiá (Hospital General Universitario de Elche, Alicante). María Dolores del Toro López (Hospital Universitario Virgen Macarena, Sevilla). María Dolores Díaz López (Complexo Hospitalario Universitario de Ourense). Nerea Carrasco-Antón (Hospital Universitario Fundación Jiménez Díaz, Madrid). Nicolás Merchante (Hospital Universitario Virgen de Valme, Sevilla). Patricia Muñoz (Hospital General Universitario Gregorio Marañón, Madrid). Rafael Torres (Hospital Universitario Severo Ochoa, Leganés, Madrid). Regino Rodríguez (Hospital Universitario de Cruces, Barakaldo, Bizkaia). Tatiana Mata-Forte (Hospital Central de la Defensa Gómez Ulla, Madrid). Vicente Abril (Hospital General Universitario, Valencia).