Over the past decades, the advent of targeted and biological therapies has revolutionized the management of cancer and autoimmune, hematological and inflammatory conditions. Although a large amount of information is now available on the risk of opportunistic infections associated with some of these agents, the evidence regarding the susceptibility to bacterial infections is more limited. Biological agents have been shown to entail a variable risk of bacterial infections in pivotal randomized clinical trials and post-marketing studies. Recommendations on risk minimization strategies and therapeutic interventions are therefore scarce and often based on expert opinion, with only a few clear statements for some particular agents (i.e. meningococcal vaccination for patients receiving eculizumab). In the present review the available information regarding the incidence of and risk factors for bacterial infection associated with the use of different groups of biological agents is summarized according to their mechanisms of action, and recommendations based on this evidence are provided. Additional information coming from clinical research and real-world studies is required to address unmet questions in this emerging field.
La introducción de terapias dirigidas y biológicas ha revolucionado a lo largo de las últimas décadas el tratamiento del cáncer y de las enfermedades autoinmunes, hematológicas e inflamatorias. Si bien existe abundante información acerca del exceso de riesgo de infección oportunista vinculado a algunos de estos agentes, la evidencia disponible sobre el riesgo de infección bacteriana específicamente es más limitada. En el marco de los ensayos clínicos pivotales y de estudios poscomercialización se ha demostrado un riesgo variable de infección bacteriana asociada al uso de terapias biológicas. Por ese motivo las recomendaciones para su minimización y abordaje terapéutico son escasas, y a menudo basadas en la opinión de expertos, con tan solo algunos escenarios de alto riesgo claramente establecidos para ciertos agentes (como la recomendación de vacunación anti-meningocócica en pacientes tratados con eculizumab). En la presente revisión se actualiza la información disponible respecto a la incidencia de infección bacteriana relacionada con las diferentes familias terapéuticas según su mecanismo de acción y sus factores de riesgo, aportándose igualmente algunas recomendaciones basadas en esta evidencia. Se requieren más estudios de investigación clínica en vida real para aclarar las preguntas sin resolver en este novedoso campo.
Over the last twenty years, the introduction of targeted and biological therapies has revolutionized the treatment of solid cancer and hematological and inflammatory diseases. The increasing experience with these agents has allowed to estimate the associated risk of developing opportunistic infections, such as invasive aspergillosis, shingles or Pneumocystis jirovecii pneumonia.1 Targeted and biologic therapies produce different blockages in the immune pathways involved in both the innate and adaptive responses related to the host-bacteria interaction, which could increase the susceptibility to severe bacterial infections.2 Nevertheless, this specific risk has not yet been completely elucidated, and recommendations are mainly based on expert opinions.
The present review summarizes the information concerning the risk and management of bacterial infection in patients receiving targeted and biological therapies. We performed a computer-based MEDLINE (National Library of Medicine, Bethesda, MD) search with no temporal or language restrictions, using the MeSH terms appropriate for each agent (always including “infection” or “infectious complications”) to capture the literature pertaining to the subject. Particular attention was given to the safety data reported across pivotal randomized controlled trials (RCTs) and relevant post-marketing surveillance studies. The references of selected articles were also examined for additional related references. We have also collected information from the recommendations issued by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Infections in Compromised Hosts (ESGICH).2–9
Which factors related to bacterial infections have to be considered before the initiation of targeted and biological therapies?
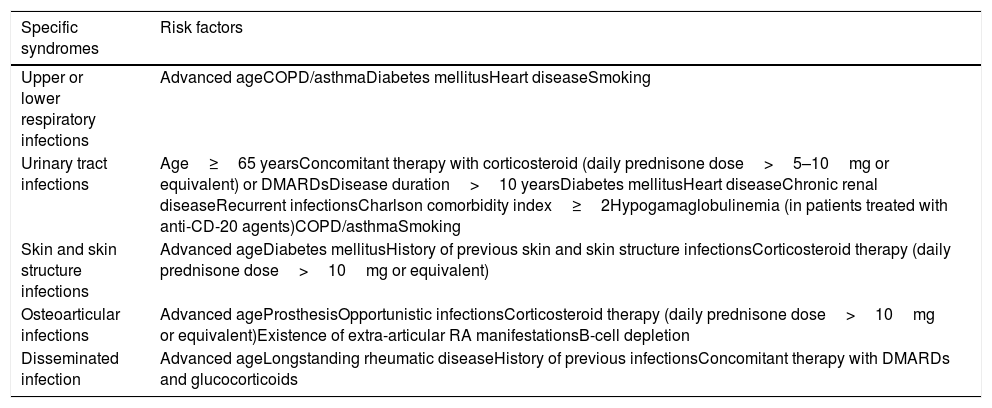
First, we should consider that many autoimmune and inflammatory diseases intrinsically confer an increased baseline susceptibility to bacterial pathogens. Therefore, those patients with severe underlying conditions could be at additional risk once the therapy is initiated.10Table 1 depicts some risk factors that could increase concomitantly the risk of bacterial infection in patients with rheumatic diseases receiving targeted therapies.11,12 The presence of previous debilitating chronic disorders (e.g. chronic obstructive pulmonary disease, interstitial lung disease or chronic kidney disease),11,12 the previous occurrence of serious infections, and high cumulative doses of corticosteroids also act as contributing factors. Despite the paucity of supporting data, numerous guidelines recommend not to initiate any biological therapy in patients with a concomitant severe active infection (which is usually defined as that requiring intravenous treatment or hospitalization).11
Risk factors for the occurrence of bacterial infections in patients with rheumatic diseases receiving biological therapy.
| Specific syndromes | Risk factors |
|---|---|
| Upper or lower respiratory infections | Advanced ageCOPD/asthmaDiabetes mellitusHeart diseaseSmoking |
| Urinary tract infections | Age≥65 yearsConcomitant therapy with corticosteroid (daily prednisone dose>5–10mg or equivalent) or DMARDsDisease duration>10 yearsDiabetes mellitusHeart diseaseChronic renal diseaseRecurrent infectionsCharlson comorbidity index≥2Hypogamaglobulinemia (in patients treated with anti-CD-20 agents)COPD/asthmaSmoking |
| Skin and skin structure infections | Advanced ageDiabetes mellitusHistory of previous skin and skin structure infectionsCorticosteroid therapy (daily prednisone dose>10mg or equivalent) |
| Osteoarticular infections | Advanced ageProsthesisOpportunistic infectionsCorticosteroid therapy (daily prednisone dose>10mg or equivalent)Existence of extra-articular RA manifestationsB-cell depletion |
| Disseminated infection | Advanced ageLongstanding rheumatic diseaseHistory of previous infectionsConcomitant therapy with DMARDs and glucocorticoids |
COPD: chronic obstructive pulmonary disease, DMARDs: disease modifying anti-rheumatic drugs, RA: rheumatoid arthritis.
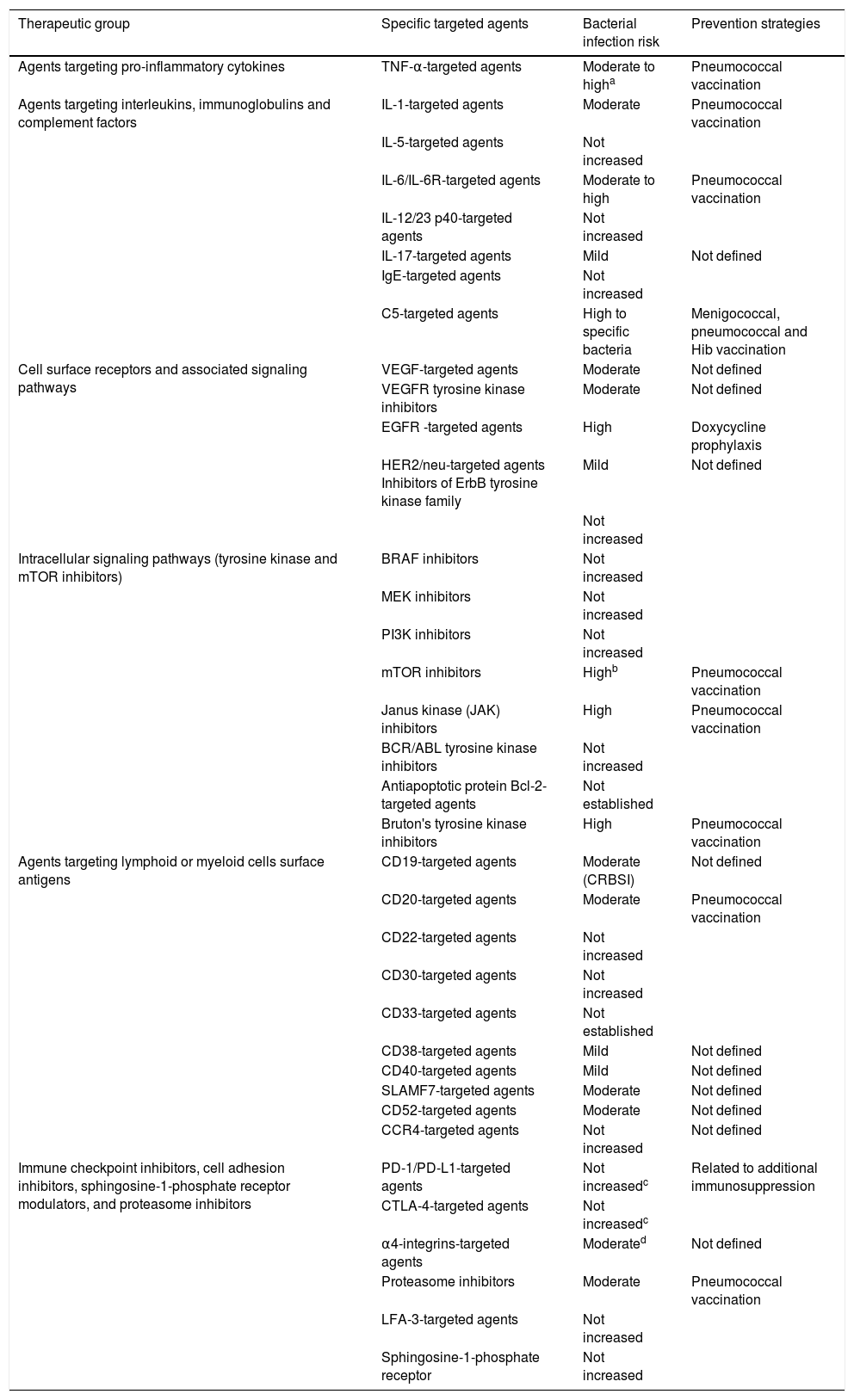
We have reviewed the existing experience by classifying the biological agents into six large categories according to their intimate mechanism of action (Table 2).
Bacterial infection risk associated to different families of targeted and biological agents and proposed prevention strategies.
| Therapeutic group | Specific targeted agents | Bacterial infection risk | Prevention strategies |
|---|---|---|---|
| Agents targeting pro-inflammatory cytokines | TNF-α-targeted agents | Moderate to higha | Pneumococcal vaccination |
| Agents targeting interleukins, immunoglobulins and complement factors | IL-1-targeted agents | Moderate | Pneumococcal vaccination |
| IL-5-targeted agents | Not increased | ||
| IL-6/IL-6R-targeted agents | Moderate to high | Pneumococcal vaccination | |
| IL-12/23 p40-targeted agents | Not increased | ||
| IL-17-targeted agents | Mild | Not defined | |
| IgE-targeted agents | Not increased | ||
| C5-targeted agents | High to specific bacteria | Menigococcal, pneumococcal and Hib vaccination | |
| Cell surface receptors and associated signaling pathways | VEGF-targeted agents | Moderate | Not defined |
| VEGFR tyrosine kinase inhibitors | Moderate | Not defined | |
| EGFR -targeted agents | High | Doxycycline prophylaxis | |
| HER2/neu-targeted agents Inhibitors of ErbB tyrosine kinase family | Mild | Not defined | |
| Not increased | |||
| Intracellular signaling pathways (tyrosine kinase and mTOR inhibitors) | BRAF inhibitors | Not increased | |
| MEK inhibitors | Not increased | ||
| PI3K inhibitors | Not increased | ||
| mTOR inhibitors | Highb | Pneumococcal vaccination | |
| Janus kinase (JAK) inhibitors | High | Pneumococcal vaccination | |
| BCR/ABL tyrosine kinase inhibitors | Not increased | ||
| Antiapoptotic protein Bcl-2- targeted agents | Not established | ||
| Bruton's tyrosine kinase inhibitors | High | Pneumococcal vaccination | |
| Agents targeting lymphoid or myeloid cells surface antigens | CD19-targeted agents | Moderate (CRBSI) | Not defined |
| CD20-targeted agents | Moderate | Pneumococcal vaccination | |
| CD22-targeted agents | Not increased | ||
| CD30-targeted agents | Not increased | ||
| CD33-targeted agents | Not established | ||
| CD38-targeted agents | Mild | Not defined | |
| CD40-targeted agents | Mild | Not defined | |
| SLAMF7-targeted agents | Moderate | Not defined | |
| CD52-targeted agents | Moderate | Not defined | |
| CCR4-targeted agents | Not increased | Not defined | |
| Immune checkpoint inhibitors, cell adhesion inhibitors, sphingosine-1-phosphate receptor modulators, and proteasome inhibitors | PD-1/PD-L1-targeted agents | Not increasedc | Related to additional immunosuppression |
| CTLA-4-targeted agents | Not increasedc | ||
| α4-integrins-targeted agents | Moderated | Not defined | |
| Proteasome inhibitors | Moderate | Pneumococcal vaccination | |
| LFA-3-targeted agents | Not increased | ||
| Sphingosine-1-phosphate receptor | Not increased |
Hib: Haemophilus influenzae type b; CRBSI: Catheter related bloodsttream infection.
Most relevant data related to the specific risk of bacterial infection in patients receiving targeted and biological agents are derived from the experience with the therapeutic blockade of tumor necrosis factor (TNF)-α in rheumatoid arthritis (RA) and inflammatory bowel disease (IBD). This theoretical background is often extensible to other indications with a more limited clinical experience. Curtis et al. analyzed the incidence of common bacterial infections with anti-TNF-α agents (abatacept, rituximab, infliximab, etanercept and adalimumab) in a large cohort (n=3111) of RA patients from the US Veterans Health Administration between 1998 and 2011, reporting high rates for pneumonia (37%), cellulitis and soft tissue infection (22%), and urinary tract infection (9%). In this study, the use of abatacept was associated with a higher rate of bacterial infection-related hospitalization, although the multivariate analysis revealed no differences across different agents. The risk of infection was greater among patients receiving corticosteroids (daily prednisone dose>7.5mg or equivalent) and for those in the highest quartile of C-reactive protein levels and erythrocyte sedimentation rate (as compared to the lowest quartile).12 More recently, Carrara et al. estimated in an Italian cohort of RA patients (n=4656) incidence rates for pneumonia, bloodstream infection, cellulitis and septic arthritis of 2.95, 2.51, 1.30 and 1.06 episodes per 1000 patient-years, respectively. Abatacept seemed to be protective for severe infection-related hospitalization as compared to etanercept, while the use of other agents was not shown to increase the infection risk compared with this anti-TNF-α agent. Additional risk factors included the occurrence of severe infection within the previous year and high corticosteroid doses.13 A Japanese cohort found a higher incidence of pulmonary infection among patients receiving adalimumab, with no differences between different biological agents in the overall risk of infection. These authors also found a significantly increased risk related to older age, RA severity, low body mass index, and presence of chronic comorbidities such as diabetes.14 A network meta-analysis for severe infection in RA patients found that standard- (odds ratio [OR]: 1.31; 95% confidence interval [CI]: 1.09–1.58) and high-dose (OR: 1.90; 95% CI: 1.50–2.39) therapy with biological agents were both associated with a higher risk for severe infection, although such difference was not significant for methotrexate (MTX) naïve and anti-TNF-α experienced patients.15 In a meta-analysis of IBD patients receiving different anti-TNF-α agents and other therapies (vedolizumab or natalizumab), the incidence of serious infection was not significantly increased as compared to placebo (OR: 0.89; 95% CI: 0.71–1.12). Nevertheless, the overall risk of infection was higher in those patients on biologic agents (OR: 1.19; 95% CI: 1.10–1.29).16 In summary, there is limited evidence suggesting an increase in the risk of bacterial infection with the use of anti-TNF-α agents. Of note, infections occur most frequently within the first year since the initiation of therapy.17
Agents targeting interleukins, immunoglobulins and complement factorsTherapy with interleukin (IL)-1-targeted agents has been shown to be associated with a moderate increase in the risk of bacterial infection, mainly in form of respiratory, urinary tract and skin and skin structure infections.18,19 The reported incidence of serious infection is 5.4 episodes per 100 patient-years and seems to be increased among older patients with previous chronic diseases.20 IL-6/IL-6R-targeted agents are associated with an increase in the risk of bacterial infection comparable to that observed with anti-TNF-α therapies, with pneumonia, urinary tract infection and cellulitis as the most commonly reported events.21,22 The pooled estimate for serious infection in phase 3 and extension RCTs is 4.9 episodes per 100 patient-years,23 with higher rates in population-based studies (9.0 episodes per 100 patient-years).24 It is important to note that most of the available experience with IL-6 blockade is restricted to tocilizumab, with limited data for sarilumab or siltuximab.
Phase 2 and 3 RCTs evaluating the use of IL-17-targeted agents in patients with psoriasis, ankylosing spondylitis and psoriatic arthritis showed a minor increase in the risk of bacterial infection (2.4 episodes of serious infection per 100 patient-years), usually mild to moderate in severity. Upper respiratory and urinary tract infections were the most common syndromes, whereas cellulitis was the predominant serious infection.25,26 IL-12/23-targeted agents have not been associated with a significant increase in the risk of bacterial infection.27
Eculizumab, a humanized monoclonal antibody targeting and preventing cleavage of the terminal complement component C5, notably predisposes to infections due to Neisseria spp., with a 10,000-fold increased risk for meningococcal infection. In addition, this agent has been associated with a significant increase in the risk of respiratory tract infection as compared to placebo.4
Cell-surface receptors and associated signaling pathwaysA large meta-analysis pooling data from 41 RCTs and more than 30,000 patients with various cancer types (mostly colorectal carcinoma) that received vascular endothelial growth factor (VEGF)-targeted agents28 concluded that the use of bevacizumab significantly increased the incidence of infection of any grade (relative risk [RR]: 1.45; 95% CI: 1.27–1.66) and serious infection (RR: 1.59; 95% CI: 1.42–1.79). In this meta-analysis, the infection risk associated to bevacizumab seemed to be mostly linked to the occurrence of febrile neutropenia, fistulae or abscesses, and pneumonia, but not sepsis or colitis.28
Therapy with VEGF tyrosine kinase inhibitors does not increase the risk of infection.29 The use of anti-VEGFR2 monoclonal antibodies (ramucirumab) is likely associated with a risk increase similar to that observed for VEGF-targeted agents, including the role of drug-induced neutropenia and gastrointestinal perforation as contributing factors.30,31
The use of epidermal growth factor receptor (EGFR)-targeted agents is associated with a meaningful increase in the risk of infection, mainly as a result of neutropenia and secondary infection in cases of severe papulopustular rash, with Staphylococcus aureus as a remarkable causative microorganism in skin and skin structure infections.32,33
Therapy with monoclonal antibodies targeting the human epidermal growth factor receptor 2 (ErbB2/HER2) might be associated with a minor increase in the risk of infection. However, the biologic rationale and clinical evidence supporting this association are weak.34 Finally, ErbB receptor tyrosine kinase-targeted agents (including either selective EGFR/HER1 and/or ErbB2/HER2 inhibitors or pan-ErbB inhibitors) does not meaningfully increase the rate of bacterial infection.5
Intracellular signaling pathways (tyrosine kinase and mTOR inhibitors)With some exceptions, this family of biologic agents does not show a significant increase in the susceptibility to bacterial pathogens.6 Ibrutinib (a Bruton's tyrosine kinase [BTK] inhibitor) exhibited a modest increase in the risk of bacterial infection. Pneumonia is observed mainly in the presence of neutropenia, whereas about 10% of patients developed urinary tract infections.35,36 The risk is influenced by other factors like additional immunosuppressive drugs or previous immune disorders related to the underlying B-cell malignancies.
Janus kinase (JAK) inhibitors are associated with a considerable increase in the risk of infection. Indeed, urinary tract infection, pneumonia, and septic shock were described for patients on ruxolitinib (a potent oral JAK1/JAK2 inhibitor) in rates of 24.6%, 13.1% and 7.9%, respectively, in one pivotal RCT for the treatment of myelofibrosis. Surprisingly, the long-term follow-up revealed a decrease in the incidence of infection, presumably resulting from the stabilization of the underlying disease with resolution of cytopenias.37
A meta-analysis evaluating the use of mammalian target of rapamycin (mTOR) inhibitors in 4,097 cancer patients from 12 RCTs (8 with everolimus and 3 with temsirolimus) reported an increased risk of infection (overall incidence of all-grade infection of 25.0%; 95% CI: 16.7–35.9%), including fatal outcomes due to sepsis and pneumonia. Sub-group analysis revealed heterogeneity across different tumor types, with lower risks for patients with giant cell astrocytoma, breast cancer and angiomyolipoma, and higher risks for those with renal cell carcinoma, lymphomas or neuroendocrine tumors.38 The variations in dose regimens are relevant in explaining these findings as compared to the more favorable safety profile observed among solid organ transplant recipients.6
BCR-ABL tyrosine kinase inhibitors, BRAF/MEK kinase inhibitors, and PI3K inhibitors do not seem to increase the risk of serious bacterial infection, whereas limited information is still available for BCL-2 inhibitors.6
Agents targeting lymphoid or myeloid cells surface antigensIn pivotal RCTs, mostly limited to blinatumomab, therapy with CD19-targeted agents does not show a significant increase in the risk of bacterial infection compared with conventional chemotherapy, with overall rates comparable to those expected in patients undergoing treatment for relapsed or refractory acute lymphoblastic leukemia.39 Of note, the need for continuous intravenous infusion for 4 weeks in the blinatumomab regimen explains the elevated rate of catheter-associated bloodstream infection in the RCTs evaluating this first-in-class bispecific T-cell engager, with rates ranging from 2.2% to 11%.40,41 Careful management of intravenous lines is therefore warranted to minimize this risk. Clinicians caring for patients receiving such therapy should be also aware of the associated risk of hypogammaglobulinemia (HGG), which is associated with a well-established increased susceptibility to encapsulated bacteria such as Streptococcus pneumoniae.42
Infection prevails as the most common non-hematological adverse effects of anti-CD20 monoclonal antibodies, including severe respiratory tract infection. Separate meta-analysis in lymphoma and RA do not show a significant increase in the risk of bacterial infection.43,44 Nevertheless, in a French cohort of patients with immune thrombocytopenia, the incidence of serious bacterial infections was 6.3 episodes per 100 patient-years, and as high as 100.5 episodes per 100 patient-years for non-serious infections. Pneumonia was the most frequently reported syndrome (42.8%), with gastrointestinal and urinary tract infections as other common infections.45 Second- and third-generation anti-CD20 monoclonal antibodies could also increase the risk of bacterial infection, although this risk is determined by the presence of concomitant comorbidities, previous and concomitant immunosuppressive therapies, and the long-term occurrence of sever HGG.7
The use of alemtuzumab (CD52-targeted agent) for different diseases shows a variable risk of bacterial infection explained in part by differences in dose regiments and the presence of previous immunosuppression. Overall, bacterial infections are higher in some scenarios such as cutaneous lymphoma, with an incidence rate of 23.3 episodes per 100 patient-years.46
Immune checkpoint inhibitors, cell adhesion inhibitors, sphingosine-1-phosphate receptor modulators, and proteasome inhibitorsThe available data from pivotal RCTs suggest that CTLA-4 (ipilimumab or tremelimumab) or PD-1/PD-L1 blockade (nivolumab or atezolizumab) is not intrinsically associated with an increase in the risk of infection.47–51 Nevertheless, cancer patients receiving immune checkpoint inhibitors may develop immune-related adverse events that may entail additional immunosuppressive therapy (such as corticosteroids or anti-TNF-α agents) impacting the susceptibility to bacterial pathogens (9). A systematic review in IBD found that patients treated with vedolizumab (an α4-integrin-targeted monoclonal antibody) experienced a moderate increase in the incidence of serious bacterial infections and surgical site infections compared to placebo.52
Pneumonia is a common bacterial infection among patients with multiple myeloma (MM) treated with proteasome inhibitors (PIs), but data do not seem to support a higher risk weighed with comparator therapies. Of note, untreated MM patients already face an increased incidence of respiratory tract infections (including pneumonia) due to the underlying impairment of humoral immune responses. However, this susceptibility seems to be influenced by the use of PIs in the induction regimen.53
Should biologic therapies be discontinued while treating a bacterial infection? When should they be reintroduced?Currently, studies evaluating these questions are lacking, and there is no clear evidence to guide the optimal management of bacterial infections in patients receiving biologic agents. Nevertheless, most guidelines concur on the need of discontinuing therapy in the presence of serious bacterial infection, to be reintroduced only once the infection has completely resolved. The decision should ideally be taken on a case-by-case basis by an experienced physician since, in some situations, the abrupt withdrawal of therapy can induce a flare of the underlying autoinflammatory disorder11 or negatively impact the prognosis of the underlying disease. Indeed, it has been reported an increased risk of progression among patients with chronic lymphocytic leukemia in which the BTK inhibitor ibrutinib has to be discontinued.54
Should asymptomatic bacteriuria be treated in patients receiving biological therapies?No studies have addressed whether patients receiving biologic therapies should be systematically screened and treated for asymptomatic bacteriuria. A case–control study performed in women with autoimmune rheumatic diseases reported no differences in the incidence of either asymptomatic or symptomatic urinary tract infection compared with the control group.55 More data are needed to establish the management in this population, although systematic treatment would not be indicated, in line with other clinical scenarios in which no apparent benefit from this strategy has been demonstrated.56,57
What prevention strategies are required to prevent bacterial infections in patients receiving biological therapies?Clear recommendations aimed at minimizing the risk of bacterial infection linked to the use of the different families of biological and targeted agents are mostly lacking, except for some specific agents. As mentioned above, in view of the increased risk of N. meningitidis infection with eculizumab use, meningococcal vaccination must be assured before the initiation of therapy.4 Vaccination against pneumococcal disease and Haemophylus influenzae type B should be provided according to current guidelines, and are recommended for various biologic targeted agents mentioned previously3,11 (Table 2). In other specific scenarios, antibiotic prophylaxis is recommended, as occurs in patients on EGFR targeted-agents who should receive oral doxycycline to prevent skin infections.6 However, it should be emphasized that there is no clear evidence to support such practices, and any potential benefit must be always balanced against the well-defined risk of inducing antimicrobial resistance, as observed in neutropenic patients.
ConclusionBacterial infections in patients on targeted and biologic therapies have a variable incidence depending on the agent used, with this risk being influenced by contributing factors such as previous comorbidities, the activity of the underlying condition, or the receipt of additional immunosuppressive therapy. There are few specific recommendations concerning the management and prevention of bacterial infections in these patient populations. The proven risk of invasive infections due to encapsulated bacteria clearly supports meningococcal and pneumococcal vaccination among patients treated with eculizumab and anti-CD20 agents. Nonetheless, evidence is mostly lacking for therapies involving interleukins and other soluble mediators, and no guidance may be provided for newer agents even in the presence of a theoretical infection risk (such as that potentially resulting from the blockade of the pro-inflammatory cytokines IL-1β or IL-6). In addition, no risk thresholds have been established to define in which patient subgroups interventions should be implemented. Multicenter post-marketing surveillance with granular information on the type and severity of infection, open-label extension studies and other sources of real-world data are necessary to elucidate the safety profile of these therapies, with the ultimate aim to define specific interventions if significant risk signals emerge.
Funding sourcesThis research was supported by Plan Nacional de I+D+I 2013–2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Spanish Ministry of Science and Innovation, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0002) – co-financed by the European Development Regional Fund (EDRF) “A way to achieve Europe”. M.F.R. holds a research contract “Miguel Servet” (CP 18/00073) from the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III.
Conflict of interestThe authors have no conflicts of interests to disclose.