Introduction
For many years, the main intervention to improve and prolong the life of HIV-infected patients was prophylaxis of opportunistic infections and healthcare by professionals with AIDS experience1,2. Despite the fact that, in developed countries, the incidence of these infections has fallen drastically in line with the use of highly active antiretroviral therapy (HAART)3,4, in countries with scarce economic resources, these infections are still common and represent the principal cause of HIV-associated mortality.
For some years now, we have known that the inhibition of viral replication by HAART prevents immune deterioration in HIV-infected patients. In patients with advanced disease, these treatments give rise to a gradual increase in naïve CD4 T and memory lymphocytes which can proliferate "in vitro" and generate cytokines in response to opportunistic pathogens. Moreover, the delayed antigen response recovers and non-specific immune activation is normalized5,6.
There can be no doubt that HAART is the best strategy for preventing opportunistic infections in these patients; this does not mean to say, however, that we can forget prophylaxis. In clinical practice, prophylaxis is still necessary in countries with scarce economic resources, in very immunodepressed patients until HAART takes effect, in patients who do not wish to or who cannot take HAART, in patients in whom HAART fails and in the small group of patients who are unable to recover sufficient CD4+ T cell counts despite good inhibition of viral replication. Finally, it is important to point out that prevention of opportunistic infections is a field in which clinical research on the withdrawal of secondary prophylaxis is still being carried out.
For the aforementioned reasons, the Board of GESIDA and the National AIDS Plan Secretariat have felt it appropriate to update their recommendations on the prevention of opportunistic infections in HIV-infected adolescents and adults7, by including those made during the last few years. In order to classify the strength and quality of the recommendations, we have followed, on this occasion, the system used by the Infectious Diseases Society of America (IDSA) and the United States Public Health System (USPHS) (Table 1)8.
Prophylaxis of infections caused by virus
Cytomegalovirus (Table 2)
Before the introduction of HAART, approximately 45% of patients co-infected by HIV and CMV developed CMV disease. Furthermore, in those co-infected patients with a CD4+ T cell count < 100/uL, 22% developed CMV retinitis within two years. This is a serious and disabling problem whose frequent relapses and complications could lead to loss of vision9.
HAART drastically reduced the incidence of this disease and made a dramatic change to its natural history with prolonged survival and reduction of relapses and complications10-12. In fact, in one large scale randomised clinical trial comparing three anti-CMV treatments, the frequency of new CMV disease was lower in patients who had received protease inhibitors, regardless of the therapeutic group to which they had been assigned13. Therefore, we can conclude that HAART currently plays an extremely important role in the prophylaxis and treatment of CMV disease regardless of the antiviral drug which patients are receiving. It is worth bearing in mind that one of the adverse events experienced by patients with CMV retinitis who begin HAART is immune recovery vitritis, which can occasionally cause severe loss of vision11,14,15.
Prevention of exposure to the pathogen
Patients belonging to population groups with a low frequency of CMV infection, and who we cannot assume to be seropositive, should undergo a serological study for CMV. These patients include those who have never injected drugs and males who have not had homosexual relations (BIII). Patients whose CMV serology is negative must not receive transfusions of blood derivatives from patients with a positive CMV serology (B3) and must avoid sexual contact without a condom (AII). The risk of acquisition of CMV can be reduced by following good hygiene practices such as hand-washing. These practices are particularly important in settings such as kindergartens, where the risk of contagion by CMV is greater (B3)16.
Primary prophylaxis
Two prospective, randomised, double-blind, placebo-controlled clinical trials have been published on primary prophylaxis with oral ganciclovir in patients co-infected with HIV and CMV. In the first, with more than 700 patients with a CD4+ T cell count ¾50/uL or < 100/uL and a history of infection indicative of AIDS, the accumulated incidence of visceral disease by CMV at 12 months was 14% in the ganciclovir group and 26% in the placebo group, and the accumulated incidence of CMV retinitis was 12% and 24%, respectively (RR 0.49, p < 0.001); there were no differences in mortality17. The second, with more than 900 patients, was different from the first in two important areas. First, the inclusion criterion in the study with respect to baseline CD4+ T cell count was ¾100 /uL. Second, when the study was under way, and the results of the previous study were known, it was accepted that all patients had access to ganciclovir. No differences were found concerning the incidence of CMV disease and mortality until the study was modified or until it finished. Nevertheless, more adverse effects, especially neutropenia, were detected in the ganciclovir group than in the placebo group18.
Primary prophylaxis with oral ganciclovir for CMV disease is not recommended due to contradictory results concerning its efficacy, its zero impact on survival, the possibility of developing resistance, toxicity and cost (CI). The best preventive strategy is administration of HAART to restore the immune system (AI).
It is very important to bear in mind that in patients who initiated HAART with CD4+ < 50/uL, there is a risk period of 3 to 4 months during which patients can suffer from CMV retinitis (and other opportunistic infections) even with a CD4+ T cell count > 100/uL. In these cases, it is advisable to carry out antigenemia studies or CMV PCR, given that the possibility of developing R-CMV is 38% for patients who test positive, compared with 2% for patients who test negative (p < 0.001) (CII)10. Patients who test positive must be reviewed by fundoscopy every 2 or 4 weeks during the first three months for early detection of the disease (CIII). In these cases, it may make sense to administer pre-emptive anti-CMV treatment, a step which is now being evaluated in a randomised clinical trial (study ACTG A5030). This study aspires to include 750 HIV-infected patients with positive CMV serology and with a CD4+ T cell count < 100/uL despite HAART. They will all be reviewed every two months using CMV DNA by PCR and every six months by an ophthalmologic examination. Those in whom CMV viremia is detected will be randomised to receive valganciclovir or placebo. At present, more than half the patients have been included and we will have to wait some years to know the results of the study.
Secondary prophylaxis
The therapeutic strategy for CMV retinitis has been well established for years; it involves an induction phase, which aims to control the infection, followed by a maintenance phase to prevent or delay relapses9,19. For this second indication, drugs are available which can be administered intravenously such as ganciclovir, foscarnet and cidofovir. These drugs have never been compared with each other and have a different toxicological profile. Available orally administered drugs include ganciclovir and valganciclovir, which is the valine ester of ganciclovir. The bioavailability of oral ganciclovir is very poor, which makes it less efficacious than intravenous ganciclovir and forces patients to take a large number of tablets20. Valganciclovir, however, is metabolized by enzymes of the digestive tract and is practically 100% transformed into ganciclovir21. In one randomised and open-label trial involving 160 patients with AIDS and recently diagnosed CMV retinitis, oral valganciclovir was proven to be as efficacious as intravenous ganciclovir in induction therapy, and easy-to-take and efficacious in the maintenance phase13. A ganciclovir implant (unrivalled in the treatment of CMV retinitis) is available for topical use, although before HAART it had to be accompanied by oral ganciclovir to avoid both disease of the other eye and extraocular disease22. In a recent study which compared the ganciclovir implant with(out) oral ganciclovir and intravenous ganciclovir, it was verified that in the subgroup of patients treated with HAART, the incidence of relapses or new disease was low and of the same size in all groups23. Also available for topical use is fomivirsen, an antisense oligonucleotide which inhibits the replication of CMV and which is administered by intravitreous injection. In the maintenance phase the dose is 330 ug per month. Its undesirable effects are increased intraocular pressure and ocular inflammation, which are transitory or reversible with topical steroid therapy. Fomivirsen is currently indicated for the treatment of relapses24.
In the light of the numerous options available for the secondary prophylaxis of CMV retinitis, it is recommended to choose the drug which is best adapted to the patient25, although given its proven efficacy and ease of use, oral valganciclovir could be considered as the drug of choice (AI). In general, maintenance treatment with prolonged use of oral ganciclovir only should not be administered to patients who cannot receive HAART or to those in whom no improvement of the immune system is expected (DIII).
Withdrawal of secondary prophylaxis
Several small-series studies have shown the possibility of withdrawing secondary anti-CMV prophylaxis in patients who recover with HAART26,27 In the last few years, four studies have been published on the withdrawal of secondary anti-CMV prophylaxis. The first included 14 patients and no relapses were detected after a median follow-up of 16.4 months28. In the second, 3 out of 22 patients who suspended secondary prophylaxis had a relapse of CMV retinitis29. In the three cases, HAART failed and patients had a CD4+ T cell count < 50/uL at the time of the relapse. The third study was multinational and included 48 patients of whom two suffered a relapse of CMV disease: retinitis in one case and peripheral neuropathy in the other. Surprisingly, the CD4+ T cell counts at the time of the relapse were 352/ul and 106/ul, respectively30. Finally, in the fourth, a Spanish multicenter study, secondary prophylaxis was withdrawn from 36 patients and, after a median follow-up of 90 weeks, no reactivation or progression of retinitis was observed in the 35 patients who responded favorably to HAART. Nevertheless, in one patient, relapse of retinitis was observed at 44 weeks after suspending prophylaxis and in the setting of immune failure. At the time of relapse, the CD4+ T cell count was 62/uL31.
Taken as a whole, the results of these four studies guarantee the safety of interrupting secondary anti-CMV prophylaxis in patients with AIDS and inactive CMV retinitis who experience an increase in their CD4+ T cell count with HAART. Nevertheless, the lowest CD4+ T cell count at which prophylaxis can be suspended is unknown. If the data from the four studies (79 patients) are combined, it can be observed that the median CD4+ T cell count at the time of withdrawing prophylaxis was 269/ul with an interquartile range of 167 360/uL. More than two thirds of the patients had CD4+ T cell counts >200/uL, less than one third had between 100 and 200/uL and only three patients had < 100/uL. This allows us to conclude that the withdrawal of secondary prophylaxis is a reasonable and safe option in patients with inactive CMV retinitis and in those who have shown a good response to HAART characterized by a CD4+ T cell count > 200/uL for at least six months (BII). In some cases, the withdrawal of secondary prophylaxis can be considered in patients who present a count of between 100 and 200/uL, given that most of the patients who relapsed in the aforementioned studies had counts of <100/uL (CIII). After secondary prophylaxis has been withdrawn, patients can be monitored with periodic determinations of their CD4+ T cell count (BIII). In those who experience immune failure, it should be decided whether to carry out frequent ophthalmologic check-ups or re-initiation of secondary prophylaxis, depending on the risk of suffering an irrecoverable loss of vision (BIII) (Table 3)
Other viruses (Table 2)
HIV-infected patients often suffer from digestive and mucocutaneous HSV infection but primary prophylaxis against these infections is not recommended (DIII). Relapses respond well to therapy, therefore suppressive therapy is not advised except for genital herpes with frequent and/or severe relapses (≥ 6 relapses per year) (AI). Consequently, the following are recommended for HIV-infected patients: acyclovir (400-800 mg two to three times per day), or famciclovir (500 mg twice per day) or valacyclovir (500 mg twice per day) (32). In infections by acyclovir-resistant HSV strains, intravenous or topical cidofovir or intravenous foscarnet should be used (AII). Given that the frequency of relapses falls with time in many patients, it is recommended to periodically evaluate (e.g. every year) the withdrawal of suppressive therapy (BII)32.
HIV-infected patients should not be vaccinated with the varicella zoster virus (VZV), although those who live with them should, in case they are susceptible to VZV (those without specific IgG antibodies) (BIII). HIV-infected patients who are susceptible to VZV should avoid contact with people with Varicella or Zoster (AII). For post-exposure prophylaxis in susceptible subjects, specific gammaglobulin is recommended within the 96 hours following contact (AIII). Another cheaper and logistically simpler option is the administration of oral acyclovir (CIII), although the efficacy of this measure has only been proven in immunocompetent children after exposure at home16,33,34.
Vaccination against the hepatitis A virus (HAV) is recommended for all HIV-infected patients who do not have anti-HAV IgG antibodies and who present a CD4+ T cell count > 200/uL (AIII)35. This practice is especially recommended in patients with chronic C hepatitis, given that there is a risk of fulminant hepatitis and death in HAV superinfection36.
Vaccination is also recommended against hepatitis B virus (HBV) in all HIV-infected patients who are HBsAg and anti-HBc-negative and who have not already been vaccinated (AIII). The standard regimen of vaccination against HBV is three injections (0, 1 and 6 months) with 20 ug of antigen. Nevertheless, the immunogenic response with the standard vaccination regimen for HBV is reduced in HIV-infected patients and is related to the CD4+ T cell count. Given that the response to the HBV vaccine in immunodepressed patients can increase to 90% with greater doses of antigen and/or by increasing the number of injections, some bodies such as GESIDA and the National AIDS Plan Secretariat recommend for this type of patient vaccination with four injections (0, 1, 2 and 6 months) and double the quantity of antigen (40 ug instead of 20 ug) (BIII)35.
Little is known about the frequency and consequences of coinfection by HIV and influenza. Some retrospective studies have found that influenza has greater morbidity and mortality in HIV-infected patients than in the general population. Nevertheless, there is evidence that hospital admissions due to influenza have fallen significantly during the HAART era, and have reached rates similar to those of other population groups considered "high risk"37. For these reasons, and given that antiflu vaccination can produce a protective antibody titer in HIV-infected patients, it is recommended that they all (even pregnant women) receive the vaccination every year (AIII)16,38.
HAART is the only intervention which can prevent progressive multifocal leukoencephalopathy (PML) and which can interrupt the lytic cycle of the JC virus. According to recent data, approximately one third of patients with AIDS and PML who receive HAART survive, and of these, approximately half experience some degree of improvement in their neurological function. Mortality is higher in those with a CD4+ T cell count <100/uL39.
Prophylaxis of infections by bacteria and mycobacteria
Mycobacterium tuberculosis (Table 4)
HIV is the most important risk factor for the progression of latent tuberculosis to active tuberculosis and it favors progression of tuberculous disease after recently acquired infection40,41. Therefore, the notification of cases of tuberculosis increases significantly in countries with a high prevalence of HIV infection. Fortunately, in several different countries, the introduction of HAART has led to a reduction in the number of cases of coinfection by HIV and tuberculosis42.
Prevention of exposure to the pathogen
HIV-infected patients should be informed about how tuberculosis, is transmitted, their risk of developing it and the meaning of the Mantoux test. As far as possible, they should avoid working in high-risk environments such as prisons, homeless shelters and hospital units with active tuberculosis patients (BIII). They should also know the advantages of consulting their doctor when they have symptoms suggestive of tuberculosis or after coming into contact with a person suffering from active pulmonary tuberculosis (BIII).
Primary prophylaxis
Evaluation of the risk of developing tuberculosis: After the first visit, the Mantoux test must be carried out (AI). Some years ago, cutaneous anergy testing was also recommended, although recent studies have shown its poor consistency and reliability, as well as the lack of benefit from chemoprophylaxis in anergic patients, especially if they can receive HAART43-46. Consequently, these tests are not currently recommended when deciding on the treatment of latent tuberculous infection47 (DII). It has been suggested that HAART-mediated immune reconstitution could give a positive Mantoux test or other skin tests in previously anergic patients. Nevertheless, in a Spanish multicenter study including HIV-infected patients with < 50 CD4+ lymphocytes/uL and cutaneous anergy, reversion of the anergy was observed in more than one third of the patients who increased their CD4+ T cell count after prolonged HAART, with no response to tuberculin observed in any cases48. Therefore, there is no basis for repeating the Mantoux test as an immune reconstitution measure after HAART. The test should be repeated to evaluate the risk of conversion in people who live in areas with a high risk of transmission of active tuberculosis (BIII).
There can be no doubt that the two groups of patients who should receive treatment for latent tuberculous infection are those with a positive Mantoux test (≥ 5 mm) (AI) and those who have come into close contact with a person with bacilliferous TB (BII). The risk of tuberculosis among anergic patients varies a great deal from one study to another, therefore universal recommendations cannot be made49-51. Prophylaxis is indicated in anergic patients with a greater risk of infection by M. tuberculosis, for example, those with a previous positive Mantoux test, those who have had prolonged contact with people with active tuberculosis and those who have spent long periods in prisons without receiving adequate prophylaxis (CIII). Before starting chemoprophylaxis, it is important to rule out active tuberculosis by clinical evaluation and chest X-ray; when there is the slightest suspicion of tuberculous disease, microbiology tests should also be performed.
Drugs and regimens. In antituberculous chemoprophylaxis, the following have proven to be efficacious: isoniazid daily or two days per week for 6-12 months52-55, rifampin with pyrazinamide daily for 2 or 3 months56 or every other day57, and isoniazid with rifampin for three months44 (AI). The latest guidelines from the American Thoracic Society and the Centers for Disease Control and Prevention recommend regimens with isoniazid for nine months and advise against regimens of 6 or 12 months58. Furthermore, direct supervision of chemoprophylaxis is recommended when it is administered on alternate days, especially in short regimens, and also when six-month isoniazid regimens are used in severely immunodepressed patients. There are no data which lead us to believe that administration of isoniazid for more than 12 months or for life provides additional advantages. Therefore, these strategies are not recommended (EIII).
It is important to point out that after reports of severe hepatotoxicity, which in some cases is fatal, with regimens of rifampin and pyrazinamide, the CDC collected information on cohorts of patients in the U.S. who had received prophylaxis with these regimens. They found an abnormally high frequency of hospital admissions and death due to hepatic toxicity from these drugs. On the basis of these findings, the American Thoracic Society, the Centers for Disease Control and Prevention and the Infectious Diseases Society of America do not recommend using this prophylaxis regimen59. Nevertheless, in the two large clinical trials which studied the regimens of rifampin and pyrazinamide in HIV-infected patients, no differences were observed in adverse effects or global mortality among groups assigned to rifampin and pyrazinamide and those assigned to isoniazid56,57. For this reason, these regimens could be used in HIV-infected patients in situations where there are clear practical advantages for the patient or in tuberculosis control programs as long as a strict clinical and analytical follow-up of the patient is carried out (DI).
In the case of infection by isoniazid-resistant M. tuberculosis, rifampin can be used for only four months. A short regimen of rifampin and pyrazinamide can also be used, but in the light of what has previously been mentioned, it is prudent to avoid this regimen when another efficacious regimen can be used60.
Interactions with antiretroviral drugs. Isoniazid can be administered with any combination of antiretrovirals. Rifampin must not be administered simultaneously with some protease inhibitors (indinavir, nelfinavir, saquinavir, amprenavir, lopinavir/ritonavir) or with some non-nucleoside reverse transcriptase inhibitors (delavirdine). The following can be administered with rifampin: all nucleoside analogs, ritonavir as the only protease inhibitor61 and the non-nucleosides nevirapine and efavirenz62,63, and perhaps also the combination of two protease inhibitors64. Rifabutin is recommended as an alternative to rifampin in patients whose antiretroviral drugs interact with it; although it should be made clear that there are no clinical studies which support this recommendation. When combined with indinavir, nelfinavir or amprenavir, rifabutin can be administered daily but at half the dose, or at the complete dose but only two or three days per week. In these cases it will also be necessary to increase the dose of the protease inhibitors65. Rifabutin in combination with ritonavir or with lopinavir/ritonavir must be administered at half-dose two or three days per week.
Rifampin increases the hepatic metabolism of methadone and usually precipitates withdrawal symptoms in patients in opiate withdrawal programs. It is important to inform the patient about this undesirable effect and increase the dose of methadone to the necessary level.
Vaccination with BCG: This vaccine is contraindicated in HIV-infected patients due to the controversial nature of its efficacy and the risk of BCG-disseminated disease66 (EIII).
Secondary prophylaxis
Secondary prophylaxis is not recommended in patients with documented tuberculosis (EIII).
Mycobacterium avium complex (MAC) (Table 4)
Prevention of exposure to the pathogen
MAC is a ubiquitous microorganism in the environment (including water and food) and no efficacious measures are known to prevent its acquisition.
Primary prophylaxis
Clarithromycin (500 mg/12 hours) or azithromycin (1200 mg, once per week) prevents disseminated MAC infection67,68 (AI). Nevertheless, this strategy is not recommended in our environment given the low incidence of this opportunistic infection even before the introduction of HAART (DIII). In a cohort study performed in Spain during the HAART era including 200 patients with CD4+ T cell counts below 50/uL, the incidence of disseminated MAC infection was 2 cases per 100 patients/year. In special situations, for example, in patients with CD4+ T cell counts constantly below 50/uL and with no possibility of receiving HAART, primary prophylaxis can only be considered with some of the abovementioned regimens. Primary prophylaxis can be interrupted safely in patients who manage to maintain CD4+ T cell counts above 100/uL for longer than 3-6 months69 (AI).
Secondary prophylaxis
Patients with disseminated MAC infection must receive therapy with clarithromycin (or azithromycin as an alternative) and ethambutol for as long as they are severely immunodepressed (AI).
Withdrawal of prophylaxis
During the pre-HAART era, lifelong maintenance treatment was recommended, although more and more data support its withdrawal in patients who maintain CD4+ T cell counts above 100/uL for more than six months (BII) (Table 3)70-73.
Other bacteria (Table 4)
Streptococcus pneumoniae
There is some controversy concerning the recommendation of pneumococcal vaccine in HIV-infected patients. Some observational studies have shown a certain degree of protection with the vaccine. Nevertheless, in a randomised, double-blind study in Africa, no beneficial effect was found with the vaccine and an association between the vaccination and a greater risk of pneumococcal disease was observed74. A recent review of studies to date concluded that pneumococcal vaccine confers no benefit and its systematic use is not advised75 (CI). If used, it is recommended in patients with a CD4+ T cell count >200/uL or even in patients with lower counts, although the response may be even less certain. Revaccination is likely to be necessary every five years, but there are no data supporting this recommendation.
Haemophilus influenzae
HIV-infected children must receive the Haemophilus influenzae vaccine in line with the habitual vaccination schedule (AI). This vaccine is neither indicated nor contraindicated in adults.
Miscellaneous
No primary or secondary prophylaxis is currently recommended in infections by Salmonella no-typhi, Campylobacter spp or Bartonella spp (EIII).
Prophylaxis of fungal infections
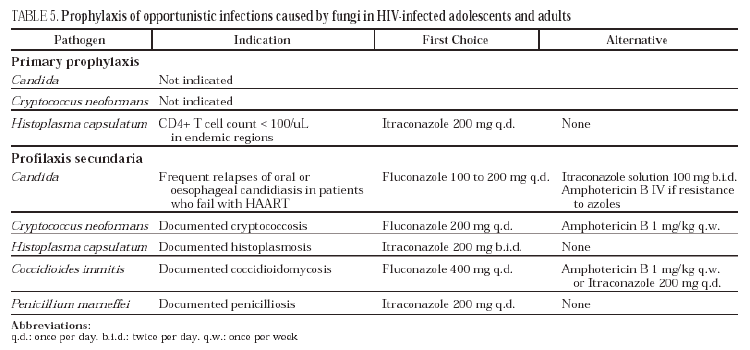
Candida (Table 5)
Prevention of exposure to the pathogen
Oropharyngeal candidiasis is the most common opportunistic infection in HIV-infected patients. Candida albicans (C. albicans) the main pathogen in this mycosis is a commensal of the human digestive tract and, therefore, does not require prophylaxis.
Primary prophylaxis
Not recommended for this mycosis (DII)
Secondary prophylaxis
Oral candidiasis responds very well to systemic antifungals but in advanced immunodepression almost 80% of patients suffer relapses during the first three months after finishing therapy. Different studies have shown that fluconazole or itraconazole in solution reduces relapses. Nevertheless, systematic use is not recommended given that the relapses are not severe, can be diagnosed easily and respond well to treatment (DII). Furthermore, secondary prophylaxis with fluconazole may favor infections by azole-resistant strains of Candida. HAART is currently the best strategy for avoiding oropharyngeal candidiasis76 (AII). Secondary prophylaxis is not recommended for oesophageal candidiasis for the same reasons as those above (DII). When HAART fails and the patient presents frequent relapses, secondary prophylaxis may be considered with daily doses of fluconazole (100 to 200 mg), as it has not been shown that weekly dosing is as efficacious in oesophageal candidiasis77 (CIII). Some patients develop azole-resistant candidiasis and require chronic suppressive therapy with amphotericin B (CIII).
Cryptococcus neoformans (Table 5)
Prevention of exposure to the pathogen
No effective avoidance measures are known despite the fact that, in most cases, Cryptococcus neoformans enters the body via the respiratory tract.
Primary prophylaxis
Many studies have been published (retrospective series, case control studies and randomised clinical trials) which have shown a reduction in the risk of cryptococcosis with daily and even weekly doses of 100 to 200 mg of fluconazole. Despite this, primary prophylaxis for this mycosis is not recommended due to its relatively low incidence in developed countries, the fact that it has not been shown to improve patient survival77,78, its cost and the possibility that it favors the development of resistant mycoses (DI).
Secondary prophylaxis
Before the introduction of HAART, relapses due to AIDS-associated cryptococcosis were very common after finishing induction treatment, and different studies showed the efficacy of secondary prophylaxis in preventing them. Recent studies have observed that in patients who show immune recovery with HAART, the risk of a relapse of cryptococcosis decreases79,80. In any case, all patients must carry out prophylaxis after treatment of the acute phase of cryptococcosis. The regimen of choice is fluconazole 200 mg/day, which reduces the frequency of relapses to 2-4% (AI)81. The alternatives are amphotericin B 1 mg/kg per week, with a relapse rate of 17%81, and itraconazole 200 mg/day with a relapse rate of 23%82.
Withdrawal of prophylaxis
There is a clinical trial83 and a cohort study84 which show that prophylaxis can be withdrawn safely in asymptomatic patients with a CD4+ T cell count above 100/uL for at least three months and a plasma viral load under 5000 copies/uL, without the need for the cryptococcal antigen to be negative (BII). After withdrawal of secondary prophylaxis, patients must receive periodic clinical and analytical check-ups. It is advisable to resume prophylaxis whenever the CD4+ T cell count falls below 100/uL or when a negative cryptococcal antigen reverts to positive (BIII).
Other fungi (Table 5)
Histoplasma capsulatum
This is the most frequent regional mycosis in AIDS patients. In endemic areas, histoplasmosis can be prevented by avoiding risk activities such as visits to caves, exposure to environmental dust, tree felling, cleaning of henhouses and demolishing or clearing of buildings (CIII). Primary prophylaxis is only indicated in patients with a CD4+ T cell count below 100/uL and with a high occupational risk in hyperendemic zones (CI)85. In Spain, primary prophylaxis could be considered for HIV-infected immigrants from endemic countries. For secondary prophylaxis, itraconazole 200 mg/12 hours is recommended (AII)86. For the withdrawal of secondary prophylaxis in patients who recover their immune function with HAART, the same criteria as for cryptococcosis can be applied (CIII).
Penicillium marneffei
Penicilliosis is an endemic mycosis in Southeast Asia which responds well to treatment with amphotericin B or itraconazole. Neither the reservoir of the fungus nor the portal of entry of the infection is well known, with the result that measures to avoid contagion cannot be recommended. In endemic areas, primary prophylaxis with itraconazole reduces incidence of penicilliosis in severely immunodepressed HIV-infected patients (especially with a CD4+ T cell count < 100/mm3), although this intervention has not been shown to prolong patient survival87 (CII). Post-therapy relapses are very frequent but a prospective, randomised and placebo-controlled study has shown the efficacy of secondary prophylaxis with itraconazole at 200 mg/day88 (AI). The impact of HAART on relapses of this opportunistic infection is not well known.
Miscellaneous
For coccidioidomycosis, secondary prophylaxis is recommended with fluconazole 400 mg/day or itraconazole 200 mg twice per day (AII)89. There are no studies on secondary prophylaxis for aspergillosis, blastomycosis or paracocccidioidomycosis16.
Prophylaxis of infections by parasites
Most parasitic infections in HIV-infected patients result from reactivations of latent infections in situations of severe immunodepression and their incidence reflects the prevalence of the different parasites in the general population90. Some of these reactivations can be prevented with chemoprophylaxis.
Pneumocystis jiroveci (previously Pneumocystis carinii) (Table 6)
Despite the fact that Pneumocystis jiroveci (P. jiroveci) is a fungus, it is included in this section because its prophylaxis and treatment are with antiparasitic drugs and not with antifungals. The taxonomy of the organism has changed. At present, P. jiroveci is the name reserved for the species which infects humans, and Pneumocystis carinii (P. carinii) is the name of the species which infects rodents91. Despite the change in nomenclature, the acronym PCP can be maintained, as it is also the abbreviation of "Pneumocystis pneumonia". P. jiroveci pneumonia can appear when the CD4+ T cell count is below 200/µL92. It has been the most common AIDS-defining disease and the first in which the efficacy of chemoprophylaxis was shown. Although its incidence has fallen during the HAART era3,93,94, it is still the most common manifestation of AIDS in patients who do not know they are infected by HIV. In countries where HAART is not available, its prevalence continues to be very high95.
Prevention of exposure to the pathogen
It has traditionally been assumed that P. jiroveci enters the body via the respiratory tract during infancy, giving rise to a latent infection which can be reactivated in situations of severe immunodepression96. Recently, there have been reports suggesting that the infection can be transmitted to susceptible persons from patients with P. jiroveci pneumonia. Nevertheless, interpatient transmission must be very low, if it actually happens97,98. Therefore, patients at risk cannot be recommended to avoid close contact with others who suffer from P. jiroveci pneumonia (CIII).
Primary prophylaxis
This should be initiated when the CD4+ T cell count is below 200/µL and in the presence of an AIDS defining disease, oral candidiasis or unexplained fever lasting more than 20 days (AI). Prophylaxis may be considered when the percentage of CD4 cells is below 14% or between 200-250 /mL and the patient cannot be monitored every three months99 (BII). The combination of trimethoprim-sulfamethoxazole (TMP-SMZ) is considered the drug of choice due to its efficacy, ease of use and cost/benefit relationship (AI). The first studies were carried out with daily doses of TMP-SMZ of 160/800 mg (1 "Forte" tablet)1, but it was later shown that tolerance is better and efficacy similar with three "Forte" tablets per week (AI) or with a "normal" tablet (80/400) every day (AI)100,101. If hypersensitivity reactions appear, desensitization must be tried before prescribing an alternative drug16. Aerosolized pentamidine is considered the second choice and must be administered using special equipment (Respigard" II or Fisoneb")(BI)102. This prophylaxis is less efficacious than oral TMP-SMZ and does not protect against extrapulmonary forms of the disease or other infections such as toxoplasmosis103. Its disadvantages include bronchospasm and metallic taste. In the care environment it can also lead to problems, such as irritability of the airway and risk of dissemination of tuberculosis. Therefore, this aerosol must be administered in an isolated, well-ventilated area. Valid, but less well studied alternatives are dapsone (BI), dapsone/pyrimethamine (BI) and atovaquone (BI)101,104,105, which may require the administration of more than one drug, thus making it difficult to adhere to prophylaxis or HAART.
Secondary prophylaxis
After P. jiroveci pneumonia, secondary prophylaxis must be administered to prevent relapses (AI). TMP-SMZ (1 "Forte" tablet daily or three days per week) is more efficacious than aerosolized pentamidine to prevent local and/or extrapulmonary relapses106 (AI).
Withdrawal of prophylaxis
Primary prophylaxis can be withdrawn in those patients receiving HAART for more than six months and who have a well controlled viral load (undetectable or <5000 copies/µL) and a CD4+ T cell count above 200/µL for at least three months107-110 (AI). These same criteria are valid for the suspension of secondary prophylaxis107,108,111-113 (AI). The withdrawal of prophylaxis reduces pharmacological toxicity, simplifies treatment and can make adherence to HAART easier as it reduces the pill burden. There have been reports of some cases of relapse of the disease after withdrawal of prophylaxis. These patients were generally elderly, had another type of immunosuppression (e.g. lymphoma), had developed P. jiroveci pneumonia with CD4+ T cell counts above 200/µL, or had abandoned HAART.
Restarting prophylaxis
Although no data are available, it is advisable to restart prophylaxis if the CD4+ T cell count falls below 200/µL or the patient presents an episode of P. jiroveci pneumonia (CIII).
Toxoplasma gondii (Table 6)
Cerebral toxoplasmosis is the most common form of encephalitis in AIDS and occurs in patients whose CD4+ T cell count is below 100/µL. This infection can complicate the course in 10-20% of HIV-infected patients with a positive T. gondii serology114, although its incidence has fallen with the use of TMP-SMZ and HAART.
Prevention of exposure to the pathogen
T. gondii is acquired through consumption of contaminated meat, eggs, greens and vegetables, and by exposure to cat feces115. Patients with a negative serology must eat meat well cooked (the inside must not be pink). Those patients who do not wish to give up rarely-cooked meat, can freeze it to a temperature below -20ºC before cooking. They must also wash fruit and vegetables well to avoid infection (BIII). Hand-washing is advised after touching raw meat, vegetables or soil (gardening) (BIII). If a cat is kept as a pet, it should be fed using commercially available products and the meat consumed by the cat should be well cooked. Furthermore, its excrement should be cleared away using gloves (BIII).
Primary prophylaxis
Prophylaxis should be initiated in patients with positive serology (anti-Toxoplasma IgG antibodies) and CD4+ T cell counts below 100/µL (AII), although some authors recommend starting it with a CD4+ T cell count below 200/µL101,116 (BII). The first studies of prophylaxis were retrospective observational studies of patients with prophylaxis for P. jiroveci with TMP-SMZ. Toxoplasmosis can be prevented with a normal daily tablet of TMP-SMZ (80/400) or a "forte" tablet (160/800) three days per week116 (AII). Nevertheless, a daily "forte" tablet is advised in patients with severe immunodepression, in those simultaneously receiving drugs which can reduce the plasma levels of TMP-SMZ (e.g. rifampin) and in those whose with a very high anti-Toxoplasma IgG antibody titer117-119 (BII). In patients who cannot tolerate TMP-SMZ, dapsone in combination with pyrimethamine and folinic acid (BI), atovaquone (alone or in combination with pyrimethamine and folinic acid) (CIII) or pyrimethamine (CI) can be administered101,116,120,121.
Secondary prophylaxis
If maintenance treatment is not administered, relapse of cerebral toxoplasmosis occurs in 60 -100% of cases between 6 12 months after finishing induction treatment122. Of the accepted regimens for secondary prophylaxis, the most efficacious is the combination of pyrimethamine with sulfadiazine, which can be administered daily or on alternate days123,124 (AI). If sulfadiazine cannot be administered, it can be replaced by clindamycin123 (BI). If there is intolerance to both drugs, there is very little experience with alternatives. In these patients it is recommended to maintain therapy with the drug used during the acute phase: pyrimethamine alone or combined with atovaquone, azithromycin, minocycline or doxycillin, 5-fluorouracil and clindamycin, and minocycline or doxycillin with sulfadiazine. Similarly, there is very little experience with dapsone and pyrimethamine or with cotrimoxazole123,125-142 (CII). Clarithromycin has also been used (1g/12 h) instead of azithromycin, but it is not recommended, given that clarithromycin at these doses has been associated with excessive mortality in a study on prophylaxis against MAC143.
Withdrawal of prophylaxis
Although there are few studies, it is considered that primary prophylaxis can be withdrawn when the requisites for withdrawal of primary prophylaxis of P. jiroveci are met: HAART for at least six months, a CD4+ T cell count above 200 µL and controlled viral load144-147 (AI). There are insufficient data in the literature which totally guarantee the withdrawal of secondary prophylaxis against this pathogen. Nevertheless, in the light of existing studies, it can be deduced that secondary prophylaxis can be suspended when the same criteria as for withdrawal of primary prophylaxis are met73,111,144,148-151 (CIII) (Table 3).
Leishmania spp (Table 6)
Visceral leishmaniasis is one of the most frequent HIV-associated parasites in Spain and other Mediterranean countries. It presents in very immunodepressed patients and its prevalence varies according to the presence of Leishmania infantum (causal agent) in reservoirs (in our environment, canids). There is some evidence that HAART has modified the incidence of visceral leishmaniasis152-154 and has reduced its relapses in HIV-infected patients152,155. Nevertheless, relapses may occur in patients who maintain a low CD4+ T cell count despite HAART155.
Prevention of exposure to the pathogen
It seems likely that leishmaniasis can be transmitted from person to person via syringe sharing154,156, thus providing yet another argument against this practice (CIII). Furthermore, in areas where the canine reservoirs present a high prevalence of infection, dogs should not be kept as pets (CIII).
Primary prophylaxis
No primary prophylaxis against this infection has been established.
Secondary prophylaxis
In the pre-HAART era, the accumulated incidence of relapses after a first episode of correctly treated visceral leishmaniasis was 60% at six months and 90% at 12 months156,157. The value of secondary prophylaxis in HIV-infected patients has been proven in a randomised, prospective and multicenter study carried out in Spain. This study compared the efficacy of amphotericin B lipid complex (3 mg/kg/d, every 21 days) with a control group. Intention-to-treat analysis at 12 months of follow-up showed that 50% of patients who received prophylaxis were free from relapses compared with 22% in the control group158 (BI). A non-randomised retrospective study found that secondary prophylaxis with a monthly dose of 850 mg of pentavalent antimony (Glucantime) reduced the frequency of relapses of visceral leishmaniasis compared with historic controls and a group treated with allopurinol157. These findings have not been confirmed in prospective studies. Miltefosine is a recently introduced oral drug which is as efficacious as amphotericin B for the treatment of visceral leishmaniasis in non-HIV-infected patients159, although there is very little experience with this drug in the treatment of leishmaniasis in HIV-infected patients both for the acute phase and for maintenance.
Withdrawal of prophylaxis
No clear recommendations can be made for the withdrawal of secondary prophylaxis against this pathogen, but its application could be considered in patients who manage to remain at least six months without relapses and who have a CD4+ T cell count above 200/uL and preferably 350/uL160 (BII) (Table 3).
Other parasites (Table 6)
Cryptosporidium spp.
This is an intracellular protozoan which produces diarrhea in animals and humans. Of the species known, C. parvum and other species (C. muris, C. maleagridis) infect humans161. The parasite is acquired via the digestive tract on ingesting water or contaminated food and by contact with infected humans or animals. In HIV-infected patients with CD4+ T cell counts below 100/uL it produces chronic diarrhea which is refractory to treatment. Its frequency varies between 10-15% in the west and up to 50% in developing countries162,163. A lower proportion of patients present biliary involvement. In order to prevent cryptosporidiasis, the patient must be informed about the ubiquitous nature of the parasite, especially in foods which are consumed raw (vegetables, oysters, etc.), water and excreta. Contact should also be avoided with infected patients or, if this is not possible, extremely strict hygiene practices should be observed16 (BIII). There is no efficacious chemoprophylaxis for this infection. Nevertheless, it has been suggested that prophylaxis for MAC with rifabutin or clarithromycin could reduce its incidence, although there are no conclusive data164,165.
Microsporidia
In severely immunodepressed patients, Microsporidiosis is the most common cause of chronic diarrhea, without a pathogen which can be identified by conventional methods166. The route of transmission is not clear and its prevalence is unknown given the difficult nature of diagnosis. In our environment, it has been found in 22% of AIDS patients with chronic diarrhea167. Most episodes are caused by Enterocytozoon bieneusi and less frequently by Encephalitozoon intestinalis which, in turn, can produce systemic infections. There is no chemoprophylaxis for this infection. Prolonged therapy with albendazole can control symptoms.
Isospora belli
This was a causal agent of chronic diarrhea during the early years of the AIDS epidemic but, at present, it has almost disappeared thanks to prophylaxis with TMP-SMZ168,169. After an episode of isosporiasis, secondary prophylaxis with TMP-SMZ must be started (CIII).
Acknowledgments
We would like to thank Luis Guerra for his contribution to previous editions of this document and Daniel Podzamczer for his comments during the time the manuscript was on the GESIDA web page. We acknowledge the work of Thomas O'Boyle in the English translation of the manuscript.
Dr. José M. Miró was a recipient of a Research Grant from the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona (Spain).