The chronic phase of Chagas disease (CD) is characterised by a low and intermittent parasitaemia. The Polymerase Chain Reaction (PCR) presents a variable sensitivity in this stage limiting its use as a diagnostic tool. Despite this, the use of PCR in untreated patients can provide information on the parasite behavior and its presence in peripheral blood.
MethodsA timely real-time PCR determination was performed on a cohort of 495 untreated chronic CD patients. Also, a subcohort of 29 patients was followed-up by serial real-time PCR during a period from 8 to 12 months in which they could not have access to the treatment due to lack of supply.
ResultsThe positive percentage of real-time PCR in our series was 42%. Nevertheless, real-time PCR positive results were significantly higher in patients with five years or less of residence in Spain (P = .041). The detection of DNA was not related to the existence of cardiac and/or gastrointestinal abnormalities.
In the follow-up subgroup, real-time PCR was consistently positive in 13.8% of patients, consistently negative in 31%, and intermittent in 55.2%.
ConclusionsThe different real-time PCR results regarding the time of residence suggests the possible relationship of external factors in the parasite presence in peripheral blood. On the other hand, specific host factors may be involved in the behavior of parasitaemia over time.
La fase crónica de la Enfermedad de Chagas (EC) se caracteriza por una parasitemia baja e intermitente. En esta fase, la sensibilidad de la Reacción en Cadena de la Polimerasa (PCR) es muy variable, limitando su utilización como técnica diagnóstica. A pesar de ello, la realización de la PCR en pacientes no tratados puede aportar datos sobre el comportamiento del parásito y su presencia en sangre periférica.
MétodosSe realizó PCR a tiempo real de forma puntual en una cohorte de 495 pacientes con EC crónica en ausencia de tratamiento. También se realizó seguimiento de una subcohorte de 29 pacientes mediante PCR a tiempo real seriadas, entre 8 y 12 meses en los que no tuvieron acceso al tratamiento por falta de suministro.
ResultadosEl porcentaje de positividad de PCR a tiempo real fue de 42%. Este porcentaje fue significativamente mayor en pacientes con 5 años o menos de residencia en España (P = .041). La detección de ADN no se relacionó con la existencia de alteraciones cardíacas y/o digestivas.
En el subgrupo de pacientes a los que se realizaron determinaciones seriadas, el resultado de PCR fue sostenidamente positivo en 13.8% de los pacientes, negativo en 31% e intermitente en 55.2%.
ConclusionesLas diferencias de resultados de PCR a tiempo real en función del tiempo de residencia apuntan que existen factores externos que pueden influir en la presencia del parásito en sangre periférica. Así mismo, factores que propios del hospedador parecen influir en la dinámica parasitaria a lo largo del tiempo.
Chagas disease (CD), caused by the flagellated protozoan Trypanosoma cruzi (T. cruzi), is the most significant parasitic disease in Latin America, where it is endemic.1 It is believed that there are 6–7 million affected people around the world and it is the cause of 12,000 deaths per year.2 In recent years, as a result of significant migration movements, there has been a change in the geographic distribution of the disease, rendering CD a global health problem. Due to its socioeconomic and cultural ties to Latin America, Spain is the leading destination in Europe for Latin American migrants, and therefore receives the most infected patients.3
Outside the endemic area, where there is no vector transmission, most patients are in the chronic phase of the disease. This phase is characterised by low, intermittent parasitemia, and patients are generally asymptomatic, although 30%–40% show cardiac and/or gastrointestinal abnormalities that can become serious. Given this low parasitemia, parasitology techniques show low sensitivity and diagnosis is based on detection of anti-T. cruzi antibodies.4
Although antiparasitic treatment in the chronic phase is of debated efficacy and has significant side effects, it is widely used due to the beneficial effects of decreasing the parasite load.5 In Spain, the most commonly used drug is benznidazole (BZL) and it is indicated in all patients with CD in the chronic phase except for those who are pregnant and those with advanced heart disease or another contraindication.6 Loss of anti-T. cruzi antibodies is the only definitive marker of recovery from CD, although it is a very slow process and its confirmation requires several years of follow-up.7
Since the 1990s, multiple protocols based on gene amplification techniques have been developed, in particular polymerase chain reaction (PCR) for the detection of T. cruzi DNA in peripheral blood. There are various PCR protocols with different sensitivity results depending on multiple factors, including amplified region (satellite or kinetoplast DNA), primer design and thermal cycler conditions.8 The advent of quantitative real-time PCR offers significant advantages, such as multiplex protocol design and parasite load measurement. It has also improved the quality of the results and the standardization of the technique.9
PCR has proven very useful in cases with high parasitemia; however, in the chronic phase, given the behavior of the parasite in peripheral blood, a negative PCR result does not rule out infection, limiting its utility as a diagnostic technique.8
Currently, PCR’s greatest utility in the chronic phase lies in follow-up of treated patients. A positive PCR result is the best and earliest marker of therapeutic failure.10
However, PCR in untreated patients may yield useful information on the behavior of the parasite in the chronic phase and its presence in peripheral blood.
The objective of this study is to analyze the results of one-time real-time PCR in a cohort of 495 patients and serial real-time PCR in a small cohort of 29 patients with chronic CD in the absence of treatment.
Material and methodsThis retrospective observational study was conducted in the Microbiology Department at Hospital Vall d'Hebron between June 2010 and May 2012. The inclusion criteria for the study patients were: age over 18 years, positive serology for T. cruzi, and presenting no causes of immunosuppression. None of the patients enrolled had been treated with any trypanocidal agent.
Clinical and epidemiological data as well as real-time PCR data were collected from all the patients enrolled. Potential cardiac complications were assessed by the Kuschnir classification, and potential gastrointestinal complications were assessed using a barium swallow test and/or a barium enema.
In the statistical analysis, qualitative variables were represented in terms of absolute numbers and percentages, and quantitative variables were represented in terms of mean and standard deviation (SD). The χ2 test was used to compare qualitative variables, and Student's t-test was used to compare quantitative variables. A univariate logistic regression model was prepared using the SPSS© software program to evaluate associations between different variables. The results were considered statistically significant if the p value was < 0.05.
This study was approved by the Hospital Vall d'Hebron Institutional Review Board.
Microbiological proceduresFor serological diagnosis of CD, all serum samples were analyzed simultaneously by two ELISAs, one using recombinant antigen (Bioelisa Chagas, Biokit, Lliçà d'Amunt, Spain) and the other using the parasite's own lysate (Ortho T. cruzi ELISA, Johnson & Johnson, High Wycombe, United Kingdom). Samples found to be reactive by both methods were considered positive.
Blood samples for real-time PCR were collected in a visit prior to starting treatment. Once the sample was received by the laboratory, it was immediately diluted in a 1:1 proportion with 6 M guanidine hydrochloride (Sigma Aldrich) for 24 h at room temperature. DNA extraction was performed from 200 μl, eluting in 50□μl using automated equipment (NucliSens easyMAG, Biomerieux, France). Amplification was performed in duplicate according to a protocol described by Piron et al.11 The real-time PCR result was considered positive when the internal amplification control (Taq Man Human RNase P detection reagent; Applied Biosystems, Foster City, CA, United States) was within normal limits (amplification cycle [Ct] 26 ± 2) and at least one of the two determinations was positive for T. cruzi (Ct < 40). All PCR panels included a positive and negative external control.
To validate the real time-PCR results, an external quality assessment (EQA) program was included. This program includes processing of panels of samples prepared with different dilutions of blood contaminated with epimastigotes from cultures of different parasite lineages (TcIa, TcId, TcV and TcVI), as well as negative samples. The panel was prepared and evaluated by an external reference laboratory.12
ResultsA total of 495 patients with chronic CD who had not received treatment were enrolled.
Most of them were women from Bolivia. Mean patient age was 37.7 (19–68) years.
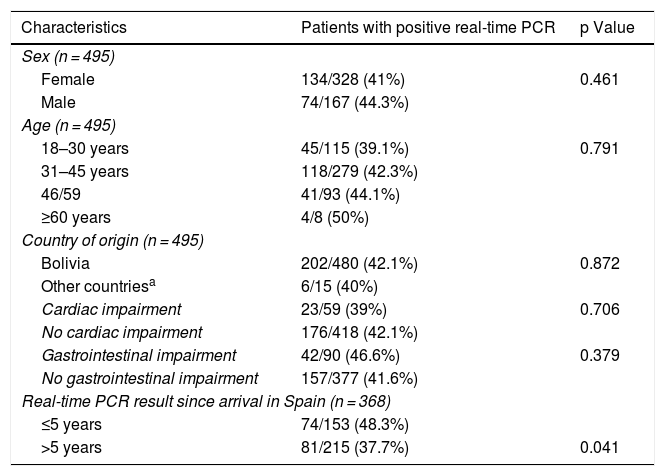
Real-time PCR was positive in a one-time determination before starting treatment in 208 (42%) of the patients. No significant differences in terms of age, sex, country of origin, or presence of cardiac and/or gastrointestinal abnormalities were found between patients with positive real-time PCR and patients with negative real-time PCR. Although a logarithmic scale was employed, the Ct value was used as an indirect indicator of parasite load. No significant differences were found in terms of Ct value between patients who showed visceral abnormalities and patients who did not (mean ± SD: 37.02 ± 2.6 vs. 36.8 ± 2.3; P = .55). Table 1 lists the clinical and epidemiological data for the patients with positive real-time PCR results.
Clinical and epidemiological characteristics of patients with positive real-time PCR results.
| Characteristics | Patients with positive real-time PCR | p Value |
|---|---|---|
| Sex (n = 495) | ||
| Female | 134/328 (41%) | 0.461 |
| Male | 74/167 (44.3%) | |
| Age (n = 495) | ||
| 18–30 years | 45/115 (39.1%) | 0.791 |
| 31–45 years | 118/279 (42.3%) | |
| 46/59 | 41/93 (44.1%) | |
| ≥60 years | 4/8 (50%) | |
| Country of origin (n = 495) | ||
| Bolivia | 202/480 (42.1%) | 0.872 |
| Other countriesa | 6/15 (40%) | |
| Cardiac impairment | 23/59 (39%) | 0.706 |
| No cardiac impairment | 176/418 (42.1%) | |
| Gastrointestinal impairment | 42/90 (46.6%) | 0.379 |
| No gastrointestinal impairment | 157/377 (41.6%) | |
| Real-time PCR result since arrival in Spain (n = 368) | ||
| ≤5 years | 74/153 (48.3%) | |
| >5 years | 81/215 (37.7%) | 0.041 |
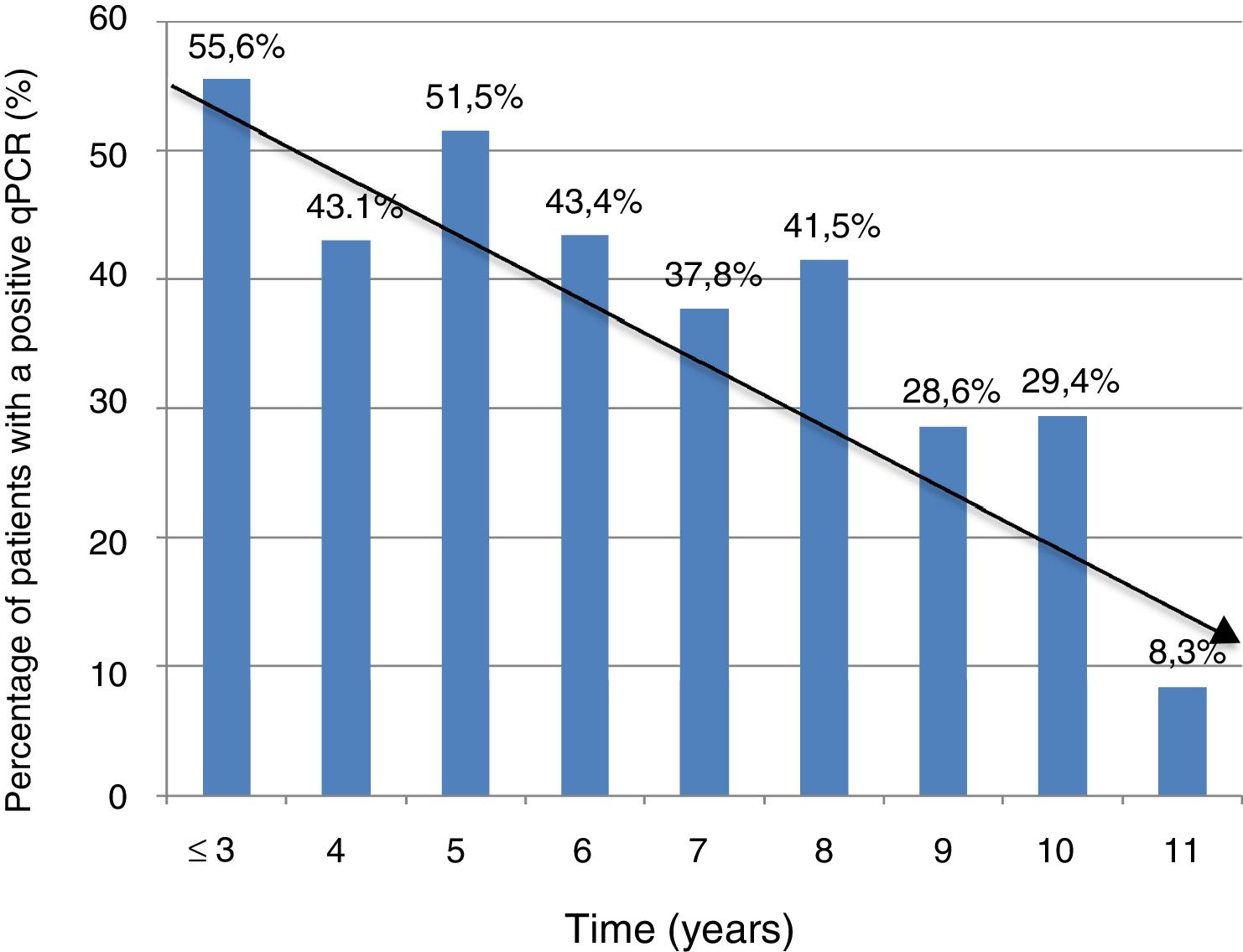
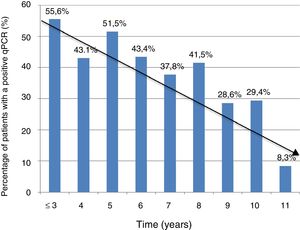
Information on time of residence in Spain was available in 368 cases (74.3%). The percentage of positive PCR was higher in patients with a shorter time of residence in Spain (5 years or less) compared to those with a longer time of residence in Spain (more than five years): 74/153 (48.3%) vs. 81/215 (37.7%); P = .041. However, there were no significant differences in terms of Ct values between the group of patients with a shorter residence time and the group of patients with a longer residence time (mean ± SD: 37 ± 2.3 vs. 36.8 ± 2.5; P = .74).
Figure 1 shows the trend towards decreased PCR positivity in relation to time of residence.
A subgroup of 38 patients could not be offered trypanocidal treatment due to lack of supply, and were given the option to have serial determinations every two months by PCR. Only 29 engaged in regular follow-up for a minimum of eight months. All patients were treated one time and the drug could be re-administered. Of these 29, 20 were women and 9 were men, and 96.5% were from Bolivia. Ten of them (34.5%) had resided in Spain for five years or more.
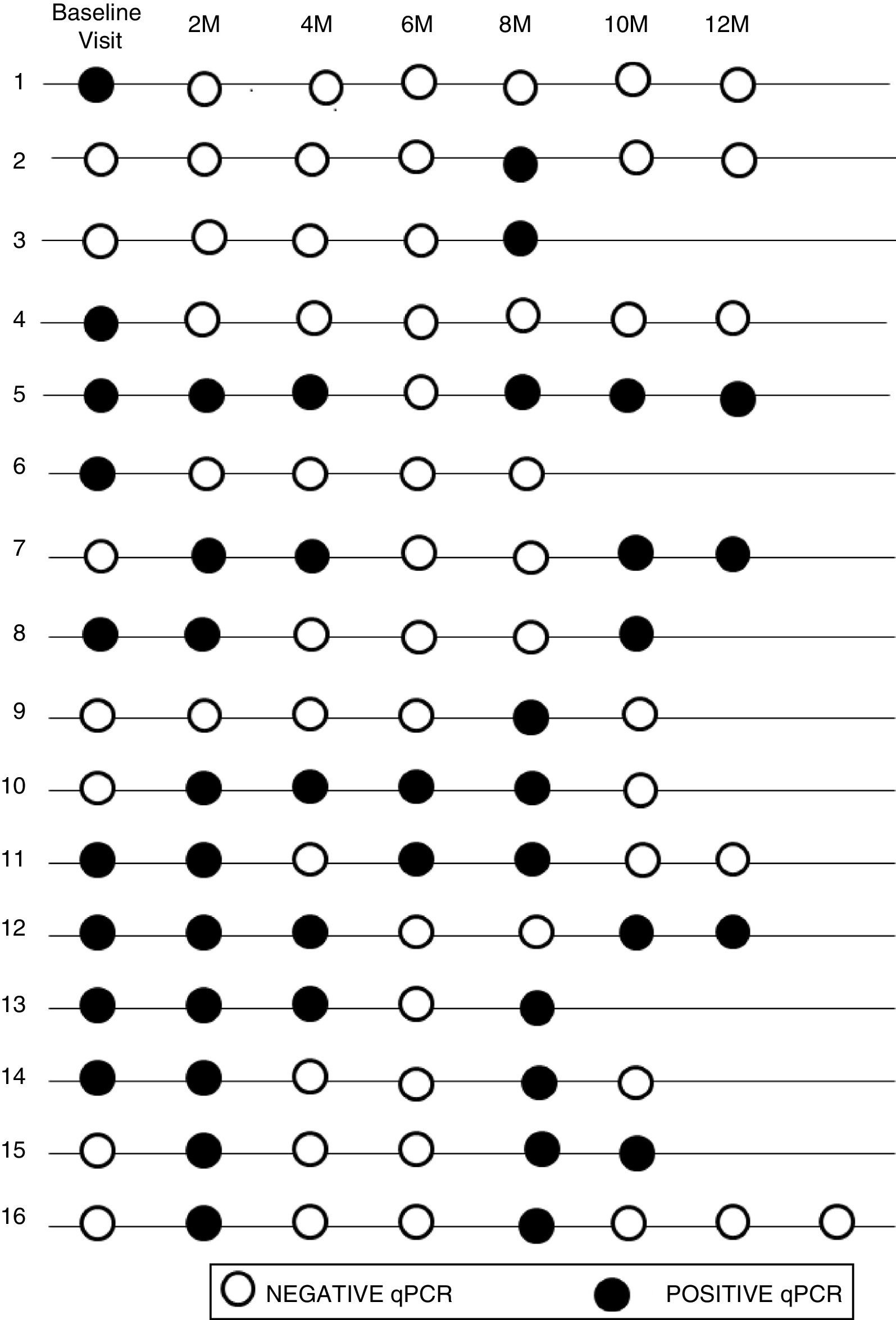
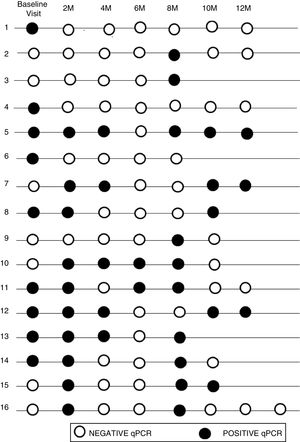
The initial real-time PCR result in this subgroup was positive in 13 patients (44.8%) and negative in 16 patients (55.2%). During follow-up, 9 (31%) of them had a sustained negative real-time PCR result, 4 (13.8%) had a positive real-time PCR result and 16 (55.2%) had intermittent parasitemia.
Figure 2 shows the timeline of real-time PCR results in follow-up of patients with intermittent DNA detection.
Of the 10 patients with a shorter time of residence in Spain, 20% presented sustained parasitemia, 2 patients (20%) were negative and 6 (60%) were intermittent. By contrast, of the 19 patients who had spent more time outside the endemic area, real-time PCR was positive in 2 cases (10.5%), negative in 7 cases (36.9%), and intermittent in 10 cases (52.6%).
Although 17/19 of the patients who had spent more time outside of the endemic area showed negative or intermittent PCR, the difference between the two groups was not significant (P = .59).
DiscussionThis study features real-time PCR results for a cohort of 495 patients with CD in the chronic phase who had not been treated with trypanocidal agents.
Real-time PCR enabled detection of parasitemia in 42% of patients. This percentage was similar to that obtained in other Spanish studies with percentages from 28% to 68%.13–16 This low level of detection reaffirms the limited diagnostic value of real-time PCR in the chronic phase, since a negative result does not rule out infection.
In addition, a positive real-time PCR result has not been linked to the presence of visceral abnormalities. These data are consistent with those obtained in other published series, both inside and outside the endemic area, and preclude one-time use of real-time PCR as a marker of disease progression.17 At present, only parasitemia detected in the third trimester of pregnancy is useful as a predictor, since it can be considered to be a risk factor for congenital transmission of the disease.18
The limit of detection of PCR depends directly on the protocols used and the number of amplifications. Currently, implementation of real-time PCR, which has allowed for the standardization of the technique, along with a EQA program, have helped to boost the quality of test results, which confirms the good sensitivity of PCR in detecting T. cruzi DNA.9,19 This study was one of the first to implement an EQA program with comparable results to those obtained by the reference laboratory.12
Setting aside the influence of the PCR protocol used, host- and parasite-related factors are the main determinants of the presence of the parasite in peripheral blood in the chronic phase and therefore the possibility of detecting parasite DNA by PCR. The fact that we did not enroll patients with potentially higher levels of parasitemia, such as pregnant women and immunosuppressed patients, might explain why we did not find higher percentages of positive results in our cohort.20
The percentage of detection of T. cruzi DNA in cohorts outside the endemic area is significantly lower than that found in endemic areas, which in some series exceeds 80%.8 These differences are also seen when comparing different Latin American countries to one another. In data drawn from the BENEFIT study, median levels of parasitemia detected in Argentina and Colombia were approximately 20 times higher than those of patients with comparable clinical characteristics in Brazil.21 This appears to be closely linked to different infection-causing parasite lineages.
The significant decrease in the percentage of positive real-time PCR results in patients who had resided in Spain for more than five years, compared to patients who had arrived in Spain more recently, may help explain the lower percentage of positive PCR results in series outside the endemic area, thus bolstering the notion of the influence of external factors on the dynamics of the parasite. These data are consistent with those reported in another Spanish series in 2010, and with the fact that rates of congenital transmission outside the endemic area are lower.15,22
Variables such as concomitant infection with other parasites, diet, and lifestyle are factors that may play a role. Concomitant infection with helminths, especially Strongyloides stercoralis, is common and significantly associated with greater detection of T. cruzi DNA in peripheral blood.23 This association reflects the immunomodulatory action of S. stercoralis in patients with CD.24 Other data suggest that T. cruzi is significantly flexible and adaptable to environmental changes and changes in nutritional status, such as those experienced by Latin Americans when they arrive in a new country.25
Real-time PCR is not a dynamic measure and only provides information on the time when the sample was taken. Regular and ongoing follow-up of a group of patients due to a BZL supply chain breakdown has allowed us to obtain information on the behavior of the parasite and its presence in peripheral blood in an area with no possibility of reinfection. In studies conducted in Brazil, both parasite load and parasite kinetics are directly linked to lineage.26 In our case, although we had not studied parasite lineage, all of them came from Bolivia, which suggests that this is not a determining factor.27 Genetic factors related to the host, associated with ongoing immunological monitoring for chronic infection, could promote the presence of the parasite in blood.28
Sustained positive, negative or intermittent detection of T. cruzi DNA, like one-time determination, has not been linked to development of visceral complications.
Despite these contributions, at present, real-time PCR's greatest utility in the chronic phase is as a marker of therapeutic failure in monitoring treatment with trypanocidal agents.29 In recent years, a positive real time-PCR result has also been used as an inclusion criterion and a marker of failure in clinical trials assessing new drugs.30
This study has certain limitations. First, it was a retrospective study; therefore, patient follow-up was not uniform. For the same reason, variables such as travel to endemic areas during follow-up to rule out possible re-infection, concomitant infection with S. stercoralis, and other diet- and lifestyle-related variables were not collected. Detection of genetic factors relating to the host and the T. cruzi lineage would enable parasitemia behavior to be better assessed.
ConclusionsDue to the low rate of positive results, real-time PCR cannot rule out T. cruzi infection in the chronic phase; this limits its use in diagnosis. This study also did not find any relationship to onset of cardiac and/or gastrointestinal visceral signs; hence, a positive PCR result is not a good marker of clinical progression. However, at present, it is the best marker of failure in treated patients.
Despite all this, real-time PCR in untreated patients provides information on the presence of the parasite in peripheral blood and points to factors that may be tied to its dynamics in the chronic phase. Better knowledge of these factors may aid in better interpreting PCR results in different situations, primarily when PCR is used to assess treatment efficacy.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sulleiro E, Salvador F, Martínez de Salazar P, Silgado A, Serre-Delcor N, Oliveira I, et al. Aportaciones de las técnicas moleculares en la fase crónica de la Enfermedad de Chagas en ausencia de tratamiento. Enferm Infecc Microbiol Clin. 2020;38:356–360.