Pre-exposure prophylaxis (PrEP) against the human immunodeficiency virus (HIV) is an effective and safe preventive measure. However, it has not reached all target users who could benefit from it.
The study aimed to understand the sociodemographic, clinical and behavioral baseline characteristics of PrEP users. As a secondary objective, the use of concomitant medication and drug consumption were described.
MethodologyObservational, retrospective and descriptive study of the sociodemographic, clinical and behavioral characteristics of the users who were included in the PrEP program of the Community of Madrid during the first two years of experience.
ResultsTwo thousand two hundred fifty-six PrEP users were included, 99.0% men, with a mean age of 36.9 years (SD 8.68). 33.1% presented a sexually transmitted infection (STI) on the first visit, highlighting chlamydiasis and rectal gonococci. 70.4% reported using drugs associated with sex, and 42.4% participated in chemsex sessions in the last 3 months. A high percentage of users with concomitant medication was observed (37.6%), highlighting drugs related to mental health and alopecia.
ConclusionsA multidisciplinary approach is required to cover all the needs of PrEP users, including mental health evaluation measures and addiction treatment with the clinical approach.
La profilaxis pre-exposición (PrEP) frente al virus de la inmunodeficiencia humana (VIH) es una medida preventiva eficaz y segura. Sin embargo, no se ha alcanzado a todos los usuarios objetivo que podrían beneficiarse de la misma.
El objetivo del estudio fue conocer las características basales sociodemográficas, clínicas y conductuales de las personas al iniciar la PrEP. Como objetivo secundario, se describió el uso de medicación concomitante y el consumo de drogas.
MetodologíaEstudio observacional, retrospectivo y descriptivo de las características sociodemográficas, clínicas y conductuales de los usuarios que se incluyeron en el programa de PrEP de la Comunidad de Madrid durante los dos primeros años de experiencia.
ResultadosSe incluyeron 2.256 usuarios en PrEP, 99,0% hombres, con una edad media de 36,9 años (DE 8,68). El 33,1% presentó alguna infección de transmisión sexual (ITS) en la primera visita, destacando las clamidiasis y gonococias rectales. El 70,4% refirió consumir drogas asociadas al sexo, y el 42,4% participó en sesiones dechemsex en los últimos 3 meses. Se observó un alto porcentaje de usuarios con medicación concomitante (37,6%), destacando fármacos relacionados con salud mental y alopecia.
ConclusionesSe requiere un abordaje multidisciplinar para cubrir todas las necesidades de los usuarios de PrEP, incluyendo al abordaje clínico medidas de evaluación de salud mental y tratamiento de adicciones.
Pre-exposure prophylaxis (PrEP) against the human immunodeficiency virus (HIV) consists of the use of antiretroviral drugs as a preventive tool with the objective of stopping the transmission of said infection. It is indicated in people with a high risk of contracting HIV infection, associated with other prevention measures.1,2
Several clinical trials have confirmed that the use of daily oral PrEP is safe and effective.3 Tenofovir disoproxil (TDF) plus emtricitabine (FTC), taken daily orally, significantly reduces the incidence of HIV in all transmission categories: men who have sex with men (MSM); transgender people (TGR); cisgender heterosexual people; HIV serodiscordant couples; and people who inject drugs (PID).4–6
The Food and Drug Administration (FDA) approved the indication of PrEP with TDF/FTC in 2012.7 The Centers for Disease Control and Prevention (CDC) recommended the daily use of a tablet co-formulated with 300 mg of TDF and 200 mg of FTC, due to its rapid diffusion and high concentration in the rectal and genital tract.8 The PROUD study, carried out in 13 sexually transmitted infection (STI) clinics in the United Kingdom, found the same preventive effectiveness of daily PrEP as the IPERGAY study with the “on demand” regimen in MSM.9,10 In both studies, the relative reduction in the risk of contracting HIV was 86%.
The European Medicines Agency (EMA) and the FDA have authorised the taking of PrEP with TDF/FTC exclusively on a daily basis. The preventive effectiveness of PrEP is closely correlated with the degree of adherence to it.4,11 In addition to TDF/FTC, other preventive pharmacological options have been approved, such as tenofovir alafenamide (TAF) co-formulated with FTC or intramuscular cabotegravir administered every two months.12,13
In Spain, PrEP began to be financed by the National Health System (SNS) in November 2019, associated with other preventive measures, in MSM and TGR of legal age with at least two of the following criteria (referring to the last year): more than 10 sexual partners; practice of anal sex without a condom; drug use associated with unprotected sexual relations; administration of post-exposure prophylaxis (PPE) on several occasions; or a bacterial STI. Also eligible were women who practice prostitution in whom use of condoms is not routine.14 On 1 December 2021, the Interministerial Commission on Drug Prices proceeded to expand the group of people eligible for PrEP, changing the age of indication to those over 16 years of age and including cisgender heterosexual people and PID with unsafe injection practices who reported non-routine condom use and also met at least two of the above criteria.15
Monitoring people without HIV infection with risk factors for contracting it helps identify people to whom PrEP can be recommended. STI clinics are ideal care centres, since they monitor HIV-seronegative patients.16 Until June 2022, the comprehensive monitoring of PrEP users in the Madrid Region was carried out in a dedicated reference clinic specialising in STI linked to a tertiary hospital.17 The implementation programme in that centre began on 20 January 2020.
PrEP is an increasingly used and widespread tool. However, the WHO considers that it still has not reached the number of users who could benefit from it.18 Accessibility to PrEP and monitoring is therefore a priority. The aim of this study was to determine the baseline sociodemographic, clinical and behavioural characteristics of PrEP users treated at an STI centre, the only centre providing care for PrEP users in Madrid Region in the time period studied. As a secondary objective, the use of concomitant medication and drug consumption were described.
Material and methodsDesignObservational, retrospective, descriptive study of the sociodemographic, clinical and behavioural characteristics of the users who were included in Madrid Region’s PrEP programme from its launch on 20 January 2020 up to 19 January 2022.
Study populationPeople not infected with HIV over 18 years of age, until November 2021, and then over 16 years of age, as of December 2021, who met the Spanish Ministry of Health inclusion criteria for the start of PrEP, in combination with other preventive measures, and who were included in the Subdirectorate General for Pharmacy and Medical Devices Centralised Registry, which is the monitoring and tracking tool for PrEP users in the Madrid Region.
People with positive HIV serology at the baseline visit, acute hepatitis B virus (HBV) infection, or glomerular filtration rate less than 60 ml/min were not included.
VariablesAge, gender, region of origin, percentage of condom use in anal/vaginal sex, number of sexual partners per month, use of drugs for sex, number of chemsex sessions in the last three months and compliance with Spanish Ministry of Health criteria for inclusion in the PrEP programme.
Although the term chemsex implies the intentional use of drugs associated with sex, the definition of chemsex used in this study refers to the use among MSM of specific substances such as mephedrone, gamma-hydroxybutyric acid/gamma-butyrolactone (GHB/GBL) and methamphetamine during prolonged sessions of sex without a condom with multiple partners.19 The use of drugs associated with sex outside of this context is not considered chemsex.
Type of regimen prescribed (daily or on demand). Use of other medicinal products, sports supplements and drug use. With regard to the regimen prescribed, although the approved indication is the daily regimen, some users opted for the on-demand regimen, supported by the literature published on the subject.10 This decision was supported in users with less frequent risk practices or with evidence of compromised kidney function.
For the diagnosis of STI/HIV, the following microbiological techniques were applied: HIV (CMIA and Western-Blot confirmation); hepatitis A, B and C viruses (HAV, HBV and HCV) (CMIA); syphilis (dark field microscopy, PCR, RPR, EIA and TPPA); Neisseria gonorrhoeae (NG) by Gram stain, culture in Thayer Martin medium and PCR; Chlamydia trachomatis, PCR and genotyping for lymphogranuloma venereum (LGV).
Statistical analysisFor the data analysis, qualitative variables were shown with relative and absolute frequencies. Quantitative variables were expressed as median and median and standard deviation (SD) or interquartile range (IQR). For all tests, significance was set at 5% (p < 0.05).
For the statistical analysis, the SPSS® Statistics 26.0 program was used.
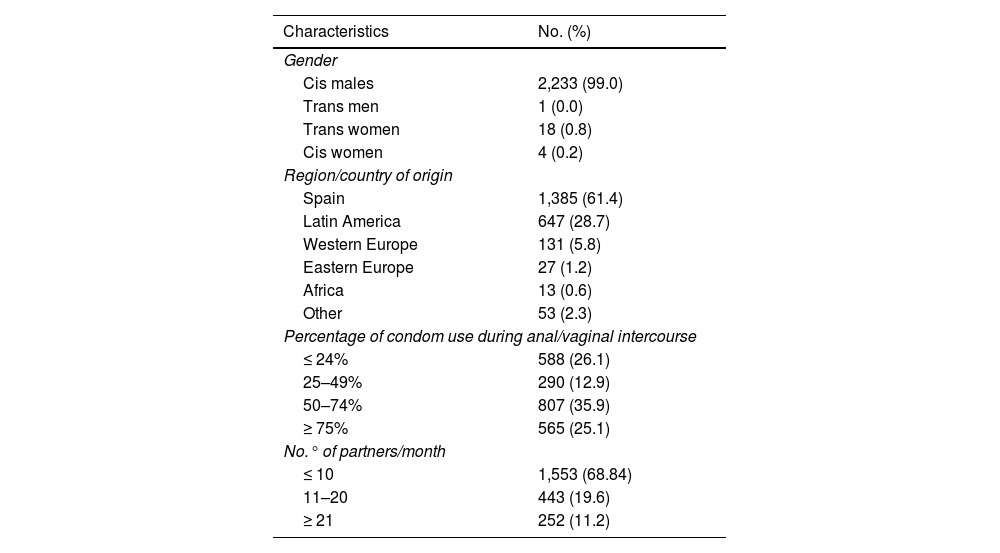
ResultsOf the 2,256 PrEP users included, 2,234 (99.0%) were male, 99.9% (n = 2,233) of whom were MSM. Mean age was 36.9 (SD: 8.68); 61.4% (n = 1,385) were of Spanish nationality and 28.7% (n = 647) were Latin American; 30.6% (n = 695) had more than 10 sexual partners per month, with inconsistent condom use (Table 1).
Baseline characteristics of users included in the PrEP programme (n = 2,256).
| Characteristics | No. (%) |
|---|---|
| Gender | |
| Cis males | 2,233 (99.0) |
| Trans men | 1 (0.0) |
| Trans women | 18 (0.8) |
| Cis women | 4 (0.2) |
| Region/country of origin | |
| Spain | 1,385 (61.4) |
| Latin America | 647 (28.7) |
| Western Europe | 131 (5.8) |
| Eastern Europe | 27 (1.2) |
| Africa | 13 (0.6) |
| Other | 53 (2.3) |
| Percentage of condom use during anal/vaginal intercourse | |
| ≤ 24% | 588 (26.1) |
| 25–49% | 290 (12.9) |
| 50–74% | 807 (35.9) |
| ≥ 75% | 565 (25.1) |
| No.° of partners/month | |
| ≤ 10 | 1,553 (68.84) |
| 11–20 | 443 (19.6) |
| ≥ 21 | 252 (11.2) |
PrEP: pre-exposure prophylaxis.
As criteria for indicating PrEP, in the last year 98.4% (n = 2,219) had more than 10 different sexual partners, 97.5% (n = 2,200) reported having had anal and/or vaginal sex without a condom, 65.9% (n = 1,486) used drugs associated with sex, 47.4% (n = 1,070) had a history of a bacterial STI and 10.8% (n = 243) had requested PrEP.
PrEP was started on a daily regimen in 96.8% (n = 2.183) and on-demand in 3.2% (n = 73).
At the first visit, 33.1% (n = 747) had an STI. Chlamydia was diagnosed in 16.3% (n = 369), with 21.5% (n = 40) being lymphogranuloma venereum (LGV), and 15.8% (n = 356) were diagnosed with gonorrhoea; the most common location for both infections was the rectum (82.7% and 75.1% respectively) (Fig. 1). Syphilis was diagnosed in 9.5% (n = 214): 10.7% (n = 22) primary; 24.3% (n = 50) secondary; 31.6% (n = 65) early latent; and 33.5% (n = 69) latent of unknown duration.
Positive serology for HAV was found in 73.1% (n = 1,637) and for HBV in 80.7% (n = 1,810), of whom 86.5% (n = 1,565) had been vaccinated, 9.7% (n = 175) had had an HBV infection and one person had chronic infection. Although all with undetectable viral load, 1.1% (n = 12) had antibodies against HCV. All those with negative antibodies against HAV and HBV were offered the possibility of vaccination.
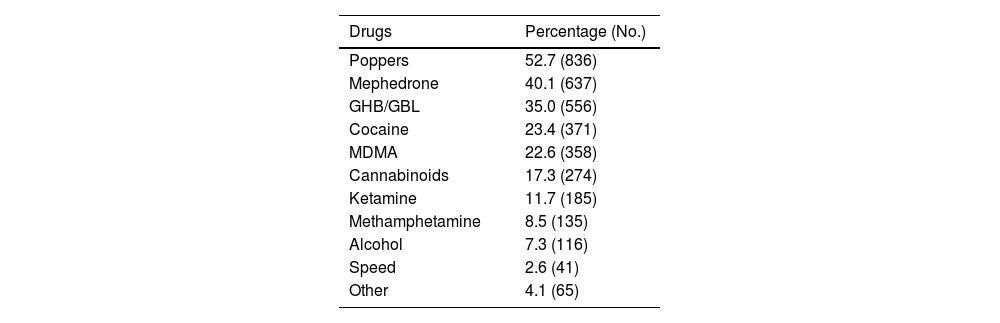
Some 70.4% (n = 1,587) used drugs for sex and 42.4% (n = 899) reported having participated in a chemsex session in the last three months. The median participation in sessions in the last quarter was three (IQR: 1–5): 68.7% (n = 618) with fewer than four sessions; 23.4% (n = 210) from four to 11 sessions; and 6.9% (n = 62) 12 or more sessions.
The most frequently consumed drugs in the last three months were: poppers (52.7%); mephedrone (40.1%); and GHB (35.0%) (Table 2).
Frequency according to each substance among the 1,587 PrEP users who used drugs in the last three months.
| Drugs | Percentage (No.) |
|---|---|
| Poppers | 52.7 (836) |
| Mephedrone | 40.1 (637) |
| GHB/GBL | 35.0 (556) |
| Cocaine | 23.4 (371) |
| MDMA | 22.6 (358) |
| Cannabinoids | 17.3 (274) |
| Ketamine | 11.7 (185) |
| Methamphetamine | 8.5 (135) |
| Alcohol | 7.3 (116) |
| Speed | 2.6 (41) |
| Other | 4.1 (65) |
GHB/GBL: gamma-hydroxybutyric acid/gamma-butyrolactone; MDMA: 3,4-methylenedioxymethamphetamine; PrEP: pre-exposure prophylaxis.
Products related to increasing sports performance were used by 43.5% (n = 982) with 88.8% (n = 872) taking protein supplements (proteins, branched chain amino acids, etc.), 30.5% (n = 300) creatine, 3.4% (n = 33) cycles with anabolic steroids (oral or parenteral) and 11.2% (n = 110) another type of supplement.
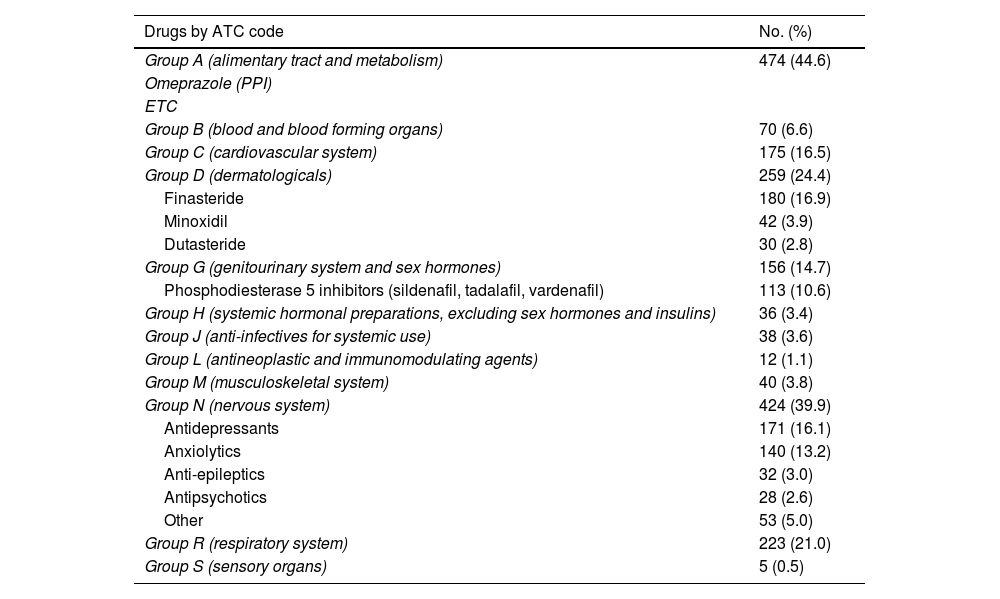
Lastly, 37.6% (n = 1,063) used some concomitant medication, the most common being those related to the gastrointestinal system (for example, antacids, multivitamins), mental health and alopecia (Table 3).
List of concomitant medicinal products by ATC code (Anatomical, Therapeutic, Chemical Classification System) among the 1,063 PrEP users who took other medicinal products.
| Drugs by ATC code | No. (%) |
|---|---|
| Group A (alimentary tract and metabolism) | 474 (44.6) |
| Omeprazole (PPI) | |
| ETC | |
| Group B (blood and blood forming organs) | 70 (6.6) |
| Group C (cardiovascular system) | 175 (16.5) |
| Group D (dermatologicals) | 259 (24.4) |
| Finasteride | 180 (16.9) |
| Minoxidil | 42 (3.9) |
| Dutasteride | 30 (2.8) |
| Group G (genitourinary system and sex hormones) | 156 (14.7) |
| Phosphodiesterase 5 inhibitors (sildenafil, tadalafil, vardenafil) | 113 (10.6) |
| Group H (systemic hormonal preparations, excluding sex hormones and insulins) | 36 (3.4) |
| Group J (anti-infectives for systemic use) | 38 (3.6) |
| Group L (antineoplastic and immunomodulating agents) | 12 (1.1) |
| Group M (musculoskeletal system) | 40 (3.8) |
| Group N (nervous system) | 424 (39.9) |
| Antidepressants | 171 (16.1) |
| Anxiolytics | 140 (13.2) |
| Anti-epileptics | 32 (3.0) |
| Antipsychotics | 28 (2.6) |
| Other | 53 (5.0) |
| Group R (respiratory system) | 223 (21.0) |
| Group S (sensory organs) | 5 (0.5) |
ATC: Anatomical, Therapeutic, Chemical Classification System; PPI: proton pump inhibitor; PrEP: pre-exposure prophylaxis.
The predominant profile of the PrEP user in our cohort was a 36.9-year-old MSM, in line with other cohorts.20–22 As in other cohorts in Spain, the region of origin was mainly Spain (61.4%) followed by Latin America (28.7%).22 Although this is a highly vulnerable population (according to data from the National Epidemiology Centre, 55.0% of new HIV diagnoses in Spain in 2022 were MSM,23) there are other population groups, not MSM, who are not accessing the use of PrEP, despite their high degree of vulnerability. An Italian cohort highlights this “unbalanced” access to PrEP among different populations that could benefit from it.20 It is worth noting that our study spans from January 2020 to January 2022, and the authorisation of PrEP in cisgender non-MSM and PID individuals was introduced in December 2021.15 Further analyses will be needed to confirm whether or not there is any variation in the majority profile of PrEP users, in addition to determining possible reasons for said imbalance.
In our cohort only 3.2% used the on-demand regimen, even though in our region only the daily regimen is recommended.24 However, other cohorts have analysed the results of encouraging both regimens equally, with up to 50% of users opting for an on-demand regimen.25 Furthermore, the Belgian cohort analyses the percentage of users who decided to change their regimen over time, with 16% of people changing from daily to on-demand.26 Our cohort may have a bias in this sense because in our region the on-demand regimen lacks indication, despite being safe and effective in real-life studies.27
The prevalence of STI at the initial visit is similar to that obtained in the Barcelona cohort,22 in which an STI was diagnosed in 30.2% of users at the baseline visit, but is higher than the data obtained in cohorts at the European level, in which 22% and 19% respectively were diagnosed with an STI.20,27 Regarding the data published in the 2023 SIPREP report (Sistema de información de programas de Profilaxis Pre-exposición [Pre-exposure Prophylaxis Program Information System]), there is some similarity regarding diagnosed syphilis, but the percentage of chlamydial and gonococcal infection is lower than that obtained in our cohort. In the SIPREP cohort, gonorrhoea was diagnosed in 7.0% of users and chlamydia in 6.0%, compared to the 15.8% and 16.3% diagnosed in our study. Of the chlamydial infections, in the SIPREP report only 5.3% were LGV, compared to 21.5% in our cohort.28 A rectal location was the most common, as in other studies, in which this was found not only at the baseline visit, but throughout the entire follow-up.20,26,27,29 Most of the STI diagnosed were asymptomatic, highlighting the importance of follow-up in these programmes, both for the user themselves and in terms of public health, by interrupting the chain of transmission of these infections.
The use of drugs associated with sex is emerging as a problem worth highlighting among the users of our cohort, as well as in others at the European level.20,22,26,27 In the Barcelona cohort, 63% participated in chemsex sessions and in the Italian cohort, the rate was 41.3%.20,22 In our case, 70.4% admitted using drugs associated with sex and 42.4% had participated in a chemsex session. However, in the SIPREP report, only 23.2% of users reported drug use in the three months prior to starting PrEP.28
To access PrEP programmes here in Spain, several criteria indicative of risk practices must be met.15 The high percentage of STI or the rate of participation in chemsex in our cohort are other factors associated with a high risk of contracting HIV infection.19 We need further analyses to determine the impact the implementation of this preventive measure against HIV infection has had in our region.
Analysing the concomitant medication of our users, we found that 15% (n = 339) used some sort of medication related to mental health problems (antidepressants, anxiolytics and antipsychotics). Although there is a lack of information in the literature regarding the use of concomitant medication in PrEP users, there are studies which analysed mental health in PrEP users.30 In the Dutch cohort they performed some kind of test to assess mental health status; 20.7% scored less than 60 points on the MHI-5 test, suggestive of anxiety and depression disorder.27 These data, combined with the problems associated with chemsex, make it essential to have mental health and addiction management professionals on the PrEP user monitoring teams.
Our study has a series of limitations. All PrEP users analysed were recruited at a single centre. The health crisis derived from the SARS-CoV-2 pandemic forced the suspension of new inclusions in the programme from mid-March to June 2020. Also, we do not know the real data on users who use drugs intravenously, as this indication was approved in December 2021, and these users were included in other categories, even though they also met this criterion.15 We do not have data on sex workers, as this criterion is restricted only to cisgender women and is not considered an indication criterion in other groups.15
In conclusion, PrEP users are largely MSM with little representation from other target populations, so access to other population groups should be a priority. Given the high incidence of STI at the first visit, especially rectal, it is essential to perform adequate STI screening throughout the individual’s follow-up. There is a high level of drug use related to sex in this group, in addition to a significant percentage of concomitant psychiatric medication. The follow-up of PrEP users must therefore have a multidisciplinary vision and not only be from a clinical point of view, and include provisions for the assessment of mental health, as well as the prevention and treatment of addictions.
FundingWe have received no funding to conduct this study. It was carried out during routine care activities.
Conflicts of interestThe authors declare that they have no conflicts of interest.