Infection with human papillomavirus (HPV) is the leading cause of sexually transmitted infection worldwide. This virus generally causes benign lesions, such as genital warts, but persistent infection may lead to cervical cancer, anal cancer, vaginal cancer, and oropharyngeal cancer, although less frequently. Cervical cancer is a severe disease with a high mortality in some countries. Screening with cytology has been very successful in the last few years, but nowadays there are numerous studies that confirm that cytology should be replaced with the detection of HPV as a first line test in population based screening. There are several commercially available FDA approved tests for screening of cervical cancer. A new strategy, based on individual detection of the high risk genotypes HPV16 and HPV18, present in 70% of cervical cancer biopsies, has been proposed by some experts, and is going to be implemented in most countries in the future.
La infección por el virus del papiloma humano (VPH) es la infección de transmisión sexual más frecuente en el mundo. Este virus ocasiona generalmente lesiones benignas, como verrugas genitales, pero también su persistencia ocasiona procesos malignos, como cáncer de cuello de útero (CCU) y, menos frecuentemente, anal, vaginal y de la cavidad orofaríngea. El CCU es una enfermedad muy severa, con alta mortalidad en muchos países. El cribado de CCU con citología ha tenido mucho éxito en estos últimos años, pero hay innumerable evidencia científica para que sea sustituida por la detección del VPH como prueba inicial. Para esto, hay en el mercado gran cantidad de técnicas, siendo aconsejable utilizar sistemas automáticos y pruebas aprobadas por la FDA. Un nuevo algoritmo basado en la detección individualizada de los genotipos 16 y 18 presentes en el 70% de los CCU ha sido propuesto por expertos y su implantación será inmediata en algunos países.
The human papilloma virus (HPV) is a DNA virus belonging to the Papillomaviridae family that causes the world's most common sexually transmitted disease. It is generally acquired sexually, but it can also be contracted vertically from mother to child, by contact with the cervical mucosa during delivery, transplacentally and, less often, by horizontal transmission during infancy.1
The prevalence of sexually active women in the Spanish population is 14%, although this can vary according to the age group studied and the associated risk factors. From the age of 40 years the number reduces, to between approximately 5% and 6%.2
HPV specifically infects the basal cells of the squamous epithelium of the uterus, and exploits the cellular division of this area to replicate. The typical koilocytes, multinucleated cells and cells with enlarged nucleii form in the upper layer of the epithelium.1 These cytopathic changes are clearly visible with Giemsa or Pap staining (cytology) in cervical cytology tests. These provide the ideal samples for detecting the virus associated with the cervical disorder it causes.
In most cases, HPV infection is asymptomatic, transitory and can go undetected. In other cases the clinical manifestations are very diverse and range from simple warts and other benign processes to anogenital neoplasias as severe as cervical cancer (CC), anal cancer (AC), penile cancer (PC), cancer of the vagina, and even cancer in other distant anatomical sites such as the oropharynx and the oral cavity (OC). There is now sufficient scientific evidence to show that there is no doubt that the persistence of HPV DNA in the infected cell is an essential condition for the development of cancer.
More than 100 HPV genotypes have been identified, and it is estimated that approximately 40 of these are found in the genital and anal area. Benign manifestations, condylomas and genital warts are caused by non-oncogenic genotypes 6 and 11 (HPV 6, HPV11). These same genotypes also cause recurrent respiratory papillomatosis (RRP), where although rare, a recurrence of papillomas in the respiratory tract can cause death in children and adolescents with the condition.
In 1983, after many doubts and much controversy in the scientific community, zur Hausen et al.3 (1983) demonstrated the involvement of HPV in the aetiopathogenesis of CC, specifically genotype 16 (HPV16). One year later, genotype 18 (HPV18) was isolated that, along with HPV16, causes 70% of CC worldwide.4 Precisely because of their level of association with CC, the HPV genotypes are classified into “high oncogenic risk” genotypes (HR-HPV), comprising genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59; “probable/possible high oncogenic risk” (pHR-HPV) (68, 26, 53, 64,65, 66, 67, 69, 70, 73, 82), and “low risk, non-oncogenic” (LR-HPV). VPH6 and VPH11 are the most common of the latter.5
There are countless direct tests to detect the virus in cervical samples. Most are based on real-time polymerase chain reaction (PCR), signal amplification and detection of E6/E7 oncogene messenger RNA. However, the Food and Drug Administration (FDA) have only approved 4 tests for use in CC screening.1 Almost all of them are automated, very reproducible and highly sensitive in detecting the virus in women with premalignant lesions. For all these reasons, it is now agreed that the HPV test should be used as the initial or “first-line” test in CC screening to replace cytology or PAP staining, traditionally used for this purpose but shown in comparative studies not to be very sensitive and to be very subjective. Similarly, considering that HPV16 and HPV18 genotypes cause 70% of CC,4 all efforts have targeted primary prevention of these specific genotypes (bi- or quadrivalent vaccines adding HPV6 and HPV11) and detection with selective or partial genotyping (individual identification of HPV16 and HPV18, in addition to other HR-HPV), advised in population screening.
CC is the third most common cancer in women worldwide, with some differences in mortality rates according to the country. There is a mortality rate of up to 22.3% in Sub-Saharan Africa. It is estimated that 2511 new cases are diagnosed every year in Spain and there are about 848 deaths, i.e., approximately 2 women every day.6 These figures are extremely high for a disease that is now completely preventable in any developed country.
Furthermore, HPV not only causes high morbidity and mortality in women, but the malignant processes associated with this virus have increased in recent years as well, principally AC, PC and OCC. Therefore cancers located in the genital areas have increased annually by approximately 3% and OCC by 1%, the latter achieving an incidence of 6.2 and 1.4 per 100,000 in men and women, respectively. These figures might be higher in individuals infected by the human immunodeficiency virus (HIV), men who have sex with men (MSM) and immunosuppressed people, in general. Likewise, HPV16 is the prevalent genotype both in AC and OCC.7 The screening and vaccination criteria approved for CC also serve for AC, although this terrain is less studied and there are still no validated algorithms to confirm their efficacy.
There are other factors in HPV infection which should be considered, principally the social impact at certain stages of life. CC causes a reduction in a woman's life expectancy estimated at 29 years, considerably greater than breast cancer. Furthermore, adolescents with benign lesions are highly contagious. Therefore, the biological reality is that frequent exposure to HPV infection and reinfection in young people can have serious consequences for their health in the long term.
Virological aspects. Human papilloma virus and oncogenesHPV belongs to the Papillomaviridae family, and is a virus that generally infects the skin and the mucous membranes. It has been classified into HR-HPV and LR-HPV according to its oncogenic capacity.5 LR-HPV only cause genital warts and condylomas and other benign skin and mucous membrane conditions and association with CC is quite rare. Therefore, from a clinical and screening perspective, only HR-HPV must be detected.
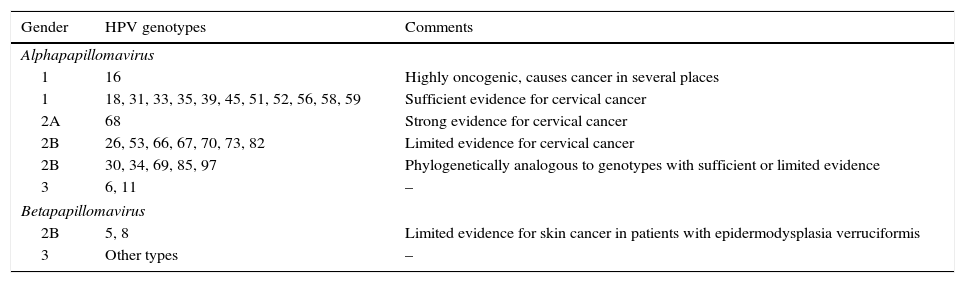
The HR-HPV group comprises genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59 (Table 1).5 Genotype 68 is considered of “probable” oncogenic risk and genotype 66 was previously considered high risk, and therefore they are included in many of the HPV DNA detection tests (HPV test) available on the market.
Classification of HPV genotypes according to their oncogenic capacity.4
| Gender | HPV genotypes | Comments |
|---|---|---|
| Alphapapillomavirus | ||
| 1 | 16 | Highly oncogenic, causes cancer in several places |
| 1 | 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 | Sufficient evidence for cervical cancer |
| 2A | 68 | Strong evidence for cervical cancer |
| 2B | 26, 53, 66, 67, 70, 73, 82 | Limited evidence for cervical cancer |
| 2B | 30, 34, 69, 85, 97 | Phylogenetically analogous to genotypes with sufficient or limited evidence |
| 3 | 6, 11 | – |
| Betapapillomavirus | ||
| 2B | 5, 8 | Limited evidence for skin cancer in patients with epidermodysplasia verruciformis |
| 3 | Other types | – |
HPV viruses are small, approximately 50–55nm in diameter, non-enveloped with an icosahedral capsid formed by 72 HPV capsomeres. Their genome comprises circular, double-stranded, covalently closed DNA, 7500–8,000pb in size. This DNA is divided into: (1) an early E region that codes various structural proteins (E1-E7); (2) a late L region that codes the capsid proteins (L1 and L2), and (3) a regulatory, non-coding region (RNC/LCR), located in direction 5′. The early region (E) represents 50% of the genome; the late region (L), 40%, and the regulatory region (RNC/LCR), 10%.
Relationship between the human papilloma virus and cancerIt has been demonstrated that in the majority of cases HPV infection is a necessary, although not unique, condition for the development of cervical intraepithelial neoplasia (CIN) and cancer. This association is based on various points8: (1) the virus is detected in more than 97% of CIN and invasive carcinomas, principally genotypes 16, 18, 31 and 45; (2) HPV infection has a greater relative risk for the development of premalignant lesions, compared with other possible associated risk factors, and the progression of the lesion is related to the type of HPV present in the lesion; (3) a grade 3 intraepithelial neoplasm (CIN3) is highly associated with chronic cervical infection by HPV 16 or HPV18 more than 2 years previously, and (4) there is an association between HPV and carcinomas of the vulva, vagina, PC and AC, and less frequently OCC, and carcinomas of the larynx, oesophagus and respiratory tract.
The integration of HPV DNA in the host cell chromosome is associated with the progression of high grade CIN to cancer.9 Integration occurs in most invasive carcinomas but is rare in premalignant and benign lesions. Integrated and episomal forms can coexist in the same cell. There are multiple insertion points for viral integration into the host genome and they can affect different chromosomes, in proximity to cellular oncogenes. The site of viral genome integration is characteristically between the end 3′ of E1 and 5′ of E2, resulting in rupture, loss or inactivation of the E2 ORF and the regulatory function of viral replication exercised by protein E2 on other proteins of the virus. Integration can also affect genes E1, E4, E5, L1 and L2 but never affects E6 and E7. Viral integration is not always necessary for malignant transformation; thus, some infections caused by LR-HPV have been associated with squamous cell carcinoma, and in those cells the virus, remaining episomal, would undergo deletions, mutations and amplifications in the genome.
Other endogenous factors associated with malignant transformation have been identified.10 Cellular oncogenes c-myc and c-ras can be activated by the integration of viral DNA in their vicinity. This is significant, since E7 can cooperate in the activation of c-ras and cause cellular transformation in vitro. Methylation can also alter the function of cellular and viral genes. Genetic abnormalities have also been found in crevical carcinomas affecting chromosome 1, and allelic losses in the short arm of chromosomes 3 and 17, and the length of chromosome 11. Suppressor genes such as p53 can be deleted in chromosome 17 and E6 and E7 proteins can cause chromosomal abnormalities in vitro. Some studies have associated HLA-DQ haplotypes with cervical cancer, suggesting that immunogenetic factors may possibly play a role.
Many exogenous factors play a cooperative role in malignant transformation associated with HPV. These include X and UV radiation, smoking, steroid hormones, vitamins A and D, retinoids, growth factors such as beta-TGF, epidermal growth factor and platelet-derived growth factor, cytokines such as TNF and alpha and gamma interferons, and viruses such as HIV and herpes.
Oncogenic proteins of the human papilloma virusThe E1 and E2 proteins are involved in replication of the virus, forming an essential complex as a transcriptional activator of the viral genome.11 E1 also helps to keep the virus in an episomal form and might be absent when the viral DNA remains integrated.
The E5 protein is bonded to the membrane and participates in the malignant transformation of the cell. However, its participation is not essential, since often the E5 gene is deleted in cervical cancer cells. It participates in the tumorigenesis process interacting with cell growth factor receptors, including the epidermal growth factor (EGF) receptor and, in the case of HPV-6, with erbB-2 and platelet-derived growth factor as well, interfering in the process of endocytosis and inactivation of these receptors. E5 also binds to a porin that participates in the endosomal proton pump, and its inactivation leads to inhibition in the acidification of the endosome, increasing the half life of the EGF receptor and promoting the functional action of the EGF of activating the c-fos and c-jun oncogenes.
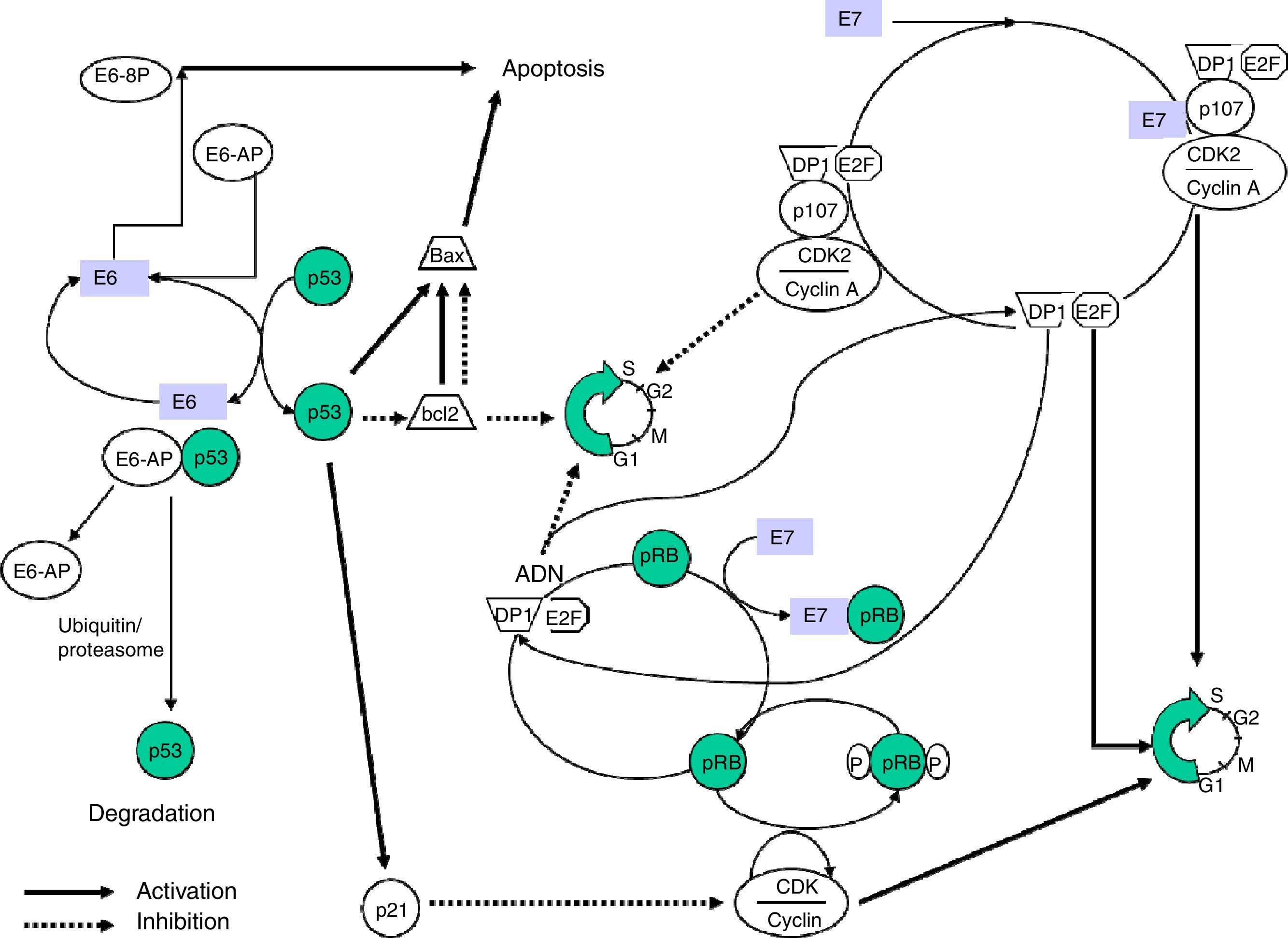
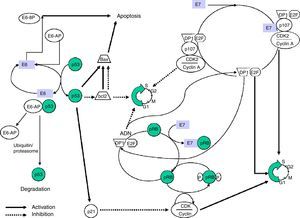
The E6 and E7 proteins play a central role in malignant transformation. Both bind to cellular factors with different affinity according to the viral genotype, which enables proteins with a high oncogenic capacity (HR-HPV) to be distinguished from others with little or no transforming power.
E6 is a protein that binds to bicatenary DNA. In combination with an associated protein, ubiquitin ligase (E6AP) it binds to p53,12 a very important protein in the regulation of the cellular cycle which is accumulated in the nucleus during the G1 phase of the cellular cycle. This protein detects damaged DNA and activates a series of genes that will inhibit the progress of the cellular cycle or will stimulate apoptosis. The p53 protein is degraded by the E6-AP complex by mechanisms linked to ubiquitin. In sum, it is a chain reaction in which, after the ubiquitin has been activated by the action of the E1 enzyme, it binds via a conjugating enzyme (E2 or UBC) with the target protein, which in turn binds to the ubiquitin ligase-protein (E3) and this complex is recognised by the proteosome and leads to proteolysis. The E6-AP linked to the HPV E6 oncoprotein and to p53 functions like an E3 enzyme and facilitates degradation of the target protein, p53 in this case, by the proteosome. The p53 protein contributes to halting the cellular cycle in phase G1, transactivating the WAF1/CIP1 gene, whose p21 protein directly interacts with the cyclin/cyclin-dependent kinase/proliferating cell nuclear antigen complex, responsible for phosphorylation and inactivation of the retinoblastoma protein (pRB).
Inactivation of p53 is not the only mechanism of carcinogenesis induced by E6.13 It has been observed that the presence of E6 reduces the capacity for p53 to bind the DNA by similar mechanisms to that used by other oncogenic viral proteins, such as the adenovirus E1A protein and the SV40 virus T antigen.
The capacity of E6 to induce genome instability has been demonstrated in cellular cultures, including aneuploidia and amplification. It has also been shown that E6 can bind to the c-Myc protein causing activation of Telomerase reverse transcriptase (hTERT). This enzyme restores the telomeres contributing towards the immortalisation of the cell.14
The E6 protein can bind to a calcium-linked protein, reticulocalbin-2 (E6-BP), associated with phenomena of apoptosis and cellular differentiation. E6 can also behave as a regulator of transcription and cooperate, along with the activated ras oncogen, in the transformation of primary rodent cells. The transformation of cell cultures infected by viral mutants incapable of degrading p53 has demonstrated that there are alternative mechanisms that are capable of turning the cell malignant, through activation of diverse cellular transcription factors (paxillin, AP-1, hDLG, IRF-3, Myc, hMCM7, Bak and E6TP-1) and activation of telomerase.
The E7 protein alters the functionality of pRB (retinoblastoma protein), a product of the tumour suppressor gene Rb-1 which, on binding to the EF-2 transcription factor and its associated DP-1 protein, curbs the cell cycle in G1 phase. E7-pRB binding modifies and inactivates its control function of the cell cycle collaborating in the transformation. E7 can also bind to the p107 and p130 proteins, with functions similar to pRB, triggering the same action. In the same way as E6, E7 has a regulatory activity of transcription of the ras oncogene in cell cultures and in transgenic animals (Fig. 1).
Obtaining transgenic mice that express E6 and E7 proteins separately has enabled their isolated effects on carcinogenesis to be examined, observing that E7 induces benign tumours, unlike E6, which causes malignant transformation of the cell.
The detection of episomal forms of HPV which cause cervical carcinoma has stimulated study of other factors that do not involve integration of the virus in the cell chromosome. Thus it has been demonstrated that mutations in the YY transcription factor binding site of the URR segment result in the progression of cervical cancer.
Natural history. Clinical forms of human papilloma virus infectionHPV is a pathogen that forms part of the human condition. It is well adapted to infect the epithelium and HPV infection is so common that its relationship with the host is almost inevitable. Unlike viruses that cause acute, serious disease, HPV infection is principally latent, subclinical and opportunist, with sporadic reproduction and transmission, and is generally found in a state of ecological balance with the host. In women, HPV infection has been associated principally with CC, cancer of the vagina and the vulva. In many industrialised countries the prevalence of HPV infections in young adult women is as high as 40–80% and the probability of becoming infected in a lifetime is 80–90%. Most of these infections disappear spontaneously with no clinical signs or symptoms. The natural history of HPV infection and the first steps of cervical carcinogenesis are well known from prospective studies.
CC is the final stage —fortunately rare— of an unresolved HPV cervical infection, in which the presence of HPV DNA persists in cervical samples.15 This persistence (more than 2 years) in the cervix is the event required for CC to develop. The fraction of persistent carriers of HPV in middle age is estimated in a range of between 4% and 10%, and these women are the real high risk group for CC, and probably for any other type of HPV-related cancer. The underlying endogenous and exogenous factors that drive the process for the infection to persist remain unclear. It takes from 2 to 4 decades from contracting the HPV infection to develop cancer,16 therefore the initial infection and cervical cancer precursor lesions are an appropriate target for screening and early detection.
HPV DNA is detected in most cancers of the vagina and their precursor lesions. Between 60% and 90% vaginal cancer cases and between 82% and 100% of vaginal lesions classified as Grade 3 intraepithelial neoplasia were HPV DNA positive. Similarly, HPV DNA has been found in cancers of the vulva, although the association with HPV infection with these cancers is lower, at around 40–50%.
In men, although PC is little known, the data reveal the participation of HPV, since HPV DNA is found in a third of PC cases and in 87.1% of high grade lesions.17
AC can be considered an emerging disease since it has been associated with HPV infection. It occurs principally in MSM but also in women whose cervices are infected. In both sexes, HPV DNA is detected in AC (88–94%).18
Finally, the malignant diseases that are associated with HPV include OCC. The prevalence of HPV DNA in these cancers varies greatly according to the different studies, the anatomical location of the tumour and geography, due to exogenous and cultural factors. The most consistent finding concerns cancer of the oropharynx, where HPV DNA has been found in between 35% and 50% in developed countries, unlike the rest of the oral cavity where HPV DNA is found in 5–15% of cases. E6/E7 mRNA expression has also been confirmed and the integration of the viral genome, and experimental models have shown the expression of E6/E7 oncogenic proteins as necessary for initiating and maintaining the malignant phonotype of these cancers.
HPV infection is also associated causally with benign disease such as warts, genital condylomas and upper respiratory tract diseases such as RRP. The HPV6 and 11 genotypes are the cause of both clinical pictures. RRP is characterised by the growth of multiple papillomas, usually in the laryngeal region. RRP can manifest in early infancy, in childhood or in adulthood and is a rare disease. The most important risk factor for childhood RRP is a maternal history of genital warts during pregnancy, whereas for adults it is their number of sexual partners and genital-oral sex. Many therapies have been tested for RRP, with limited success and, often, with serious side effects. It could be that the most effective therapy long term would be one that stimulates an efficient and persistent immune response through vaccination.
TransmissionHPV is the agent responsible for a highly contagious disease that affects human beings because of their sociable behaviour. Current data indicates that HPV transmission between heterosexual partners is extremely common, principally through contact with the skin of the genital area, but it can also be transmitted through contact with mucosa and biological fluids. Some cases from sharing sex toys have also been described.
Self-inoculation between the genital and anal regions is common in women. Studies show that HPV infections in the anal region in women and MSM are very common, especially in people infected with HIV. Likewise, anal HPV clearance is also common and few individuals display persistence, unless they are infected by HIV. This is a factor that strongly influences the development of the precursory stage of invasive anal cancer.
A high number of sexual partners will increase the risk of HPV infection. Other risk factors include unprotected sex—although it has been demonstrated that using condoms does not offer 100% protection – MSM relationships and reduced immunity.
EpidemiologyThe global prevalence of HPV infection in women with normal cytology is around 11–12%, with higher levels in Sub-Saharan Africa (24%), Eastern Europe (21%) and Latin America (16%). The maximum HPV rates are observed in women under the age of 25 years and reduce in the older age groups in many populations. Although in some populations there is a secondary spike of incidence in women during the early peri-menopause or the menopause. In other populations, like China, the prevalence of VPH is relatively independent of age. The explanation for the difference in these prevalence patterns and the clinical significance is not clearly understood. The 5 types of virus that are most common worldwide are HPV16 (3.2%), HPV18 (1.4%), HPV52 (0.9%), HPV31 (0.8%) and HPV58 (0.7%), although these estimations represent the point incidence and not the cumulative incidence of exposure, and can be underestimated.19
A recent study demonstrated that approximately 85% of CIN3 lesions, more than 70% of grade 2 intraepithelial neoplasia (CIN2) and half of grade 1 intraepithelial lesions (I1) are associated with infections by HPV6/11/16/18/31/33/45/52/58, from which it can be deduced that a nonavalent vaccine would offer protection against most oncogenic infections.
The incidence increases in women with cervical pathology directly proportionate with the level of development of the lesion determined by cytology, at around 90% in women with CIN3 and CC. Retrospective research studies have demonstrated that HP Vis found in almost 100% of all CC.
CC is the fourth most common cancer in women and the seventh in general, with an estimated 528,000 new cases in 2012 and 609,000 for 2020. Like liver cancer, a great majority (around 85%) of cases occur in the least developed regions, where it represents almost 12% of all cancers that affect women. The high risk regions, with an age-standardised rate above 30 per 100,000, include East Africa (47.7), Melanesia (33.3), Southern Africa (31.5) and Central Africa (30.6). The lowest rates are in Australia/New Zealand (5.5) and Western Asia (4.4). In Spain there were 2511 new cases in 2012 and 2710 are estimated for 2020. In terms of mortality, there were 848 deaths in 2010, and it is estimated that there will be 949 in 2020.20
Sample collection, transport, preservation and handlingSample collectionAppropriate samples for detecting HPV are taken by brush cytology or biopsy—depending on the location—usually collected in a liquid medium, in sterile, hermetically sealed containers.
The brushes used are sterile and of inert material. Natural materials such as cotton or wooden spatulas are avoided because they can inhibit PCR. It should also be remembered that samples with more than 2% v/v blood should be discarded, since haemoglobin can inhibit PCR.
Cervical brush cytology uses a brush specifically designed for collecting cells from the cervical canal (endocervical brush or cytobrush). The brush is inserted two thirds into the endocervical canal and gently rotated between 90 and 180 degrees or 5 times clockwise. If lesions are seen on the exocervix, a sample is taken from that region. Samples with blood are avoided, since haemoglobin can inhibit PCR. The brush is then inserted into a liquid transport medium. If the brush can be easily cut or the head breaks off, it is left inside. Otherwise, it is rubbed 10 times against the bottom of the vial with the transport medium and discarded.
Urine samples in women, although very easy to take, have been demonstrated to be less sensitive, and therefore are not recommended for CC screening.
In anal brushing, in women and in men, samples are taken from various areas at random.
In males, triple sampling from the glans penis, coronal groove and distal urethra (inserting the three brushes into the same vial) is recommended by most authors. Brush cytology, urine and semen can be used, but the results are poorer.
Brush cytology can be collected from other locations to detect the presence of HPV, but their positive or negative predictive value has not been established: brush cytology of the tonsils, inside the mouth and edges of the tongue, or mouth rinses with commercial antiseptic solutions in the case of a diagnosis of oral infection. It should be clarified that finding HPV in the oral cavity should not be used as screening for oropharyngeal cancer. In this case, the biopsy taken during surgical treatment of the lesion is a suitable sample for detecting HPV and this should be flagged up as particularly useful. Given that the prognosis is different according to whether or not HPV is detected, the test result will help to decide the most suitable duration and dose of chemo and radiotherapy.
In some clinical contexts biopsying warts can be useful (for example warts that are resistant to topical treatment or warty lesions of uncertain aetiology that require surgical removal). They will be sent in a sterile container on gauze with a saline solution base or in liquid medium. Formalin should be avoided, since it can inhibit PCR. If these are paraffinised biopsies, they can be extracted after deparaffinising with the solution indicated for the purpose.
Liquid media are routinely recommended (liquid cytology media) in which each commercial technique has been evaluated and that are occasionally the only vials recognised by automatic extraction/PCR equipment. The most widely used is Thin Prep® PreservCyt® Solution (Hologic Inc., Marlborough, MA, USA). BD SurePath™ (BD Diagnostics) is another liquid cytology medium which, unlike the former, contains formalin. This promotes bonding of the HPV DNA to the proteins, which can trigger fragmentation of said DNA interfering with its amplification and therefore reducing analytical sensitivity. As a consequence, SurePath™ samples should be pretreated before HPV detection.
Other liquid media that are suitable for PCR can be used, such as molecular biology grade TE buffer solution, pH 8.0. Although there are few studies that have compared the effect of diverse liquid transport media, some authors conclude that the result is not influenced by the transport media but by the analytical sensitivity of the tests used.
Sample transport and preservationBefore taking the sample, the liquid cytology vials are kept at ambient temperature. The samples are hermetically sealed, labelled with the patient's name and accompanied by clinical data request. The samples are transported as soon as possible to the microbiology laboratory at ambient temperature.
Handling of the sample in the laboratoryBecause this is an urgent test, the samples are processed in groups of greater or lesser size according to the system used. However, the DNA—especially the mNRA—can degrade after repeated cycles of freezing and defrosting, therefore it is always recommended not to freeze, keep in the fridge or delay processing of the sample. In general the transport media should be kept a maximum of 4–6 weeks at ambient temperature (15–30°C) before processing. Nucleic acid extracts should be kept at −20°C.
The biopsies will be crushed for subsequent nucleic acid extraction and frozen at −20°C.
Very mucosal samples can be homogenised beforehand with sterile glass balls and spun before extraction taking the necessary precautions to prevent contamination.
An appropriate work load, personnel training and frequency of PCR testing will make the results more reliable.
Commercial techniques for detecting human papilloma virusThere are more than 125 commercialised techniques for detecting HPV, with more than 84 variants, and the offer increases annually by approximately 20%. This is one of the most numerous groups of diagnostic techniques, but also the least regulated. In general, any technique can be used that has been validated for the use indicated, and will be selected depending on the processing capacity, training of personnel and the general organisation of the laboratory. However it is highly recommended that FDA approved, automated techniques are used because they offer advantages in terms of standardisation and quality control.
Clinical validation and FCA marking for use in screeningAny new technology that is going to be used in diagnosing cancer in general must have been demonstrated in controlled and randomised clinical trials to reduce the incidence of invasive cancer over time. HPV DNA detection has been demonstrated to do so with data from 8 years’ follow-up of patients included in 4 European trials.
Because it is not feasible to undertake longitudinal, randomised controlled studies with all the HPV tests that are marketed, an international committee of experts21 proposed in 2009 that all tests must be at least as precise as the techniques used in the abovementioned trials (gold standard: PCR GP5+/GP6+ and hybrid capture) and very reproducible in order to be used in primary screening for CC in women aged 30 years and over. To be specific, these validation criteria are based on clinical sensitivity and specificity, i.e., sensitivity and specificity in detecting cervical lesions. They must demonstrate sensitivity and specificity relative to the gold standard ≥0.90 and ≥0.98, respectively. Very high sensitivity will result in a very high negative predictive value (NPV) for the test, enabling the screening intervals in women with a negative result to be extended. These are the majority of the participants in a screening programme. As a counterpart, given the low incidence of premalignant lesions expected in this programme, minimal reductions of specificity might give false positive results the major repercussions of which would be increased unnecessary gynaecological follow-ups and their associated costs. These non-inferiority studies are usually undertaken by reference laboratories where intralaboratory reproducibility and interlaboratory concordance are also evaluated.
Furthermore, the VALGENT project (Validation of HPV genotyping tests)22 is an international network and a forum for validating HPV genotyping tests. It has collected 1300 cervical cytology samples provided by a large European laboratory with sufficient capacity in its biobank. These include a sufficient number of cytology samples of premalignant lesions and normal cytology samples to calculate the specificity and sensitivity of the HPV test in primary screening. The general reports of the evaluations are published in a list which clearly shows whether or not they meet the thresholds for use in clinical practice. The VALGENT project will enable screening programme organisations to make the best decisions as to which test is the most appropriate to use.
Likewise, in order to achieve FDA approval, a test must establish its sensitivity and clinical specificity through prospective studies completed in 3 or more different places that are evaluated by various committees in a lengthy and costly process. Its reproducibility must also be proved, the absence of cross-reactivity, contamination and carryover, and it must be verified that these characteristics are maintained through the maximum storage period specified by the manufacturer.
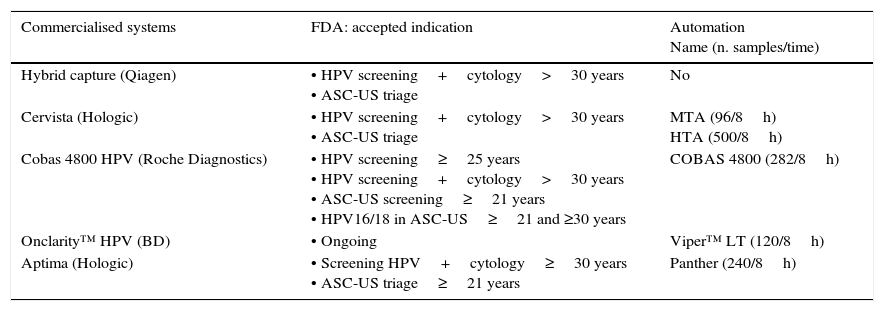
Below is a brief description of techniques approved by the FDA (Table 2). The 4 are clinically validated for primary screening and also have been approved by the FDA for ASCUS triage. It must be highlighted that only one technique has currently been approved for primary screening based on the detection of HPV (Cobas® HPV Test). None currently have been approved for post-treatment monitoring of cervical cancer, AC diagnosis, or detection in males or in the oropharynx.
Clinical indications of the FDA-approved HR-HPV techniques.
| Commercialised systems | FDA: accepted indication | Automation Name (n. samples/time) |
|---|---|---|
| Hybrid capture (Qiagen) | • HPV screening+cytology>30 years • ASC-US triage | No |
| Cervista (Hologic) | • HPV screening+cytology>30 years • ASC-US triage | MTA (96/8h) HTA (500/8h) |
| Cobas 4800 HPV (Roche Diagnostics) | • HPV screening≥25 years • HPV screening+cytology>30 years • ASC-US screening≥21 years • HPV16/18 in ASC-US≥21 and ≥30 years | COBAS 4800 (282/8h) |
| Onclarity™ HPV (BD) | • Ongoing | Viper™ LT (120/8h) |
| Aptima (Hologic) | • Screening HPV+cytology≥30 years • ASC-US triage≥21 years | Panther (240/8h) |
The Thin Prep® PreservCyt transport medium was used in all the FDA approved tests.
This is the first test approved by the FDA (March 2003) for detecting carcinogenic HPV genotypes, and is the method that has been most evaluated in the literature. It is a signal amplification technique that uses the high-risk probe cocktail, the latest version of which includes 13 types of HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68), and another for the low-risk group that includes genotypes 6, 11, 42, 43 and 44, which detects any of these genotypes in 2 unique reactions, although in CC screening only the HR-HPV should be detected. It does not distinguish the genotype present. It has some limitations, such as cross-reactivity with some LR-HVP that causes false positive results.
Cervista HPV HR® (Hologic)In 2009, the FDA approved the Cervista® HPV HR and Cervista® HPV16/18 tests for use in CC screening. This is a signal amplification technique using Invader technology. It comprises 2 isothermal reactions: the first is produced in the HPV DNA sequence and the second produces a fluorescent signal. It includes an internal control, the human histone gene 2. The result reports the presence of any of the 14 HR-HPV genotypes but does not detect them in an individualised way. Cervista HPV 16/18 identifies VPH16 and VPH18 individually.
Cobas® HPV test (Roche diagnostics)Cobas 4800 (Roche Diagnostics, Mannheim, Germany) is a completely automated system that consists of the Cobas Z thermocycler, Cobas X, and the software necessary for achieving a real-time PCR with primers targeting the L1 region of the HPV. Batches of 22–94 samples can be processed and it can perform 1344 tests over 24h using primary vials. The results appear differentiated in 4 channels: HPV16, HPV18, other non 16/18 HR-HPV 16, 18 (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 66) and beta-globin, which is utilised as internal control in each sample. It has high clinical sensitivity and has been evaluated in multicentre studies in Europe23 and in the United States (ATHENA study)24 with high concordance with the results obtained by other benchmark techniques. It is worth noting that no cross-reactivity with LR-HPV was observed in the positive results. The reproducibility and the degree of automation are very high. All these characteristics make it one of most widely used and preferred, totally automated systems for CC screening.
Aptima HPV Assay® (Hologic)Aptima® detects the presence of mRNA of the E6 and E7 oncogenes of 14 genotypes. It utilises TMA technology and detection of products amplified by hybridisation. It can be undertaken on the Panther automated platform. If ThinPrep® is used; the sample must be transferred to specific tubes. It does not perform partial genotyping, but there is already a prototype that identifies HPV16 and HPV18/45. It has no human cellularity control. In published studies, this method has been demonstrated to be as sensitive as tests that detect HPV DNA yet with greater specificity, which is promising. Data from randomised clinical trials with this mRNA test were analysed at 3 years. However, we should wait for the low risk of >CIN3 after a negative mRNA result to be demonstrated over a longer period of time so that there is no doubt as to its usefulness in cervical cancer screening at intervals of 5 years or more.
QualityThe microbiology laboratory where the HPV test is performed must have certain essential requirements22 as well as the appropriate infrastructure and must follow the directives for good laboratory practice and standard procedures. It is also recommended that the laboratory is accredited for clinical molecular tests, and participates in regular intra and interlaboratory assessments.
The laboratories that for years have processed large amounts of molecular biology tests for diagnosing various pathogens, usually easily meet these quality requirements because they have the appropriate conditions for handling samples and the facilities to prevent contamination. General precautions will also apply to them for handling infectious substances, common to all microbiology services.
Internal quality control, external quality evaluation and ongoing quality improvement are important for quality assurance. The WHO network of HPV laboratories (WHO HPV LabNet) has established, among others, the first international standard for DNA of HPV16 and HPV 18. It also periodically leads genotyping evaluation studies where a test is considered adequate if it detects 50IU of HPV16 and HPV18, as well as 500 genome equivalents from the other 12 types included on the panel in both coinfection and monoinfection. To consider a test adequate it must also have a zero false positive rate. Although it is a control of the analytical validation of genotyping tests, the creation of a panel to evaluate screening tests is not ruled out in the future. Other noteworthy external quality controls are UKNEQAS (United Kingdom National External Quality Assessment Service) and QCMD (European Quality Control for Molecular Diagnostics).
ChallengesThe first challenge has to be that only clinically validated or FDA-approved tests can be used for CC screening. We also have to bear in mind the specificity and sensitivity discrepancies between the different tests when used in screening programmes, even when comparing totally automated and FDA approved commercial tests.25
Fully assessed tests with high clinical sensitivity and specificity must be used. It is also advisable for them to be totally automated and reasonably priced.
Another major challenge is to obtain scientific evidence in vaccinated populations, since all the studies of sensitivity and specificity of screening have been undertaken in non-vaccinated populations. The performance of diagnostic tests could vary in vaccinated population screening.
Improving the specificity of the HPV test is an objective for the near future, since, due to the high incidence of HPV in people younger than 30, HPV detection cannot be used for screening women younger than this age.
Finally, it would be very useful from a clinical perspective to have validation criteria to predict the risk of recurrence of premalignant lesions in the first 2 years post treatment. This risk is high, approximately 18%, and the sensitivity of the different HPV tests in predicting this failure can vary widely.26
Cervical cancer screeningThere is unanimous agreement among experts that there must be population screening for CC, since opportunistic screening with a view to solving an individual problem for women who request it has been demonstrated to be totally “inefficient, ineffective and unfair”.27 The principal objective of CC screening is to rule out the presence of premalignant lesions in healthy women, but it has a secondary objective, also very important, to detect women with grade 2 intraepithelial cervical neoplasia or more severe lesions (>CIN2) according to the Bethesda28 system, in order to start their treatment. It is worth remembering that cervical lesions can be defined as mild (CIN1) moderate (CIN2) or severe (CIN3).
Utilisation of the human papilloma virus test in cervical cancer screeningThe major advantages of the HPV test are (a) it has a very high NPV, almost 100%. This means that if the result of the test is negative, that woman has almost no probability of developing premalignant lesions in a minimum period of 5 years. This will limit the screening rounds to from 5 to 7 depending on the age started or the protocols adopted by the different countries. And (b) it has very high sensitivity, much higher than that of cytology, (c) it is very reproducible and less subjective, since sometimes the result of cytology is influenced by the experience of the examining cytologist, (d) it is totally automated, therefore a large number of samples can be processed with reliable results, and (e) the influence on the cost is also significant, according to studies undertaken.
For all these reasons, there is currently no doubt that the HPV test should replace cytology as the initial test in CC screening. But it should also be considered that the weakness of the HPV test is its specificity, slightly lower than that of cytology. As a consequence, if a positive result is obtained in the initial HPV detection test, a second test is required, termed triage test, with a view to stratifying women according to their risk for premalignant lesions in the years to come. The objective of this triage test is to save unnecessary colposcopies and treatments for women with negative cytology and positive HPV, since they have a minimal risk of suffering CC in the future. Cytology is the triage test that is advised in the Spanish Obstetrics and Gynaecology Society Guidelines, and has been used in Holland and all the countries with the most experience and best effectiveness in CC screening. However, several studies have been published recently evaluating other options with very good results, such as partial genotyping (separate detection of genotypes 16 and 18) or dual staining, although the latter needs further studies to support the good results achieved.
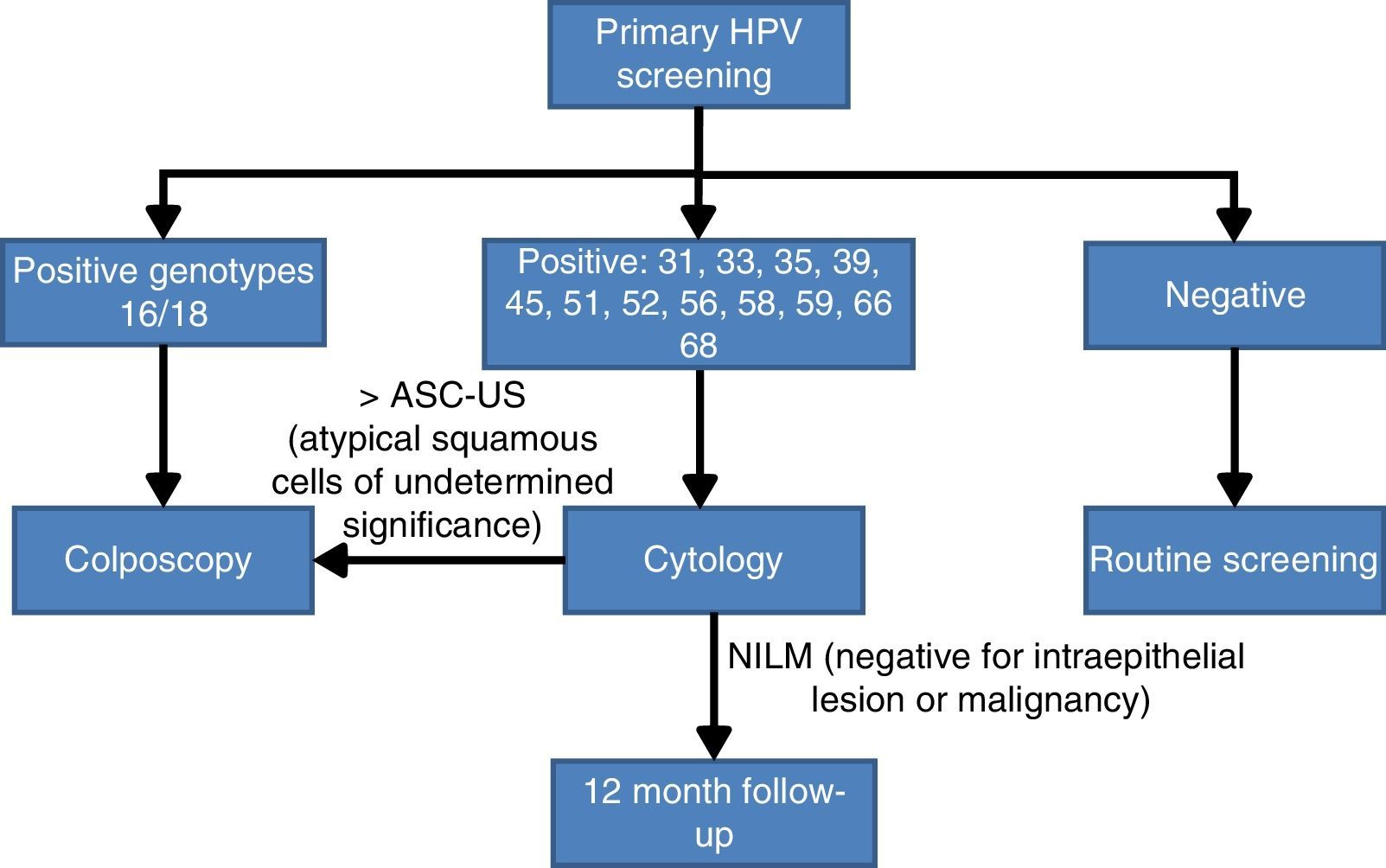
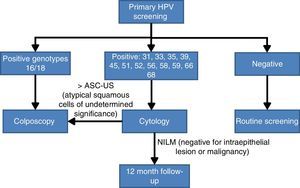
Screening algorithm based on partial human papilloma virus genotypingThe choice of partial HPV genotyping as a triage test after a positive result from the HPV detection test is a consequence of the importance of the persistence of genotypes HPV16 and HPV 18 in the development of premalignant lesions. One of the first studies was that of Khan et al.,29 that demonstrated that the cumulative incidence of lesions >CIN3 at 12 years follow-up is 20% when HPV 16 is detected, 15% when HPV 18 is present and only 2% if the result is positive for other non 16/18 HR-HPV genotypes. A second very recent study with similar conclusions is the ATHENA study,24 whose principal objective was the approval of the Cobas 4800 system by the FDA for use in population screening for CC. In this study performed in the United States, 42,209 healthy women were included and the conclusions clearly confirm the importance of detecting the presence of genotypes 16 and 18 in cervical brushings and distinguish them from other non 16/18 HR-HPV. The most conclusive results were that women with negative cytology but with a positive result for HPV16 or HPV18 have an estimated absolute risk in the following 3 years of presenting premalignant lesions, and grade 3 or more severe intraepithelial neoplasias (>CNI3) of 9.8%. By contrast, if the genotypes present are non 16/18 HR-HPV, the estimated risk reduced to 2.4%.
Bearing in mind the abovementioned scientific evidence on the importance of establishing whether HPV16 and HPV18 are present in the cervix, a panel of experts30 reached an agreement on the CC screening algorithm based on the HPV test with partial genotyping as an initial test. Women with a positive result for the most oncogenic genotypes HPV 16 or HPV 18 will be studied directly with colposcopy. By contrast if they are infected by other HR-HPV, cytology will be used as the triage test for follow up at 12 months if the result is negative for intraepithelial lesions or malignancy (NILM), or monitoring with colposcopy (Fig. 2).
Population screening algorithm based on partial genotyping.30
This algorithm seems to meet the requirements of being “acceptable, effective, efficient and based on current best scientific evidence”, established by the health authorities for CC screening.
Situation of cervical screening in SpainThere is no national CC screening plan in our country, which results in what could be described as a chaotic situation. A National Health System report on the situation of CC screening in Spain in 2014 (Spanish Health Ministry, Social and Equality Services); www.cribadodecancer.es/images/archivos/PAISVASCO2015/28 indicates that:
- -
Screening is opportunist in 16 autonomous communities and 2 autonomous cities.
- -
Cytology is the most commonly used first screening test, although the HPV test is gradually being implemented, which is used as primary screening in 6 autonomous communities (cotest) and as the initial test in one autonomous community.
- -
Devolved regional screening programmes are undergoing a process of review in 13 autonomous communities, and primary screening is already planned in 7 using the HPV test as the initial test.
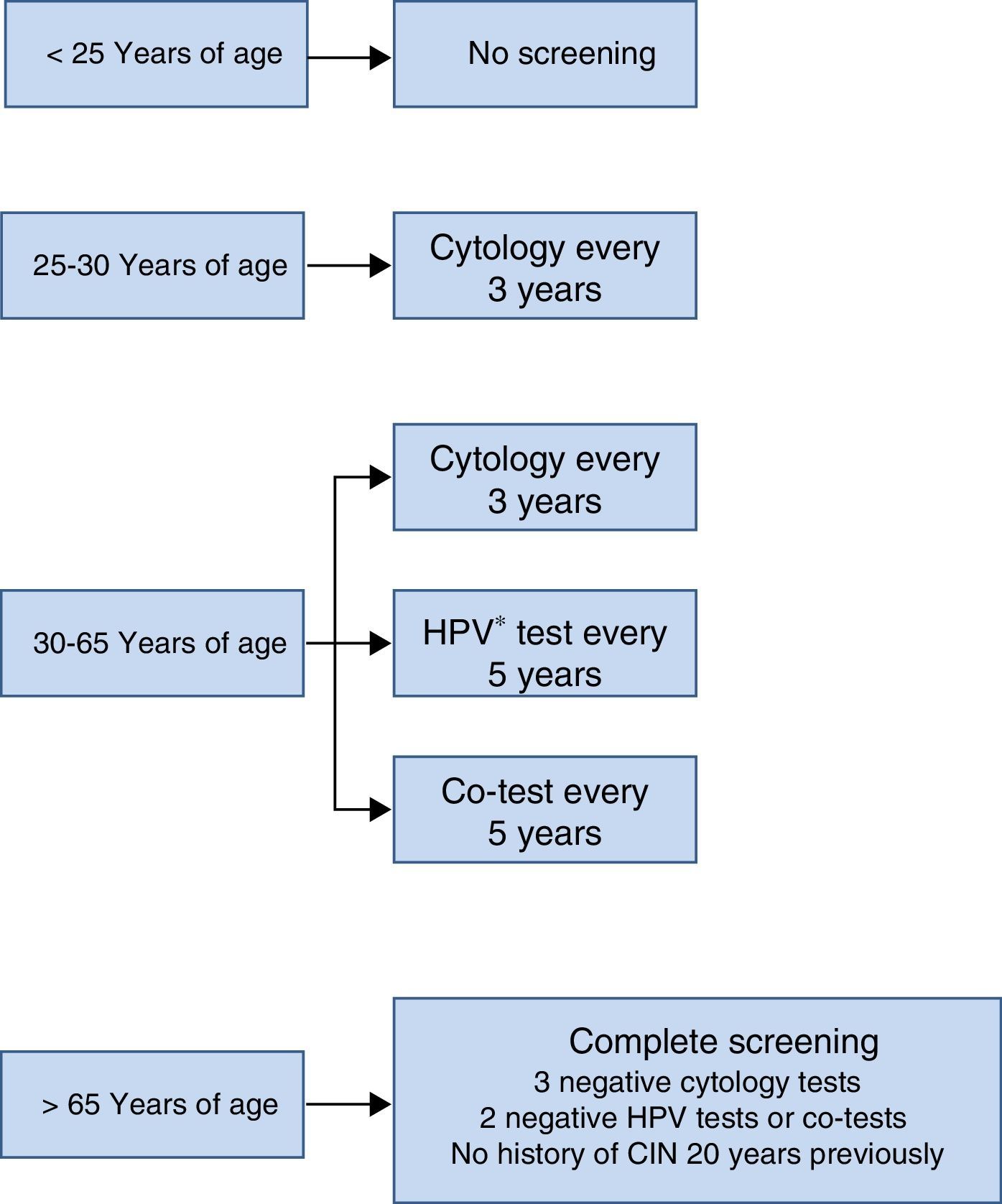
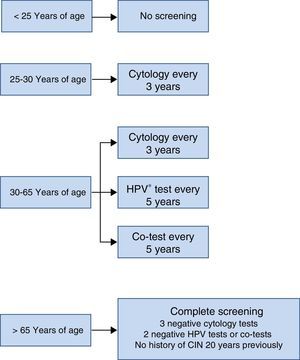
The screening scheme recommended in the Consensus Document prepared by various scientific societies in 2014 is summarised in Fig. 3. Most noteworthy is that screening should not start under the age of 25 years, that between the ages of 25 and 30 cytology is recommended as the initial test due to the high incidence of HPV infection in that age group and that from the age of 30, as mentioned earlier, the HPV detection test is the preferred option, because it provides greater benefits.
That said, whatever the chosen screening strategy, it should be remembered that 50% of women with CC have never participated in a screening programme and 10% have done so more than 5 years ago. Therefore, whether by cytology, if there are no better methods available, by HR-HPV testing, with or without partial genotyping, our primary objective must be to implement population screening. This means covering the entire target population, women aged between 30 and 60 years. Screening must be easy to access, have wide coverage and be performed under the same conditions in all of Spain's autonomous communities. The principal function of microbiologists as a collective should be to help achieve the aforementioned public health objectives, applying our knowledge and using the most sensitive and specific method of detecting HPV according to best scientific evidence. Only thus will there be any hope of eradicating this serious, totally preventable disease from most countries.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Mateos-Lindemann ML, Pérez-Castro S, Rodríguez-Iglesias M, Pérez-Gracia MT. Diagnóstico microbiológico de la infección por virus del papiloma humano. Enferm Infecc Microbiol Clin. 2017;35:593–602.