Pre-Exposure Prophylaxis (PrEP) is a biomedical intervention to prevent HIV infection in seronegative people at high risk of becoming infected. This strategy was endorsed in October 2019 by the Spanish Ministry of Health.
ObjectiveTo present the PrEP initial experience in the HIV Unit of the Hospital Clínic of Barcelona, paying special attention to the analysis of the vulnerability factors in the cohort.
Materials and methodsRetrospective, descriptive study. The epidemiological, sociodemographic, and clinical characteristics of the users included in the program during the first year are analyzed, paying particular attention to Infections, risky practices, and substance use.
Results190 individuals were included, 177 men and 12 trans women with a mean age of 35 years (8 SD). 70% had higher education, and half had Spanish nationality. An average of 10 couples per trimester and 60% reported unprotected anal sex. 31% had at least one positive PCR for STIs, with N. gonorrhoeae being the most prevalent microorganism (51%) and the rectal sample the most affected (21%). 63% reported chemsex use, 19% polydrug use, and 8% “slamming”. Half expressed concern about consumption and/or sexual practices and 25% the need for help.
ConclusionsThe PrEP user profile attended in our Hospital Unit justifies the creation of multidisciplinary teams that allow us to provide holistic attention to the sexual life of these people.
La Profilaxis Pre-Exposición (PrEP) es una intervención biomédica dirigida a prevenir la infección por VIH en personas seronegativas con alto riesgo de contraer la infección. Esta estrategia fue aprobada por el Ministerio de Salud de España en octubre del 2019.
ObjetivoPresentar la experiencia inicial de la PrEP en la Unidad de VIH del Hospital Clínic de Barcelona, poniendo especial atención en el análisis de los factores de vulnerabilidad de la cohorte.
Materiales y métodosEstudio retrospectivo, descriptivo. Se analizan las características epidemiológicas, socio demográficas y clínicas basales de los usuarios incluidos en el programa durante el primer año de funcionamiento prestando particular atención a las Infecciones, las prácticas de riesgo y el consumo de sustancias.
ResultadosSe incluyeron 190 individuos, 177 hombres y 12 mujeres transexuales con una edad media de 35 años (8DE). El 70% tenían estudios superiores y la mitad nacionalidad española. Informaron 10 parejas de media al trimestre y el 60% practicar sexo anal desprotegido. El 31% presentaron al menos una PCR positiva para ITS, siendo laN. gonorrhoeae el germen más prevalente (51%) y la muestra rectal la más afectada (21%). El 63% reportaron uso de chemsex, 19% policonsumo y 8% “slamming”. La mitad expresaron su preocupación por el consumo y/o prácticas sexuales y un 25% la necesidad de ayuda.
ConclusionesEl perfil del usuario de PrEP visitado en nuestra Unidad hospitalaria justifica la creación de equipos multidisciplinares que permitan prestar una atención holística de la vida sexual de estas personas.
Pre-exposure prophylaxis (PrEP) is a biomedical intervention based on the use of antiretroviral drugs, aimed at preventing infection by the human immunodeficiency virus (HIV) in seronegative people at high risk of contracting the infection. Multiple clinical trials have demonstrated the efficacy of oral PrEP for prevention against HIV, as long as there is proper adherence to the regimen1–4.
PrEP was initially approved in the United States in April 20125. In 2014 UNAIDS recommended its implementation as one more tool to contribute to the end of the HIV epidemic6. In October 2019, the Spanish Sistema Nacional de Salud (SNS) [National Health System] agreed to include PrEP in its portfolio of services7. In 2017, the National AIDS Plan launched a study to assess the feasibility of implementing PrEP in different healthcare settings in Spain. The results of this study have been published very recently8.
The approved regimen consists of the daily administration of a tablet that combines two active ingredients: tenofovir disoproxil fumarate and emtricitabine (TDF/FTC)9. This strategy must be accompanied by other measures of prevention against HIV and other sexually transmitted infections (STIs).
In Catalonia, the Departament de Salut [Department of Health] established that PrEP would be carried out in hospital functional AIDS units and in two community centres with a long history of monitoring vulnerable people.
The HIV unit of the Hospital Clínic de Barcelona [Barcelona Clinical Hospital] monitors 6000 people with chronic HIV infection, and annually treats 900 people who request post-exposure prophylaxis (PEP) after risky sexual contact. In November 2019, PrEP was started in our centre.
In this article, we present the experience of the first year of operation of the PrEP programme, the baseline characteristics of the users served, and the opportunities for improvement.
Materials and methodsDesignA retrospective and descriptive study of the baseline characteristics of the people included in the PrEP programme of the Hospital Clínic de Barcelona from its launch on 1 November 2019, until 31 October 2020. The study was evaluated and approved by the Hospital Ethics Committee.
Inclusion criteriaAdults without HIV infection, who reported having an untreated HIV-positive partner or multiple sexual partners in the past year, practising anal sex without protection, using drugs in the sexual context, being a sex worker, having required PEP or treatment for a bacterial sexually transmitted infection (STI) in the past year. Those people with a positive HIV test at baseline, with active hepatitis B virus (HBV) infection requiring treatment, or with any contraindication to taking TDF/FTC were excluded.
VariablesInformation collected at the baseline visit: demographic data (sex, age, place of birth, level of education and origin of referral) and clinical data (pathological history, concomitant medication, drug use and risky relationships); serologies: HIV, HAV, HBV, HCV and syphilis; results of a multiplex PCR performed on urine, throat and rectal swabs; and the responses to two self-completed questionnaires on risky sexual practices and drug use to prolong and/or improve sexual relations (Chemsex).
Statistical analysisQualitative variables have been described with absolute frequencies and percentages, and quantitative variables with median and interquartile range. The Stata (StataCorp. 2019. Stata: Release 16.1. Statistical Software. College Station, TX: StataCorp LLC) program was used for the analysis.
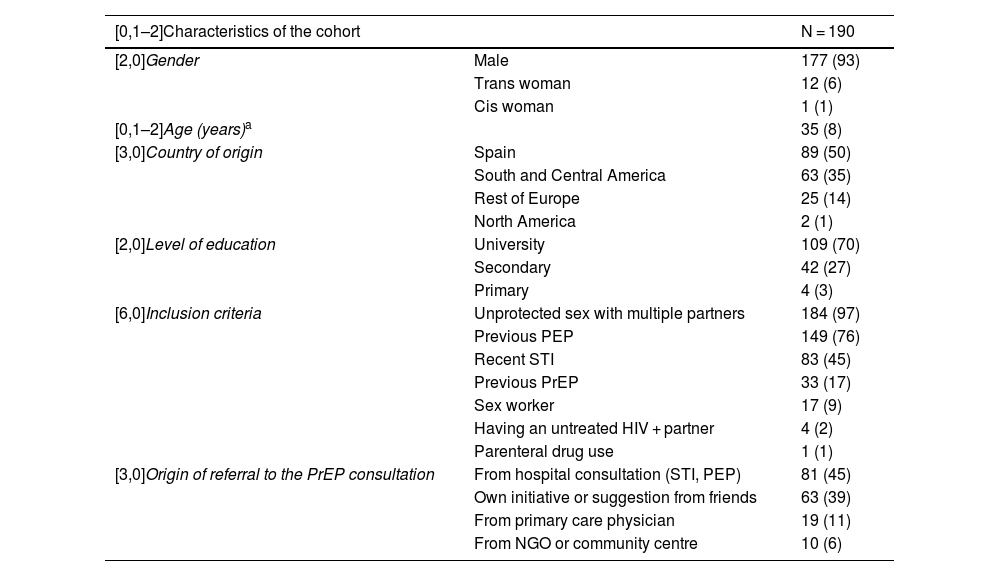
ResultsDemographic characteristics (Table 1)194 baseline visits were made and 190 people were finally included. Three individuals decided not to participate, and one person was diagnosed with HIV infection at the baseline visit.
Baseline characteristics of users included in the PrEP programme.
| [0,1–2]Characteristics of the cohort | N = 190 | |
|---|---|---|
| [2,0]Gender | Male | 177 (93) |
| Trans woman | 12 (6) | |
| Cis woman | 1 (1) | |
| [0,1–2]Age (years)a | 35 (8) | |
| [3,0]Country of origin | Spain | 89 (50) |
| South and Central America | 63 (35) | |
| Rest of Europe | 25 (14) | |
| North America | 2 (1) | |
| [2,0]Level of education | University | 109 (70) |
| Secondary | 42 (27) | |
| Primary | 4 (3) | |
| [6,0]Inclusion criteria | Unprotected sex with multiple partners | 184 (97) |
| Previous PEP | 149 (76) | |
| Recent STI | 83 (45) | |
| Previous PrEP | 33 (17) | |
| Sex worker | 17 (9) | |
| Having an untreated HIV + partner | 4 (2) | |
| Parenteral drug use | 1 (1) | |
| [3,0]Origin of referral to the PrEP consultation | From hospital consultation (STI, PEP) | 81 (45) |
| Own initiative or suggestion from friends | 63 (39) | |
| From primary care physician | 19 (11) | |
| From NGO or community centre | 10 (6) | |
NGO: non-governmental organisation; PEP: post-exposure prophylaxis; PrEP: pre-exposure prophylaxis; STI: sexually transmitted infection.
Of the 190 included, 177 (93%) were men and 13 women, of whom 12 were transgender (6%). The mean age was 35 years old (SD: 8). 50% (n = 89) were born in Spain and 35% (n = 63) came from Central and South America. 70% (n = 109) had higher education.
45% (n = 81) had accessed the programme directly after a hospital visit for PEP or an STI during the previous year. 39% (n = 69) requested a visit on their own initiative or at the suggestion of friends. 11% (n = 19) were referred from primary care and 6% were referred from community organisations that work with the LGTB community in our environment.
Regarding the eligibility criteria: 140 (76%) individuals had a history of previous PEP, 184 (93%) reported unprotected sexual relations with multiple partners, and 83 (45%) had had a bacterial STI in the last year.
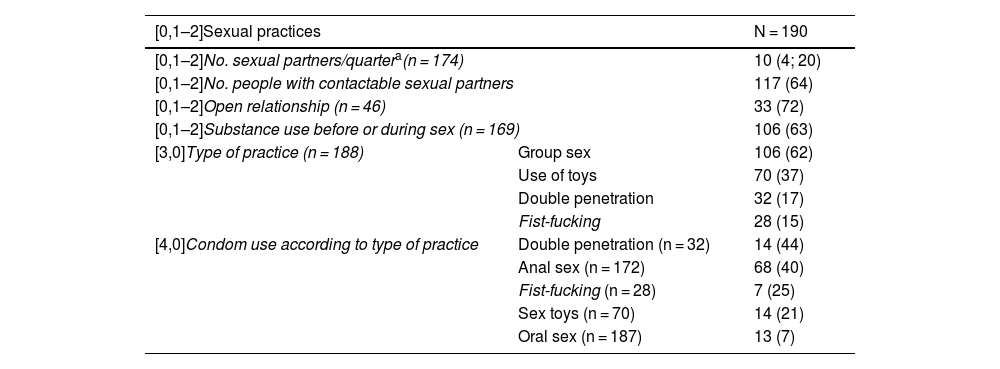
Sexual habits (Table 2)99% were gay, bisexual, and men who have sex with men (gbMSM) or transgender women with an average of 10 sexual partners per quarter and 62% engaged in group sex. 64% responded that they had easily contactable partners. 7% of users reported protecting themselves during oral sex and 40% during anal sex. The percentage of condom use regarding the most aggressive practices, such as double penetration and fisting, decreased to 25%.
Risky sexual practices of users included in the PrEP programme.
| [0,1–2]Sexual practices | N = 190 | |
|---|---|---|
| [0,1–2]No. sexual partners/quartera(n = 174) | 10 (4; 20) | |
| [0,1–2]No. people with contactable sexual partners | 117 (64) | |
| [0,1–2]Open relationship (n = 46) | 33 (72) | |
| [0,1–2]Substance use before or during sex (n = 169) | 106 (63) | |
| [3,0]Type of practice (n = 188) | Group sex | 106 (62) |
| Use of toys | 70 (37) | |
| Double penetration | 32 (17) | |
| Fist-fucking | 28 (15) | |
| [4,0]Condom use according to type of practice | Double penetration (n = 32) | 14 (44) |
| Anal sex (n = 172) | 68 (40) | |
| Fist-fucking (n = 28) | 7 (25) | |
| Sex toys (n = 70) | 14 (21) | |
| Oral sex (n = 187) | 13 (7) | |
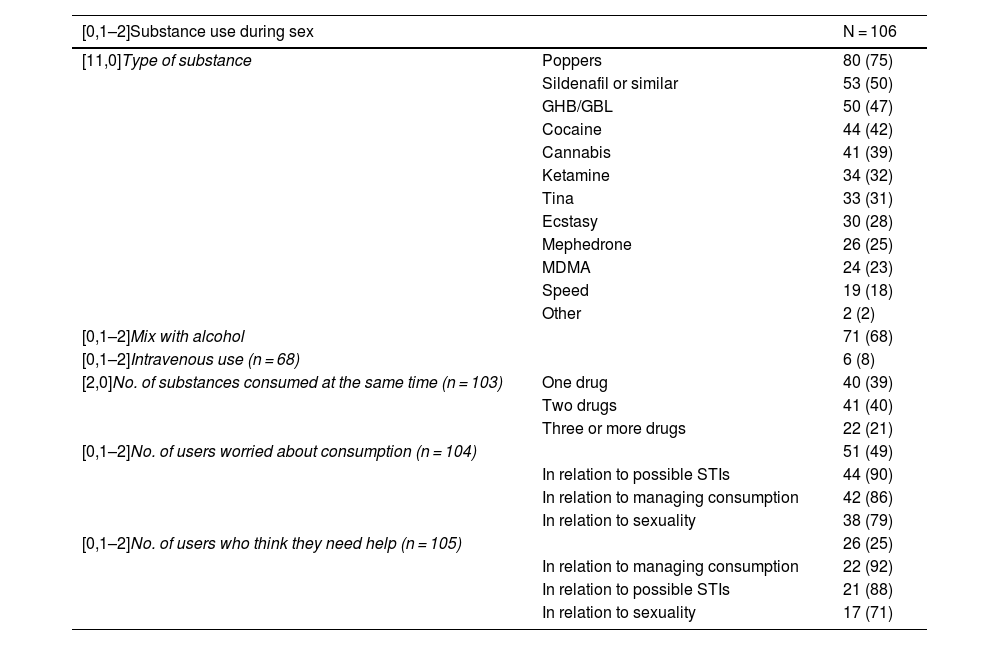
63% (n = 106) reported chemsex in the last year, the most commonly used substances being poppers, sildenafil, gamma-hydroxybutyrate (GHB/GBL) and cocaine. 40% combined two or more drugs, 19% reported polydrug use (use of three or more drugs) and 8% practiced slamming, that is, intravenous use. 49% (n = 51) of users reported being concerned about consumption, both in relation to the possible toxic effects of drugs, their sexuality or the risk of contracting STIs, and 25% (n = 26) stated they needed help managing these issues.
Substance use in the sexual context by users.
| [0,1–2]Substance use during sex | N = 106 | |
|---|---|---|
| [11,0]Type of substance | Poppers | 80 (75) |
| Sildenafil or similar | 53 (50) | |
| GHB/GBL | 50 (47) | |
| Cocaine | 44 (42) | |
| Cannabis | 41 (39) | |
| Ketamine | 34 (32) | |
| Tina | 33 (31) | |
| Ecstasy | 30 (28) | |
| Mephedrone | 26 (25) | |
| MDMA | 24 (23) | |
| Speed | 19 (18) | |
| Other | 2 (2) | |
| [0,1–2]Mix with alcohol | 71 (68) | |
| [0,1–2]Intravenous use (n = 68) | 6 (8) | |
| [2,0]No. of substances consumed at the same time (n = 103) | One drug | 40 (39) |
| Two drugs | 41 (40) | |
| Three or more drugs | 22 (21) | |
| [0,1–2]No. of users worried about consumption (n = 104) | 51 (49) | |
| In relation to possible STIs | 44 (90) | |
| In relation to managing consumption | 42 (86) | |
| In relation to sexuality | 38 (79) | |
| [0,1–2]No. of users who think they need help (n = 105) | 26 (25) | |
| In relation to managing consumption | 22 (92) | |
| In relation to possible STIs | 21 (88) | |
| In relation to sexuality | 17 (71) | |
N (%).
Negative HIV serology was confirmed in all users before starting TDF/FTC. 86% of users were immunised against HAV, 76% had antibodies to HBV surface antigen (HBsAg) and in 13 people there was also a resolved HBV infection with positive HBcAb. Three individuals had chronic non-replicative HBV infection HBsAg positive, but HBV-DNA not detectable). One user had antibodies to HCV, but with undetectable HCV-RNA. 10% of subjects had an active syphilis at the baseline visit.
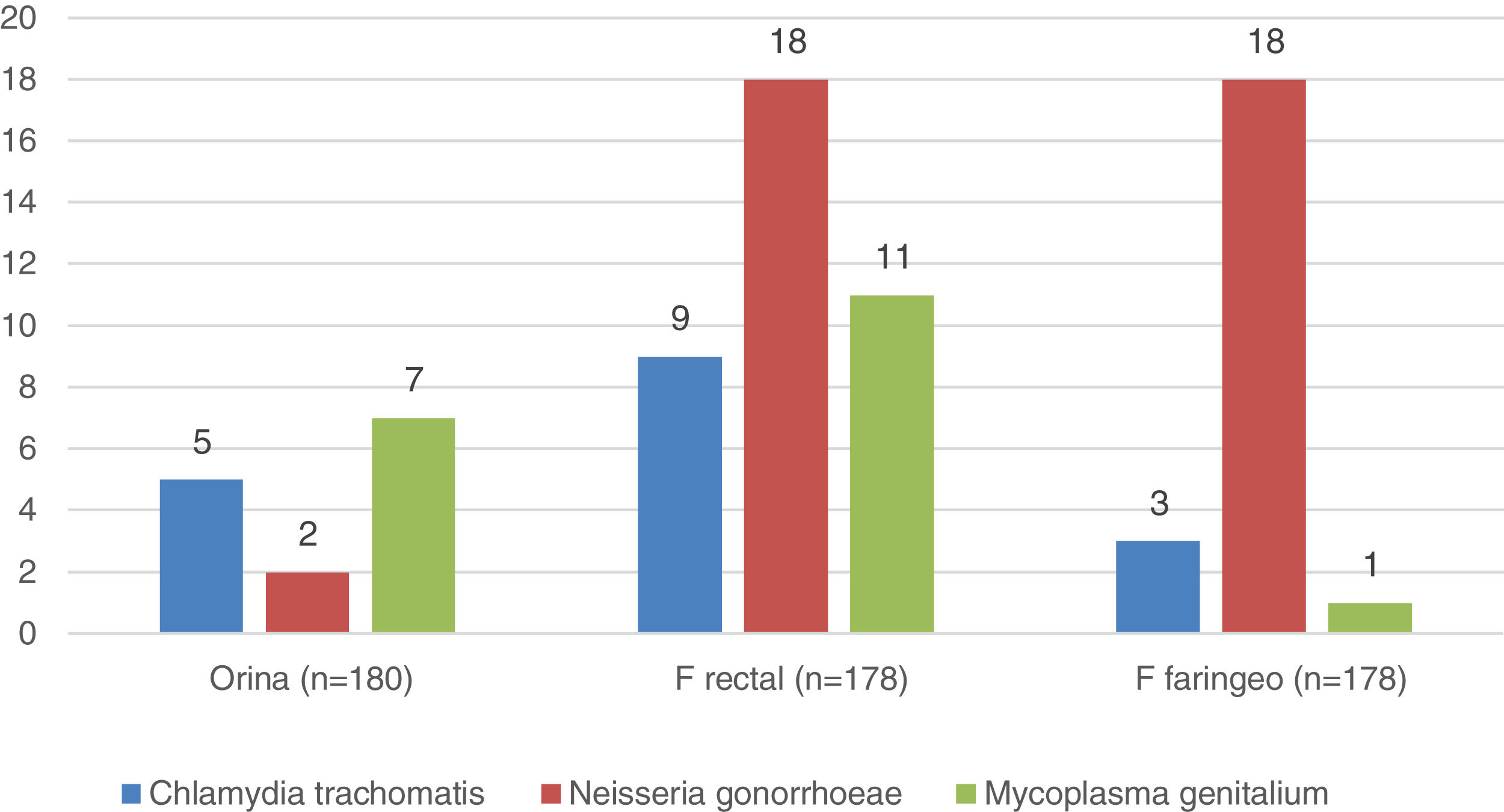
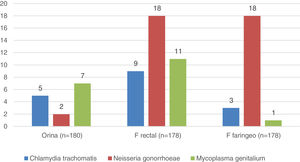
Microbiology (Fig. 1)In 56 (31%) of the 180 individuals from whom samples were successfully collected, at least one of the three samples was positive. The rectal sample was the most affected, positive in 38 individuals (21%), and Neisseria gonorrhoeae the germ most frequently isolated (51% of positive PCR). All users with PCR diagnoses were informed, advised to notify their sexual partners, and treated for their STIs before starting PrEP.
20% had a history of genital or anal warts.
Discussion190 people were included in the first year of operation of the PrEP programme at the Hospital Clínic de Barcelona. In 2020, the SARS-CoV-2 pandemic resulted in changes in hospital care activity, leading to the forced temporary suspension of the inclusion of new users in the PrEP programme for three months, with the correct follow-up of people already in treatment maintained.
The profile of the PrEP user in our hospital is a 35-year-old gbMSM with university studies, similar to that described in other PrEP cohorts from Europe10–12 Canada13 or the USA14. 50% were born in Spain, but the high percentage of users from Central or South America stands out.
In our cohort, priority was given to the inclusion of people previously identified as high risk for having undergone PEP in the last year in the hospital. Thus, the percentage of individuals with this history is clearly higher than that described in other series10,12.
17% of users reported having received PrEP informally before arriving at the hospital, despite it not being authorised in Spain. This reveals the interest aroused by this preventive strategy and the active attitude of the at-risk group of taking care of themselves.
The high prevalence of trans women and sex workers in our cohort stands out, and is clearly higher than that reported in a hospital in another large European city, such as Paris12, which highlights the significant vulnerability of our users.
We would like to emphasise the value of PrEP programmes as part of a global health prevention strategy. On the one hand, there is an insistence on promoting vaccination in all susceptible users; in our case for HAV, HBV and human papillomavirus. On the other hand, baseline STI screening makes it possible to demonstrate infections, mostly silent, which are treated immediately, obtaining a double benefit: for the individual and for the community by reducing the risk of future infections. In addition, this benefit has been extended in our cohort by offering treatment to users' contactable partners, thus contributing to blocking the chain of transmission.
In this sense, the high prevalence of STIs in our cohort compared to other European series11,12 stands out. It is possible that difficulty accessing the health system during this year of the pandemic could have contributed to more people undertreated for STIs and an increase in infections in our environment.
Similar to data reported in the literature1–4,10–14, users included in the programme are involved in high-risk practices for contracting HIV, such as a very high prevalence of anal intercourse without a condom, group sex, and a notable percentage of aggressive sexual practices that can lacerate the rectal mucosa.
Another factor clearly related to an increased risk of contracting HIV infection is chemsex15, which in our series rises to almost two thirds of the users. A priori, poor adherence to the programme might be expected in these individuals, but a recent study16 has demonstrated correct adherence to the PrEP prescribed in these people, possibly related to a higher perception of risk of contracting the infection and their interest in taking care of themselves17–20.
Finally, we believe it is relevant that half of the chemsex users in our cohort say that they are concerned about their consumption, about their sexuality, or about the risk of contracting STIs. In addition, one in four stated that they need help to manage these problems. Based on these results, we believe it is necessary to bring in specialists in psychiatry/psychology, sexology and addictions to form part of a multidisciplinary team, along with infectologists, to ensure a holistic approach to sexual health for the PrEP user.
There are conflicting data in the literature on the role that PrEP programmes may play in relation to the increase in the number of STIs21–24, possible changes in sexual behaviours12,13,24,25 and substance use26 among users during follow-up, which will be very interesting to analyse in our future cohort.
This study had some limitations. In the first place, the size of the sample presented is obviously lower than the annual recruitment that was initially planned, and this is due to the healthcare restrictions experienced during this year of the SARS-CoV-2 pandemic. Secondly, it is possible that there is a selection bias of the users included that could magnify certain baseline characteristics of vulnerability of the cohort, such as the high number of sex workers, STIs or chemsex. Finally, it is worth remembering that this work shows an initial picture of the start-up of the PrEP programme in an HIV unit of a tertiary care hospital. It will be necessary to closely monitor the efficacy of this intervention in our environment in the long term, analysing adherence to treatment, permanence in the programme, the rate of HIV seroconversion, STI diagnoses, and to determine how a global intervention regarding the sexual health of our users may impact sexual practices and the use of chemsex.
Conflicts of interestThe authors declare that they have no conflicts of interest.