Some studies have observed an increased incidence of necrotizing pneumonia (NP) in recent years. This might be related to the emergence of non-vaccine S. pneumoniae serotypes after PCV7 introduction although it is suggested that evolutionary factors may have modified the virulence and the interactions of pneumococci. The aim of this study was to clinically and microbiologically define NP in the population served by the three major paediatric hospitals in Barcelona (Catalonia, Spain).
MethodsA prospective observational study was conducted in patients <18 years hospitalized due to invasive pneumococcal disease (January 2012–June 2016). Data of confirmed cases of pneumococcal NP (diagnosed by culture or DNA detection and serotyped) were collected. PCV13 was not systematically administered in Catalonia during the study period, but was available in the private market so the vaccination coverage in children increased from 48.2% to 74.5%.
Results35 cases of NP were identified. 77.1% of cases were associated with empyema. In the first 4 years, a trend to a decrease in NP incidence was observed (p=0.021), especially in children <5 years (p=0.006). Serotype 3 was responsible for 48.6% of NP cases. Five patients with NP due to serotype 3 were fully vaccinated for their age with PCV13.
ConclusionsSerotype 3 has a preeminent role in pneumococcal NP and was associated with all PCV13 vaccination failures. Although in our series the incidence does not seem to be increasing, evolution of pneumococcal NP rates should be monitored after inclusion of PCV13 in the systematic calendar.
En algunos estudios se ha observado un aumento de la incidencia de neumonía necrosante (NN) en los últimos años. Dicho aumento podría estar asociado a la aparición de serotipos de S. pneumoniae no vacunales después de la introducción de la PCV7, aunque se sugiere que factores evolutivos podrían haber modificado la virulencia y las interacciones de los neumococos. El objetivo de este estudio fue definir clínica y microbiológicamente la NN en la población tratada en los 3 hospitales pediátricos principales de Barcelona (Cataluña, España).
MétodosSe llevó a cabo un estudio observacional prospectivo en pacientes <18 años hospitalizados a causa de una enfermedad neumocócica invasiva (enero de 2012-junio de 2016). Se recopilaron datos de casos confirmados de NN neumocócica (diagnosticada mediante cultivo o detección de ADN y serotipado). La PCV13 no se administró de forma sistemática en Cataluña durante el periodo del estudio, pero se encontraba disponible en el mercado privado, por lo que la cobertura de vacunación en niños pasó del 48,2 al 74,5%.
ResultadosSe identificaron 35 casos de NN. El 77,1% de los casos estuvieron asociados a un empiema. En los primeros 4 años se observó una tendencia decreciente de la incidencia de NN (p=0,021), especialmente en niños <5 años (p=0,006). El serotipo 3 causó el 48,6% de los casos. Cinco pacientes con NN debida al serotipo 3 estaban completamente vacunados para su edad con la PCV13.
ConclusionesEl serotipo 3 desempeña un papel prominente en la NN neumocócica y se asoció a todos los fracasos de vacunación de la PCV13. Aunque en nuestra serie la incidencia no parece estar aumentando, debe controlarse la evolución de las tasas de NN neumocócica tras la inclusión de la PCV13 en el calendario sistemático.
Necrotizing pneumonia is a complication of bacterial pneumonia in which areas of necrosis develop within pulmonary condensation. Streptococcus pneumoniae is the most frequent etiologic agent in children, but bacteria such as Staphylococcus aureus,Mycoplasma pneumoniae and Streptococcus pyogenes, among others, have also been reported as causal agents.1–4 The pathophysiology of necrosis consists of the production of bacterial toxins and vascular changes related to inflammation, including intravascular thrombosis, leading to liquefaction and the subsequent cavitation of the lung parenchyma affected.5,6 Recently, an association between the severity of necrosis and bacterial load and high levels of some proinflammatory cytokines (TNF-ɑ, IL-1β and IL-8) in pleural fluid has been demonstrated.7
The clinical course varies in severity, although generally patients show alterations in the health status and prolonged fever in spite of antibiotic treatment. Necrotizing pneumonia is frequently associated with pleural effusion or empyema and may be complicated by respiratory failure and/or sepsis and bronchopleural fistulas.8–10 Infrequent late complications such as pneumothorax or tension pneumatocele are reported in patients with residual air cavities.11
Chest X-ray shows radiolucent areas within the consolidated areas that allow necrotizing pneumonia to be suspected, although the frequent association with pleural effusion or dense consolidations may make visualization difficult. Thoracic ultrasound is a very useful complementary examination to confirm the diagnosis and assess vascularity in the area of pneumonia. Occasionally, thoracic computed tomography (CT) may be necessary.
Although necrotizing pneumonia is a relatively infrequent complication, studies have shown an increased incidence in recent years, analogously to cases of pneumonia with pleural effusion or empyema.1,8,12,13 This might be related to the emergence of non-vaccine S. pneumoniae serotypes after the introduction of the 7-valent vaccine3,14 although it is suggested that evolutionary factors may have increased the virulence of pneumococci in their interaction with the host.7 The aim of this study was to define, clinically and microbiologically, necrotizing pneumonia in the population served by three paediatric hospitals in Barcelona (Catalonia, Spain) during a period when the 13-valent pneumococcal conjugate vaccine (PCV13) was already available in the private market but not yet part of the public health system-financed Catalan vaccination calendar. The work also tries to provide the first epidemiological data of necrotizing pneumonia in the PCV13 era.15
Materials and methodsA prospective observational study of cases of invasive pneumococcal disease (IPD) was carried out in patients aged <18 years admitted to three paediatric hospitals in Barcelona (Hospital de Nens, Hospital Sant Joan de Déu and Vall d’Hebron University Hospital, the last two of them being the largest tertiary paediatric hospitals in Catalonia). The three hospitals represent 31.9% of total paediatric admissions in Catalonia, an autonomous community located in the northeast of Spain. The estimated reference population aged <18 years of these hospitals is 442,761. An active IPD case surveillance protocol was implemented to collect the patients.
The study period was January 2012 to June 2016. PCV13 had been available on the private market since 2010 but was not included in the public health system-financed immunization schedule of Catalonia until July 1, 2016.16 According to data previously published by our group, the estimated PCV13 vaccination coverage in the reference paediatric population for the three hospitals was 48.2% in 2012, 63.9% in 2013, 68.5% in 2014 and 74.5% in 2015.17
Information was collected on demographic, epidemiological, clinical and microbiological variables of cases diagnosed, and the vaccination status (verified using vaccination card and/or electronic medical record). Informed consent was obtained in all cases.
Cases of IPD were defined according to the parameters of clinical diagnosis in daily practice in addition to microbiological diagnosis by culture or real-time polymerase chain reaction (PCR) in normally-sterile samples (blood, pleural fluid). The S.pneumoniae DNA was detected by RT-PCR, amplifying the pneumolysin (ply), autolysin (lytA), and wzg (cpsA) genes. Only samples positive for ply/lytA or wzg were included in the study.18
Strains isolated by culture were serotyped using the Quellung reaction or dot blot by the National Centre for Microbiology, Majadahonda, Madrid, Spain. Culture-negative and PCR-positive samples with a cycle threshold (Ct) >30 cycles were serotyped using a previously-described, real-time multiplex PCR technique.18 PCR-positive samples with a Ct ≤30 cycles were serotyped using sequential multiplex PCR combined with fragment analysis and automated fluorescent capillary electrophoresis.19
Pneumococcal necrotizing pneumonia was defined as a clinical entity in which areas of cavitation were observed on imaging studies. In all cases, a paediatric radiologist endorsed the diagnosis of necrotizing pneumonia with the information available in each patient.
Any dose of PCV13 given after six weeks of age, at least four weeks after the previous dose, and at least 15 days before the hospital admission date, was considered valid. According to the PCV13 data sheet,20 we distinguished between unvaccinated patients, completely-vaccinated patients, patients with incomplete but age-appropriated vaccination, and not up-to-date for age vaccinated patients (children who could have received the full age-appropriate regimen but had received an incomplete regimen).
Statistical analysisThe characteristics of necrotizing pneumonia cases were compared with those of other forms of pneumonia. Three groups were distinguished: necrotizing pneumonia, pneumonia with pleural effusion or empyema without signs of necrosis (complicated pneumonia), and uncomplicated bacteremic pneumonia. Quantitative variables were compared using the non-parametric Kruskal–Wallis test. Categorical variables were compared using Pearson's Chi-square test or Fisher's exact test. The incidence of necrotizing pneumonia was calculated for each semester of the study period in all patients and in children aged <5 years. The reference population of both age groups was used as the denominator. The chi-square trend test was used to determine differences in the incidence during the study period. A value of p<0.05 was considered statistically significant. The analysis was performed using the SPSS v24 statistical package and Stata.
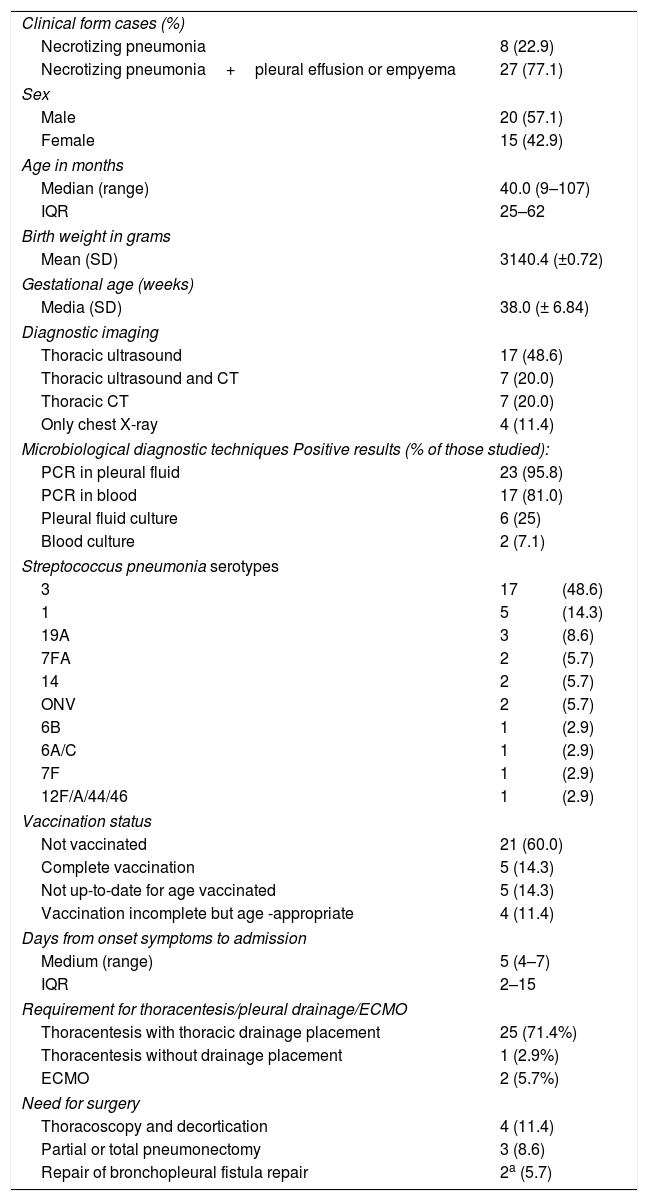
ResultsDescription of the casesDuring the study period, 35 cases of necrotizing pneumonia (18%) were identified out of a total of 194 cases of pneumococcal pneumonia. This proportion is higher than that described in other previous studies since not all cases of community-acquired pneumonia are taken into account, but only those cases of pneumonia with microbiological confirmation of pneumococcal aetiology (PCR or culture of pleural fluid or blood).21Table 1 shows the main characteristics of patients with necrotizing pneumonia: 74.3% of patients were aged <5 years. Seven patients (20%) had received home oral antibiotic treatment before hospitalization. No patient had risk factors for IPD or relevant comorbidities: 77.1% of necrotizing pneumonia cases had pleural effusion or empyema. No patient with necrotizing pneumonia was vaccinated against influenza in the season corresponding to the diagnosis.
Characteristics of patients with pneumococcal necrotizing pneumonia.
| Clinical form cases (%) | ||
| Necrotizing pneumonia | 8 (22.9) | |
| Necrotizing pneumonia+pleural effusion or empyema | 27 (77.1) | |
| Sex | ||
| Male | 20 (57.1) | |
| Female | 15 (42.9) | |
| Age in months | ||
| Median (range) | 40.0 (9–107) | |
| IQR | 25–62 | |
| Birth weight in grams | ||
| Mean (SD) | 3140.4 (±0.72) | |
| Gestational age (weeks) | ||
| Media (SD) | 38.0 (± 6.84) | |
| Diagnostic imaging | ||
| Thoracic ultrasound | 17 (48.6) | |
| Thoracic ultrasound and CT | 7 (20.0) | |
| Thoracic CT | 7 (20.0) | |
| Only chest X-ray | 4 (11.4) | |
| Microbiological diagnostic techniques Positive results (% of those studied): | ||
| PCR in pleural fluid | 23 (95.8) | |
| PCR in blood | 17 (81.0) | |
| Pleural fluid culture | 6 (25) | |
| Blood culture | 2 (7.1) | |
| Streptococcus pneumonia serotypes | ||
| 3 | 17 | (48.6) |
| 1 | 5 | (14.3) |
| 19A | 3 | (8.6) |
| 7FA | 2 | (5.7) |
| 14 | 2 | (5.7) |
| ONV | 2 | (5.7) |
| 6B | 1 | (2.9) |
| 6A/C | 1 | (2.9) |
| 7F | 1 | (2.9) |
| 12F/A/44/46 | 1 | (2.9) |
| Vaccination status | ||
| Not vaccinated | 21 (60.0) | |
| Complete vaccination | 5 (14.3) | |
| Not up-to-date for age vaccinated | 5 (14.3) | |
| Vaccination incomplete but age -appropriate | 4 (11.4) | |
| Days from onset symptoms to admission | ||
| Medium (range) | 5 (4–7) | |
| IQR | 2–15 | |
| Requirement for thoracentesis/pleural drainage/ECMO | ||
| Thoracentesis with thoracic drainage placement | 25 (71.4%) | |
| Thoracentesis without drainage placement | 1 (2.9%) | |
| ECMO | 2 (5.7%) | |
| Need for surgery | ||
| Thoracoscopy and decortication | 4 (11.4) | |
| Partial or total pneumonectomy | 3 (8.6) | |
| Repair of bronchopleural fistula repair | 2a (5.7) | |
ONV: other non-vaccine serotypes not specified in serotyping.
ECMO: Extracorporeal membrane oxygenation.
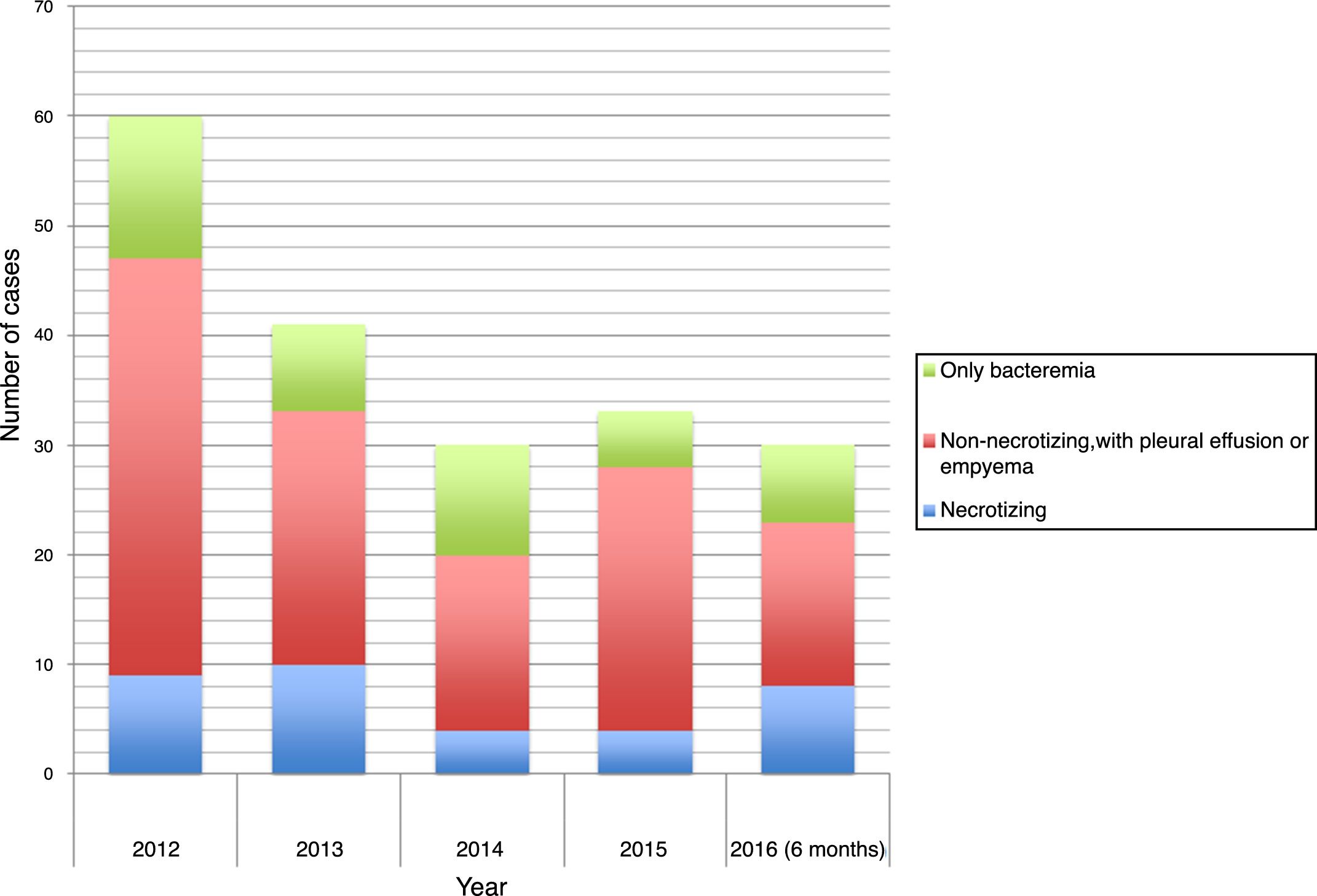
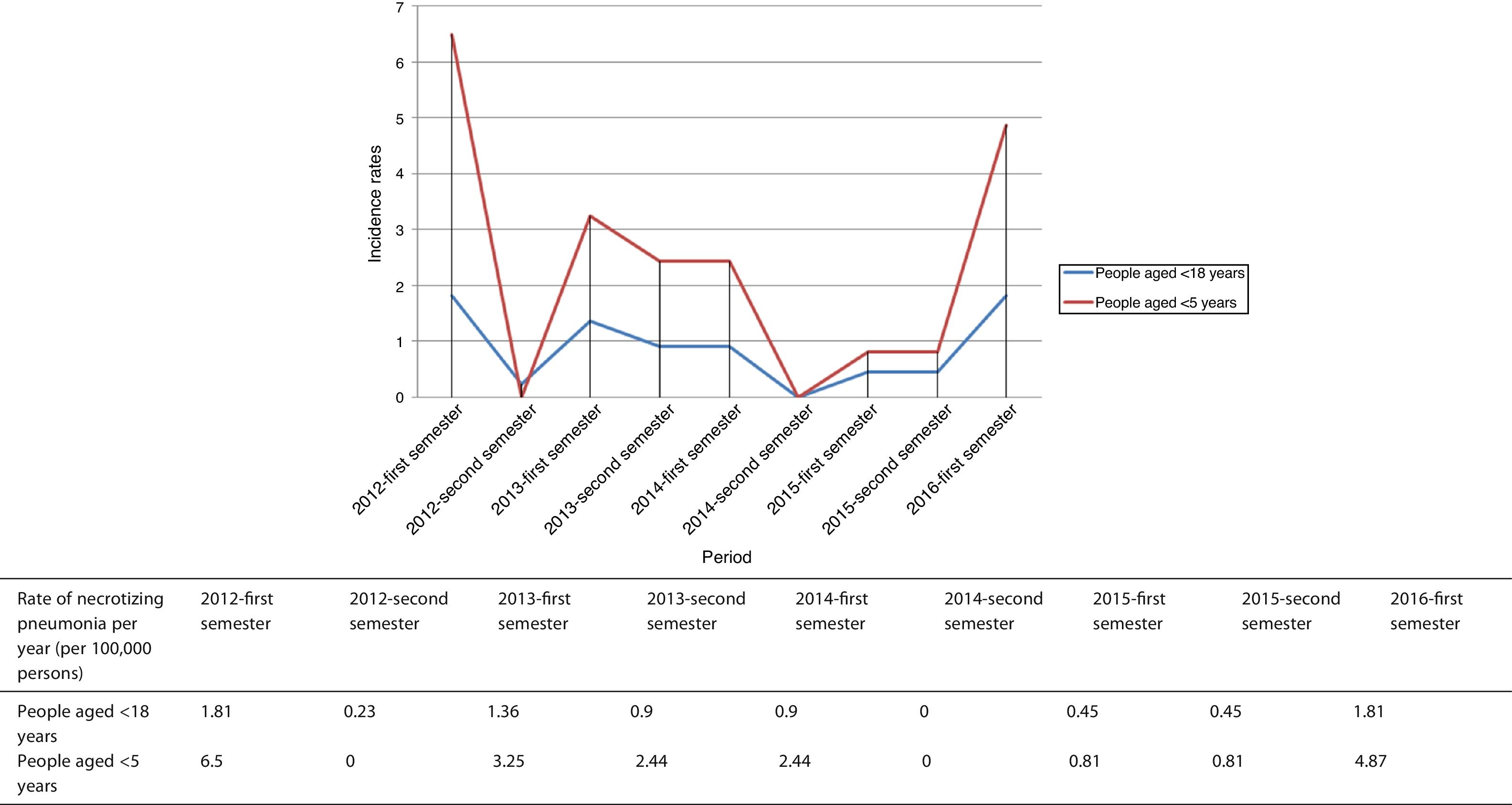
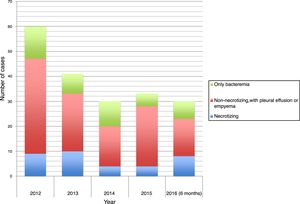
The time distribution of necrotizing pneumonia cases showed a clear winter predominance, with almost one third of cases occurring in February and 60% in January-March. Fig. 1 shows the evolution of the number of cases of necrotizing pneumonia, complicated pneumonia with effusion or empyema and uncomplicated bacteremic pneumonia. Fig. 2 shows necrotizing pneumonia incidence rates for each semester of the study in persons aged <18 years and those aged <5 years. In the first four years of the study, there was a significant decrease (even reaching zero cases in some periods) in necrotizing pneumonia incidence rates, (p=0.021), especially in patients aged <5 years (p=0.006), although there was a rebound in the number of cases in the last six months of the study period.
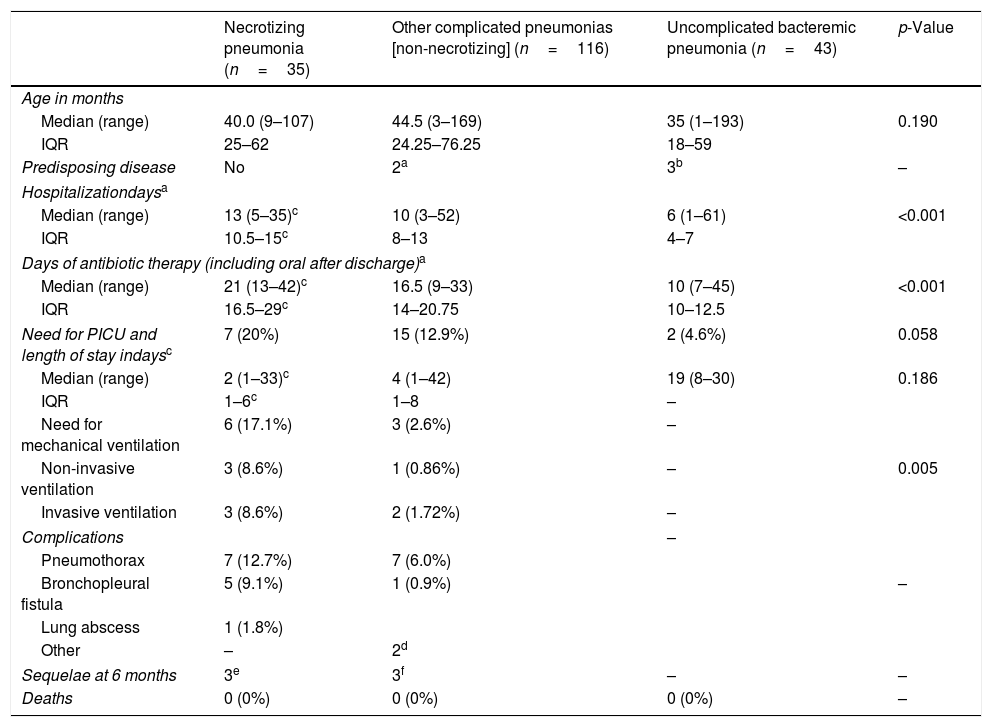
Table 2 compares clinical characteristics of cases of necrotizing pneumonia, pneumonia with pleural effusion or empyema without signs of necrosis (complicated pneumonia) and uncomplicated bacteremic pneumonia.
Epidemiological and clinical characteristics of types of pneumonia.
| Necrotizing pneumonia (n=35) | Other complicated pneumonias [non-necrotizing] (n=116) | Uncomplicated bacteremic pneumonia (n=43) | p-Value | |
|---|---|---|---|---|
| Age in months | ||||
| Median (range) | 40.0 (9–107) | 44.5 (3–169) | 35 (1–193) | 0.190 |
| IQR | 25–62 | 24.25–76.25 | 18–59 | |
| Predisposing disease | No | 2a | 3b | – |
| Hospitalizationdaysa | ||||
| Median (range) | 13 (5–35)c | 10 (3–52) | 6 (1–61) | <0.001 |
| IQR | 10.5–15c | 8–13 | 4–7 | |
| Days of antibiotic therapy (including oral after discharge)a | ||||
| Median (range) | 21 (13–42)c | 16.5 (9–33) | 10 (7–45) | <0.001 |
| IQR | 16.5–29c | 14–20.75 | 10–12.5 | |
| Need for PICU and length of stay indaysc | 7 (20%) | 15 (12.9%) | 2 (4.6%) | 0.058 |
| Median (range) | 2 (1–33)c | 4 (1–42) | 19 (8–30) | 0.186 |
| IQR | 1–6c | 1–8 | – | |
| Need for mechanical ventilation | 6 (17.1%) | 3 (2.6%) | – | |
| Non-invasive ventilation | 3 (8.6%) | 1 (0.86%) | – | 0.005 |
| Invasive ventilation | 3 (8.6%) | 2 (1.72%) | – | |
| Complications | – | |||
| Pneumothorax | 7 (12.7%) | 7 (6.0%) | ||
| Bronchopleural fistula | 5 (9.1%) | 1 (0.9%) | – | |
| Lung abscess | 1 (1.8%) | |||
| Other | – | 2d | ||
| Sequelae at 6 months | 3e | 3f | – | – |
| Deaths | 0 (0%) | 0 (0%) | 0 (0%) | – |
IQR: interquartile range; ECMO: extracorporeal membrane oxygenation; PICU: pediatric intensive care unit.
The case of a 3-year-old patient with septic shock and necrotizing pneumococcal pneumonia who required haemodynamic and respiratory support with ECMO and pneumonectomy with an 11-month paediatric ICU stay excluded from the analysis.
Pleural drainage was required by 71.4% of patients with necrotizing pneumonia during hospitalization. Thoracentesis without drainage placement was made in one patient and 9 patients (25.7%) required surgical intervention (Table 1). The most frequent complications were pneumothorax (7 cases, 12.7%) and bronchopleural fistula (5 patients, 9.1%).
Thoracic ultrasound, which is an important imaging test in the diagnosis of necrotizing complications, was performed in 24 cases (68.6%). The PCR for pneumococcus in pleural fluid was the fundamental complementary test for the etiological diagnosis of 23 patients (65.7%), and was positive in 95.8% of patients in whom a pleural fluid sample was obtained, while cultures were positive in only 25% of patients (Table 1).
Overall, the characteristics of the cases of necrotizing pneumonia in the series are consistent with data from previous studies, despite some differences in terms of hospital stay and treatment.22
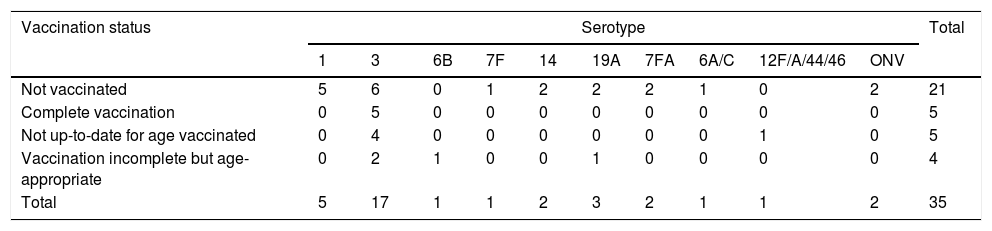
S. pneumoniae serotypes, vaccination status and vaccine failuresThe most frequent serotype was serotype 3, which was identified in 17 cases of necrotizing pneumonia (48.6%), 27 cases of complicated pneumonia (23.3%) and 5 cases of bacteremic pneumonia (11.6%) (p=0.002). The association between necrotizing pneumonia and serotype 3 was statiscally significant (OR 3.74, 95% CI 1.73–8.07).
Serotype 1 was the second most frequent serotype identified in cases of necrotizing pneumonia (5 cases, 14.3%) but was the most frequent in pneumonia with pleural effusion or empyema without signs of necrosis (36 cases, 31.0%) and was detected in 6 cases of uncomplicated bacteremic pneumonia (14%).
Serotypes 3 and 1 were responsible for 22 cases of necrotizing pneumonia (62.9%). The remaining serotypes are shown in Table 2. Data are consistent with previous reports.12
PCV13 vaccine serotypes were dominant in both necrotizing pneumonia and complicated pneumonias (85.7% and 79.3%, respectively), and were responsible for 51.4% of cases of bacteremic pneumonia.
Five patients with necrotizing pneumonia were fully vaccinated for their age with PCV13, constituting vaccination failures, all of whom had serotype 3 disease (29.4% of the total of necrotizing pneumonia due to serotype 3).
Table 3 shows the vaccination status of patients with necrotizing pneumonia according to the serotypes involved.
PCV13 vaccination status in patients with necrotizing pneumonia by serotype.
| Vaccination status | Serotype | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6B | 7F | 14 | 19A | 7FA | 6A/C | 12F/A/44/46 | ONV | ||
| Not vaccinated | 5 | 6 | 0 | 1 | 2 | 2 | 2 | 1 | 0 | 2 | 21 |
| Complete vaccination | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Not up-to-date for age vaccinated | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 5 |
| Vaccination incomplete but age-appropriate | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 4 |
| Total | 5 | 17 | 1 | 1 | 2 | 3 | 2 | 1 | 1 | 2 | 35 |
ONV: other non-vaccine serotypes not specified in serotyping.
This study, carried out between 2012 and 2016, when PCV13 was not included in the public health system-financed vaccination calendar in Catalonia, shows a significant trend to a decrease in the incidence of necrotizing pneumonia due to S. pneumoniae (especially in patients aged <5 years) during most of the study period, but a rebound of the disease during the last months of the study. However, the total observation period of the study is relatively short and normal temporal fluctuations should also be taken into account. It's relevant to highlight the central role played by serotype 3 in necrotizing pneumonia. These factors support the need to reinforce epidemiological surveillance.
In part of the literature it is suggested a trend towards more locally-aggressive forms of pneumococcal disease other than occult bacteremia (such as necrotizing pneumonia) after PCV7 vaccination introduction.3,23,24 One of the reasons stated is the probable replacement of S. pneumoniae serotypes (with the emergence of serotypes such as 3, 19A and 1, among others).3,17 Previous results from our same population show that serotype 3 was the most frequent in patients with IPD aged 7–59 months hospitalized in Catalonia in 2012–2016, especially in the 24–59 months age group, which accounted for 75.6% of cases.17
The preeminent role of serotype 3 as a cause of necrotizing pneumonia and the low effectiveness of PCV13 to prevent this serotype (25.9%; 95% CI: −65.3 to 66.8, according to data published by our group) may partly explain the persistence of the disease.17,25–27 In this study, all cases of vaccine failure in necrotizing pneumonia patients were due to serotype 3. Recent studies suggest that PCV13 vaccination may have a greater impact preventing serotype 3 related sepsis and meningitis rather than pneumonia.28
Our results underline the known importance of PCR in pleural fluid, which had a high rate of positivity when it was requested (patients with effusion who were candidates for pleural drainage).29,30 PCR in blood also offered a high diagnostic yield. Thoracic ultrasound was the most requested complementary examination for the diagnosis of the necrotizing complication, probably because the technique can provide valuable data at the bedside and without irradiation.
Patients with necrotizing pneumonia required longer hospitalization times, more days of antibiotic administration and, non-significantly, more frequently required intensive care (results are consistent with previously published data31). In contrast to other reports,1,6,32,33 the proportion of surgical interventions was low, reinforcing the value of conservative approach.15,22,34 Overall, patients evolved well and there were no deaths, as other studies report.22
The study has three major limitations. First, only patients admitted to three hospitals were included; however, these hospitals serve 31.9% of the paediatric population in Catalonia. In addition, the study only included cases in which S. pneumoniae was identified in blood or pleural fluid but no other probable cases that were not confirmed microbiologically. Finally, the study period could be relatively short to securely point out a clear trend in a low-incidence disease such as necrotizing pneumonia, as was discussed before.
ConclusionsPneumococcal necrotizing pneumonia is a serious condition that is often associated with pleural effusion or empyema and which requires prolonged antibiotic therapy and hospitalization.
We found a trend to a decreased incidence of necrotizing pneumonia, whose continuation should be verified. Despite relatively high rates of PCV13 vaccination, serotype 3, which even occurred in fully vaccinated children was the most frequent cause of necrotizing pneumonia and persistence of the disease during the entire study period.
In future years, epidemiological surveillance will be key to monitoring the evolution of IPD in general and pneumococcal necrotizing pneumonia in particular, in order to observe trends after the inclusion of PCV13 in the public health system vaccination schedule. The evolution of disease-causing serotypes is relevant due the association of some serotypes with complicated forms of the disease.
DeclarationsAll authors have read and approved the manuscript.
Ethics approval and consent to participateThe study has the approval of Sant Joan de Déu Hospital investigation ethics committee.
Consent for publicationWe have obtained written informed consent of the patients’ parents or guardians.
Availability of data and materialsThe datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
FundingThis work was supported by the National Plan of R + D + I 2008–2011 and ISCIII Sub-Directorate General for Evaluation and Promotion of Research (Projects PI11/02081 and PI11/2345), and cofunded by European Regional Development Fund (ERDF) and the Catalan Agency for the Management of Grants for University Research (AGAUR grant number 2014/SGR 1403).
The sources of funding had no role on data collection, analysis, interpretation and writing.
Conflict of interestsSebastià González-Peris, MD; Fernando Moraga-Llop, MD; Magda Campins, PhD; Ángela Domínguez, PhD; Álvaro Díaz-Conradi, PhD; Mariona F. de Sevilla, PhD; PhD; Sergi Hernández, PhD; Pilar Ciruela, PhD; Gemma Codina, PhD; Sonia Uriona, MD; Núria Soldevila, PhD; Conchita Izquierdo, PhD; Anna Solé-Ribalta, MD; Ana María Planes, PhD; Cristina Esteva, PhD; Johanna Martínez-Osorio, MD and Luis Salleras, PhD, have nothing to disclose.
Juan José García-García, PhD reports grants from Plan Nacional I+D+I, ISCIII - Subdirección General de Educación y Fomento de la Investigación Sanitaria (Project PI 11/02081) and Cofounded by Fondo Europeo de Desarrollo Regional, grants from AGAUR (Grant 2014 SGR 1403), during the conduct of the study; personal fees from Pfizer Inc., outside the submitted work.
Carmen Muñoz-Almagro, PhD reports grants from Instituto de Salud Carlos III, during the conduct of the study; personal fees from GSK Laboratories, grants from Pfizer Laboratories, outside the submitted work.
The members of the Barcino Working Group (Projects PI11/02081 and PI11/2345) are: Conchita Izquierdo, Pilar Ciruela, Sergi Hernández (Public Health Agency of Catalonia), Àngela Dominguez, Luis Salleras, Nuria Soldevila (University of Barcelona), Anna Solé-Ribalta, Carmen Muñoz-Almagro, Cristina Esteva, Johanna Martínez-Osorio, Juan José García-García, Mariona F. de Sevilla, (Hospital Sant Joan de Déu Barcelona, University of Barcelona, Barcelona), Ana María Planes, Fernando Moraga-Llop, Gemma Codina, Magda Campins, Sebastià González-Peris, Sonia Uriona, (Vall d’Hebron University Hospital, Barcelona), Alvaro Díaz (Hospital de Nens, Barcelona).