To determine the proportion of people infected by HIV or AIDS under follow-up in the VACH Cohort in 2012 who were lost to follow-up from 2013 to 2014, and to establish the sociodemographic features relating to this loss.
MethodsWe considered subjects with less than one recorded consultation per year studied to be lost to follow-up. We built logistic regression models to calculate the odds ratios (OR) and their 95% confidence intervals (95% CI), of the variables relating to loss to follow-up.
ResultsThe overall percentage of losses to follow-up was 15.5% (95% CI 14.9–16.1). The variables associated with loss to follow up were: not receiving antiretroviral treatment (ART) (OR: 1.948, 95% CI: 1.651–2.298), being an immigrant (OR: 1.746, 95%CI: 1.494–2.040), intravenous drug consumption being the mechanism for HIV transmission (OR: 1.498, 95% CI: 1.312–1.711), being unemployed (OR: 1.331, 95% CI: 1.179–1.503), being without a partner (OR: 1.948, 95% CI: 1.651–1.298), belonging to a low socioeconomic class (OR: 1.279, 95% CI: 1.143–1.431), and being attended in a hospital with fewer than 1000 patients under follow-up (OR: 1.257, 95% CI: 1.121–1.457), as well as being under age and having spent less time under follow-up in the Cohort.
Conclusions15.5% of the patients were lost to follow-up over a period of 2years in the VACH Cohort. This was associated with a series of sociodemographic and epidemiological variables that it might be useful to identify to design initiatives targeting the populations most likely to abandon the circuits of care, and guide strategies towards achieving Objective 90-90-90.
Determinar la proporción de personas con infección por VIH o sida que se encontraban en seguimiento en la cohorte VACH en 2012 y que resultaron perdidas del mismo en 2013 y 2014, así como establecer las características sociodemográficas relacionadas con dicha pérdida.
MétodosConsideramos perdidos del seguimiento a los sujetos con menos de un registro de consulta por año analizado. Construimos modelos de regresión logística para la estimación de las razones de ventajas (odds ratio [OR]) y sus intervalos de confianza del 95% (IC del 95%) de las variables relacionadas con la pérdida de seguimiento.
ResultadosEl porcentaje global de pérdidas en seguimiento fue del 15,5% (IC del 95%: 14,9-16,1). Las variables asociadas con la pérdida de seguimiento fueron no recibir tratamiento antirretroviral (TAR) (OR: 1,948; IC del 95: 1,651-2,298), ser inmigrante (OR: 1,746; IC del 95: 1,494-2,040), el consumo de fármacos por vía intravenosa como mecanismo de transmisión del VIH (OR: 1,498; IC del 95: 1,312-1,711), encontrarse en situación de desempleo (OR: 1,331; IC del 95: 1,179-1,503), no tener pareja (OR: 1,948, IC del 95: 1,651-1,298), pertenecer a un estrato socioeconómico bajo (OR: 1,279; IC del 95: 1,143-1,431) y ser atendido en un hospital con menos de 1.000 pacientes en seguimiento (OR: 1,257; IC del 95%: 1,121-1,457), además de menor edad y menos tiempo de seguimiento en la cohorte.
ConclusionesEl 15,5% de los pacientes fueron perdidos del seguimiento en un periodo de 2años en la cohorte VACH. Ello se asoció a una serie de variables sociodemográficas y epidemiológicas, cuya identificación puede ser útil para diseñar iniciativas focalizadas sobre las poblaciones más susceptibles de abandonar los circuitos asistenciales y a orientar estrategias diseñadas a la consecución del objetivo 90-90-90.
Antiretroviral treatment (ART) prevents the progression of HIV infection by blocking viral replication and secondarily immune reconstitution and the reduction of inflammatory activity, markedly decreasing the incidence of opportunistic diseases, other diseases and mortality.1–3 In addition, it reduces the infectivity of infected subjects, effectively preventing (under optimal conditions) vertical transmission and transmission via sexual relations,4–6 as well as probably transmission associated with the use of injected drugs.7 The benefit of ART used without interruption has been proven at any time during the evolution of the infection,2,8 which justifies the already universal recommendation to treat all infected persons.9,10 In addition to this individual benefit, and in support of the reduction of infectivity, there are mathematical models that indicate that expanded access to treatment could contribute to reducing the incidence of HIV infection and its eventual elimination,11–15 while also reducing the financial costs of the pandemic.16,17 For these reasons, UNAIDS has formally endorsed “treatment as prevention” and proposed the so-called 90-90-90 target as a global strategy for the treatment of HIV.18
Access to HIV treatment is only one part of the continuous spectrum of health and social care, which is a long-term process. Some people do not start it because they have not yet been diagnosed. In other cases the diagnosis is known but contact with any healthcare mechanism has not been established. Lastly, other people, after an initial connection, are not permanently retained and are lost to follow-up.
Loss to follow-up is one of the critical issues in trying to achieve the 90-90-90 target. The information available about this phenomenon is difficult to analyse since there is no consensus on its definition or on the methodology for its estimation.
Most of the information available comes from research on clinical databases. Very variable percentages of losses to follow-up have been reported. The United States and Canada have put it between 22 and 60%, depending on the regions analysed.19–22 In a multinational study, the proportion of people with HIV or AIDS (PHAs) lost to follow-up ranged from 54% in Georgia to 19% in Denmark.21 In Spain, another two large multicentre cohorts have reported their data. In the Catalan PISCIS cohort, 85% of the patients recruited in 2010 had at least one additional visit, a circumstance defined by the investigators as “retained in care”.23 In the CoRIS cohort, 19.8% of patients did not have any visits in the last year of data collection (October 2008–October 2009), so they were classified as losses to follow-up. The authors also describe some socio-demographic factors associated with these losses,24 an analysis that had been uncommon or limited to ethnicity and gender in the previously cited studies conducted in other countries.25
The primary objective of this study is to estimate the proportion of losses to follow-up and identify the main socio-demographic variables related to patients in the Spanish VACH cohort (an acronym for HIV and AdvanCedHIV, the name of the electronic medical record shared by the hospitals that make up the cohort), with data from real practice, in a country with a public health system.
MethodsStudy population and designThe VACH cohort is made up of people with confirmed HIV infection in follow-up at 24 Spanish hospitals. The inclusion criteria and work methodology have been described previously.26 The study protocol was approved by the research committee from the principal investigator's hospital.
Study subjectsThe study population consists entirely of subjects over 16 years of age from the VACH cohort in follow-up between January and December 2012 at the participating hospitals.
Data collectionThe data specified in the study was collected through an electronic case report form (eCRF). The encrypted eCRFs were sent to the data coordinating site for entry into the central database (VACH database). Duplicate controls, quality controls and data validation were used by means of computer algorithms implemented in the central database.
Statistical analysisWe refer to the primary outcome variable of this study as “loss (or losses) to follow-up”, which we define as the absence of (those patients without) at least one record of a check-up visit in the period analysed (2013–2014).
We established this definition of loss to follow-up based on the frequency of consultation visits recommended in the clinical practice guidelines, consensus among project investigators with extensive experience in the field of care for HIV infection and the available evidence.27–29
We do not consider as losses to follow-up instances of imprisonment, death, change of hospital or province of residence or admission to a therapeutic community. We do not consider as follow-up visits those visits made by outpatients to other departments, to emergency services, pharmacies, social workers or hospitalisations.
We collected information on the following explanatory variables: current age, sex (male, female), transmission mechanism (men who have sex with men, heterosexual transmission, intravenous drug user, other/unknown), socio-economic stratum (high, medium, low, unknown), level of education (higher, secondary, primary, without formal education, unknown), employment status (active, not working, retired, non-contributory benefit, housewife, unknown), pre-existing AIDS (yes, no), immigrant (yes, no, unknown), region of origin (Spain; Sub-Saharan Africa; North Africa and the Middle East; South America and the Caribbean; Eastern Europe; Western Europe, North America and Australia; Asia; unknown), stable partner at time of entry in the cohort (yes, no, unknown), ART at time of loss to follow-up (yes, no), CD4+ lymphocyte count nadir, duration of previous follow-up and type of hospital defined based on the number of patients included in the cohort (<600, 600–1000, >1000).
We developed logistic regression models for the estimation of the odds ratios (OR) and the 95% confidence intervals (95% CI) of the variables related to loss to follow-up, using the conditional elimination steps procedure based on the overall model with the variables associated with loss to follow-up in the bivariate analysis (with p<0.05). We used the SPSS programme (IBM SPSS Statistics for Windows, Version 19.0. IBM Corp., Armonk, NY, USA).
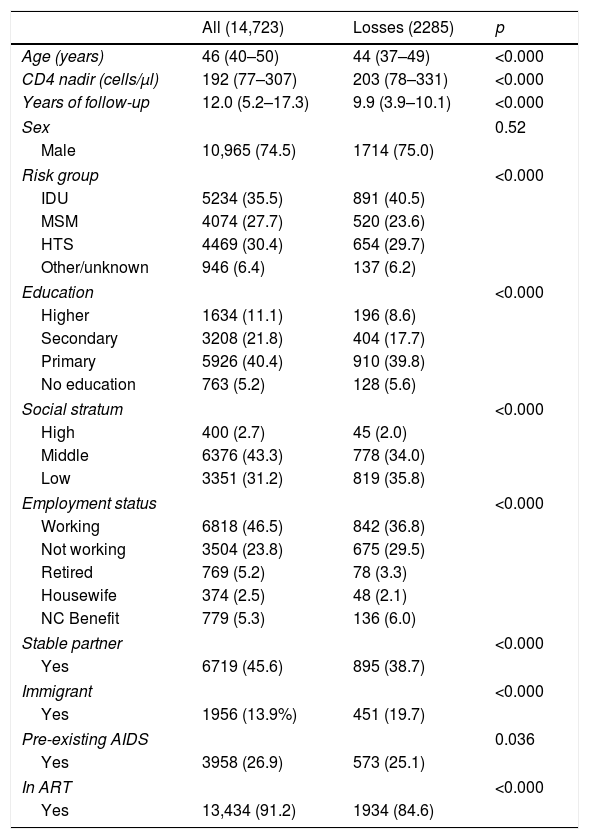
ResultsIn 2012, the total number of PHAs in follow-up in the VACH cohort was 16,440. We excluded from the analysis 1717 (10.4%), for whom we did not have updated information on the variables that were the object of the study. We therefore analysed the data from 14,723 PHAs with a median age of 46 years (quartiles: 39–50), of whom 25.5% were women and 86.1% were of Spanish origin. The main socio-demographic characteristics of the population analysed are summarised in Table 1. Ten thousand eight hundred and forty-seven patients (73.7%) were Spanish-born, 487 (3.3%) came from sub-Saharan Africa, 83 (0.6%) from North Africa or the Middle East, 954 (6.5%) from Central America, South America or the Caribbean, 128 (8.7%) from Eastern Europe, 268 (1.8%) from Western Europe, North America or Australia, 15 (0.1%) from Asia and for 1941 (13.2%) country of origin was not recorded.
Main clinical and socio-demographic characteristics of all patients in follow-up in the VACH cohort in 2012 and losses during 2013–2014.
| All (14,723) | Losses (2285) | p | |
|---|---|---|---|
| Age (years) | 46 (40–50) | 44 (37–49) | <0.000 |
| CD4 nadir (cells/μl) | 192 (77–307) | 203 (78–331) | <0.000 |
| Years of follow-up | 12.0 (5.2–17.3) | 9.9 (3.9–10.1) | <0.000 |
| Sex | 0.52 | ||
| Male | 10,965 (74.5) | 1714 (75.0) | |
| Risk group | <0.000 | ||
| IDU | 5234 (35.5) | 891 (40.5) | |
| MSM | 4074 (27.7) | 520 (23.6) | |
| HTS | 4469 (30.4) | 654 (29.7) | |
| Other/unknown | 946 (6.4) | 137 (6.2) | |
| Education | <0.000 | ||
| Higher | 1634 (11.1) | 196 (8.6) | |
| Secondary | 3208 (21.8) | 404 (17.7) | |
| Primary | 5926 (40.4) | 910 (39.8) | |
| No education | 763 (5.2) | 128 (5.6) | |
| Social stratum | <0.000 | ||
| High | 400 (2.7) | 45 (2.0) | |
| Middle | 6376 (43.3) | 778 (34.0) | |
| Low | 3351 (31.2) | 819 (35.8) | |
| Employment status | <0.000 | ||
| Working | 6818 (46.5) | 842 (36.8) | |
| Not working | 3504 (23.8) | 675 (29.5) | |
| Retired | 769 (5.2) | 78 (3.3) | |
| Housewife | 374 (2.5) | 48 (2.1) | |
| NC Benefit | 779 (5.3) | 136 (6.0) | |
| Stable partner | <0.000 | ||
| Yes | 6719 (45.6) | 895 (38.7) | |
| Immigrant | <0.000 | ||
| Yes | 1956 (13.9%) | 451 (19.7) | |
| Pre-existing AIDS | 0.036 | ||
| Yes | 3958 (26.9) | 573 (25.1) | |
| In ART | <0.000 | ||
| Yes | 13,434 (91.2) | 1934 (84.6) | |
All numbers are absolute numbers with percentages in parentheses with respect to the column total, except for “Age”, “CD4 nadir” and “Years of follow-up”, which are medians with 25th and 75th centiles in parentheses. The p value was obtained using the Mann–Whitney test for “Age” and “CD4 nadir” and the χ2 test for the remainder of the variables. The sum of the sub-columns corresponding to some variables does not match the total due to incomplete data.
NC: non-contributory; MSM: men who have sex with men; HTS: sexual relations between people of different sexes; ART: antiretroviral treatment; IDU: intravenous drug users.
During the period studied, 2285 (15.5%, 95% CI: 14.9–16.1) patients were lost to follow-up. The percentage of losses was higher in the subjects infected through intravenous drug use (17.0%, 95% CI: 16.3–18.1), in people without formal education (23.4%, 95% CI: 20.5–23.4), in those belonging to a low socio-economic stratum (24.4%, 95% CI: 23.0–25.9), in the unemployed (19.3%, 95% CI: 18.0–20.6) and in those without a stable partner (17.5%, 95% CI: 16.7–18.3). Losses to follow-up were also higher in HIV-infected immigrants than in those of Spanish origin (23.1% vs. 13.5%), particularly in immigrant women (17.7% vs. 25.4%). Except for the group of 15 subjects identified as of “Asian” origin, among whom there were no cases of loss to follow-up (OR: 0.135, 95% CI: 0.066–0.276), losses were significantly more frequent in all other groups of patients defined by their region of origin, so, after excluding the “Asians”, we grouped the rest as “immigrants” for the multivariate analysis. The proportion of losses to follow-up was higher in patients who did not receive ART (27.2% vs. 14.4%). We did not observe differences between the groups defined by pre-existing or no pre-existing AIDS. The percentage of losses to follow-up was greater in hospitals that contributed fewer than 1000 cases to the cohort (16.8% in hospitals with <1000 patients vs. 13.6%).
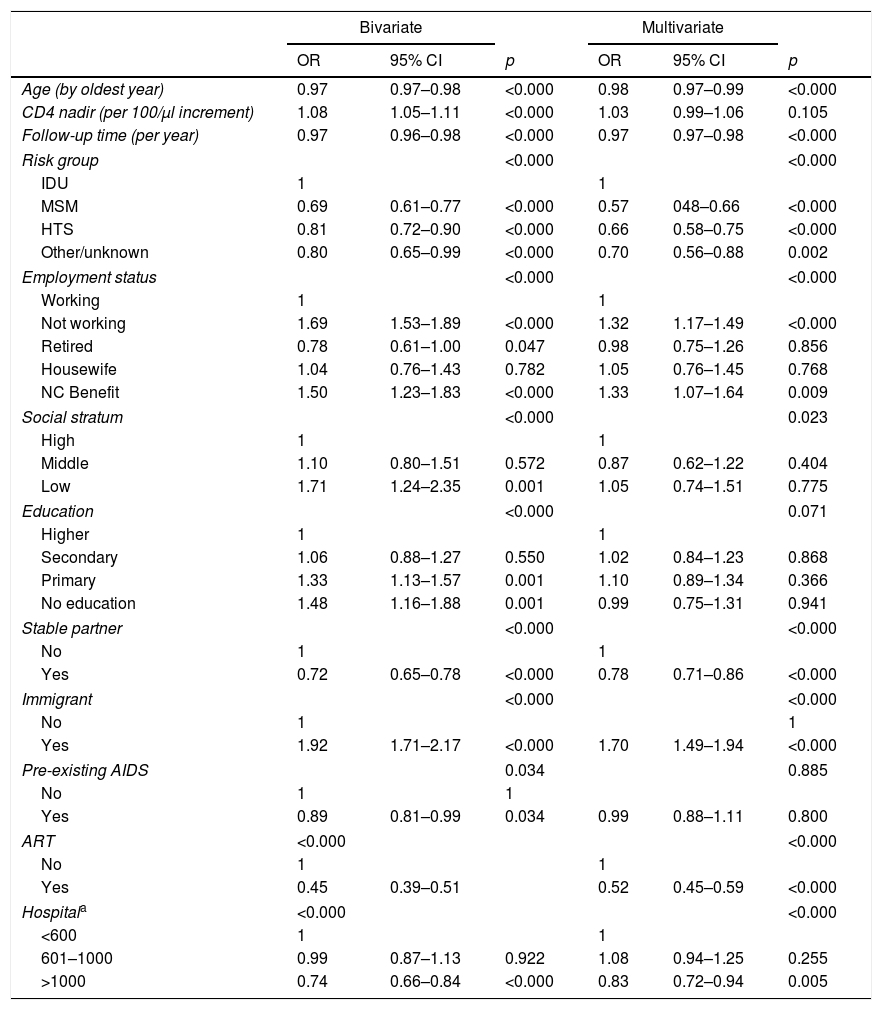
Table 2 shows the final regression model with the socio-demographic variables related to loss to follow-up. In this model, age, ART, immigrant status, risk group for the acquisition of HIV, employment situation, not having a partner, social stratum, classification of the hospital according to the number of patients in follow-up and follow-up time in the cohort before 2012 demonstrated an association with loss to follow-up.
Risk factors for being lost to follow-up during 2013–2014, among the socio-demographic and clinical characteristics studied in patients in follow-up in the VACH cohort in 2012.
| Bivariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age (by oldest year) | 0.97 | 0.97–0.98 | <0.000 | 0.98 | 0.97–0.99 | <0.000 |
| CD4 nadir (per 100/μl increment) | 1.08 | 1.05–1.11 | <0.000 | 1.03 | 0.99–1.06 | 0.105 |
| Follow-up time (per year) | 0.97 | 0.96–0.98 | <0.000 | 0.97 | 0.97–0.98 | <0.000 |
| Risk group | <0.000 | <0.000 | ||||
| IDU | 1 | 1 | ||||
| MSM | 0.69 | 0.61–0.77 | <0.000 | 0.57 | 048–0.66 | <0.000 |
| HTS | 0.81 | 0.72–0.90 | <0.000 | 0.66 | 0.58–0.75 | <0.000 |
| Other/unknown | 0.80 | 0.65–0.99 | <0.000 | 0.70 | 0.56–0.88 | 0.002 |
| Employment status | <0.000 | <0.000 | ||||
| Working | 1 | 1 | ||||
| Not working | 1.69 | 1.53–1.89 | <0.000 | 1.32 | 1.17–1.49 | <0.000 |
| Retired | 0.78 | 0.61–1.00 | 0.047 | 0.98 | 0.75–1.26 | 0.856 |
| Housewife | 1.04 | 0.76–1.43 | 0.782 | 1.05 | 0.76–1.45 | 0.768 |
| NC Benefit | 1.50 | 1.23–1.83 | <0.000 | 1.33 | 1.07–1.64 | 0.009 |
| Social stratum | <0.000 | 0.023 | ||||
| High | 1 | 1 | ||||
| Middle | 1.10 | 0.80–1.51 | 0.572 | 0.87 | 0.62–1.22 | 0.404 |
| Low | 1.71 | 1.24–2.35 | 0.001 | 1.05 | 0.74–1.51 | 0.775 |
| Education | <0.000 | 0.071 | ||||
| Higher | 1 | 1 | ||||
| Secondary | 1.06 | 0.88–1.27 | 0.550 | 1.02 | 0.84–1.23 | 0.868 |
| Primary | 1.33 | 1.13–1.57 | 0.001 | 1.10 | 0.89–1.34 | 0.366 |
| No education | 1.48 | 1.16–1.88 | 0.001 | 0.99 | 0.75–1.31 | 0.941 |
| Stable partner | <0.000 | <0.000 | ||||
| No | 1 | 1 | ||||
| Yes | 0.72 | 0.65–0.78 | <0.000 | 0.78 | 0.71–0.86 | <0.000 |
| Immigrant | <0.000 | <0.000 | ||||
| No | 1 | 1 | ||||
| Yes | 1.92 | 1.71–2.17 | <0.000 | 1.70 | 1.49–1.94 | <0.000 |
| Pre-existing AIDS | 0.034 | 0.885 | ||||
| No | 1 | 1 | ||||
| Yes | 0.89 | 0.81–0.99 | 0.034 | 0.99 | 0.88–1.11 | 0.800 |
| ART | <0.000 | <0.000 | ||||
| No | 1 | 1 | ||||
| Yes | 0.45 | 0.39–0.51 | 0.52 | 0.45–0.59 | <0.000 | |
| Hospitala | <0.000 | <0.000 | ||||
| <600 | 1 | 1 | ||||
| 601–1000 | 0.99 | 0.87–1.13 | 0.922 | 1.08 | 0.94–1.25 | 0.255 |
| >1000 | 0.74 | 0.66–0.84 | <0.000 | 0.83 | 0.72–0.94 | 0.005 |
Results of the logistic regression models of the probability of being lost to follow-up during 2013–2014 on socio-demographic and clinical characteristics.
NC: non-contributory; MSM: men who have sex with men; HTS: sexual relations between people of different sexes; ART: antiretroviral treatment; IDU: intravenous drug users.
The percentage of losses to follow-up in this study, which included 14,723 PHAs in follow-up during 2012 in Spanish hospitals from the VACH cohort, is lower than that reported in other previous studies performed in the USA, Canada, Denmark or Australia.19–22 In these studies, the authors considered lost to follow-up those patients with <2 determinations of CD4 lymphocytes or viral load of HIV, with an interval >3 months in the year analysed. In our work, we have considered lost to follow-up those people with <1 attendance at a consultation in the year analysed, in accordance with the criteria used in the Spanish studies cited above.23,24 The results of recent research indicate that more frequent laboratory tests may not be necessary for good infection control,27,28 a recommendation endorsed in a recent observational study with data from European and American cohorts.29 If we had used <2 visits/year as a criterion of loss, the percentage of losses would have been 24% (CI: 23.3–24.8, data not shown). The percentage of losses to follow-up is very similar to the 15% reported in another Spanish study carried out in Catalonia23 and lower than that recorded in CoRIS.24 Although the defining criterion of “loss” chosen for our study is closer to those of the other 2 Spanish studies, without a standardisation of the definition and recording methods it is not appropriate to venture speculative explanations. It must be taken into account that loss to follow-up is associated with suboptimal adherence, a lower probability of having a suppressed HIV viral load, a greater risk of transmission, the appearance of resistance to drugs and worse health outcomes.30–33
Several previous studies have analysed gender and race or ethnicity, along with other variables such as age or treatment, against retention in health care.19,23,30,31 The inclusion of other socio-demographic variables not previously analysed, such as social stratum, level of education, employment status or partner, is, in our opinion, one of the strengths of this study. This information allows the identification of more vulnerable populations that possibly have greater barriers to access to the health system.
In our study, in contrast to a similar one carried out in the Netherlands between 2007 and 2013,32 losses to follow-up were higher in hospitals with fewer than 1000 patients in follow-up (16.8% in hospitals with <1000 patients vs. 13.6%, OR 1.257). This could be due to technical or structural differences related to the size of hospitals (differing accessibility of specialists and technicians for psychosocial issues), socio-economic factors (internal migration to pockets of greater economic activity in the context of the economic recession) and even differences in the use of the computer application (in real time or deferred).
One limitation of our study is the external validity of the data when generalising the results for the Spanish population infected with HIV. Since 2000, the VACH cohort has prospectively recruited patients in follow-up in 24 Spanish hospitals with a wide geographical representation. As a whole, these hospitals cover the specialised healthcare of 16% of the population, so we think that the results of the study could be representative at a general level, taking this premise into account. Regarding internal validity, like all observational studies, it can be compromised by the quality and degree of completion of the collected data.
In our opinion, knowledge of the magnitude and characteristics of loss to follow-up can contribute to our knowledge and dynamic monitoring of the epidemic, and so help to guide a more efficient distribution of resources for prevention of losses and the attainment of the 90-90-90 target. The information generated can also be useful to determine the impact of losses to follow-up, and design general improvement plans and plans focused on the most vulnerable populations.
FundingThis study was financed by ViiV Healthcare.
Conflicts of interestRamón Teira, Nuria Espinosa, M. Mar Gutiérrez, Marta Montero, Elisa Martínez, Francisco González, Fernando Lozano de León, Francisco Téllez, M. José Galindo, Joaquim Peraire, Elisabeth Deig and Pepa Muñoz-Sánchez declare at least one of the following: has carried out consultancy work, or has participated in scholarships for clinical research, or has received financial compensation for talks, or has collaborated in the preparation of educational materials, or has received travel grants for attendance at conferences, from at least one of the following: Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, MSD, ViiV Healthcare or AbbVie.
To Dr. Ignacio Suárez Lozano, for inspiring and driving this study and many others in two decades of leadership of the VACH Study Group.
Belén de la Fuente and María Campoamor (Hospital de Cabueñes [Cabueñes Hospital], Gijón), Pere Domingo and Teresa Puig (Hospital Arnau de Vilanova [Arnau de Vilanova Hospital], Lleida), Esteban Ribera (Hospital Vall d́Hebron [Vall d́Hebron Hospital], Barcelona), Bernardino Roca (Hospital General [General Hospital], Castellón de la Plana), Josefina García (Hospital Santa Lucía [Santa Lucía Hospital], Cartagena), Manuel Castaño and M. Isabel Mayorga (Hospital Carlos Haya [Carlos Haya Hospital], Málaga), Alberto Terrón and Ángel Álvarez (Hospital de Jerez [Jerez Hospital], Jerez de la Frontera), Ignacio Suárez-Lozano and Lola Merino (Complejo Hospitalario de Huelva [Huelva Hospital Complex]), Paloma Geijo and Olga Belinchón (Hospital Virgen de la Luz [Virgen de la Luz Hospital], Cuenca), M. Antonia Sepúlveda (Hospital Virgen de la Salud [Virgen de la Salud Hospital], Toledo), Vicente Estrada (Hospital Clínico de San Carlos [San Carlos Clinical Hospital], Madrid), Agustín Muñoz-Sanz (Hospital Infanta Cristina [Infanta Cristina Hospital], Badajoz), M. Gracia Mateo (Hospital Santa Creu i Sant Pau [Santa Creu i Sant Pau Hospital], Barcelona), Consuelo Viladés (Hospital Joan XXIII [Joan XXIII Hospital], Tarragona), Iván Castro (Hospital La Fe [La Fe Hospital], Valencia), Luis López-Cortés (Hospital Virgen del Rocío [Virgen del Rocío Hospital], Sevilla), Fernando Mateos (Complejo Hospitalario [Hospital Complex], Albacete).
Please cite this article as: Teira R, Espinosa N, Gutiérrez MM, Montero M, Martínez E, González F, et al. Pérdidas de seguimiento de personas con infección por el VIH en la cohorte española VACH en el periodo 2013-2014: importancia de los factores sociodemográficos. Enferm Infecc Microbiol Clin. 2019;37:361–366.