The purpose of this study was to determine the prevalence of IgG antibodies against Bartonella sp. in a randomly selected sample from the population of the patients of North Sanitary District of Jaén.
MethodsWe used a commercially available immunofluorescent test (Focus-Technology IFA Bartonella quintana and B. henselae test).
ResultsSix hundred five healthy individuals were divided by sex into three age groups. We detected that 13.55% and 11.07% subjects were IgG seropositive to B. henselae and B. quintana, respectively.
ConclusionsOur data show that the prevalence of both Bartonella species in Andalusia (Southern Spain) is relatively high. No statistical difference in the seropositivity was observed among these groups. In both cases, the IgG antibody titers ranged from 1/128 to 1/512.
El propósito de este estudio fue determinar la prevalencia de anticuerpos IgG frente a Bartonella sp. en una muestra escogida al azar de la población de pacientes del Distrito Sanitario Norte de Jaén.
MétodosSe ha utilizado una prueba de inmunofluorescencia disponible comercialmente (Focus-Technology IFA Bartonella quintana y prueba de B. henselae). Seiscientos cinco individuos sanos se dividieron por sexo en 3 grupos de edad.
ResultadosDetectamos que el 13,55% y el 11,07% de los sujetos eran IgG seropositivos a B. henselae y B. quintana, respectivamente. En ambos casos, los títulos de anticuerpos IgG variaron de 1/128 a 1/512.
ConclusiónNuestros datos muestran que la prevalencia de ambas especies de Bartonella en Andalucía (sur de España) es relativamente alta. No se observaron diferencias estadísticas en la seropositividad entre grupos de edad.
Bartonella infections have gained considerably importance in international public health during the last decades, mainly due to their worldwide distribution and their morbidity rate among human populations. In addition, these bacteria cause a growing clinical spectrum, which often results in a challenge for clinicians due to the limitation of the microbiological tests used for diagnosis.1
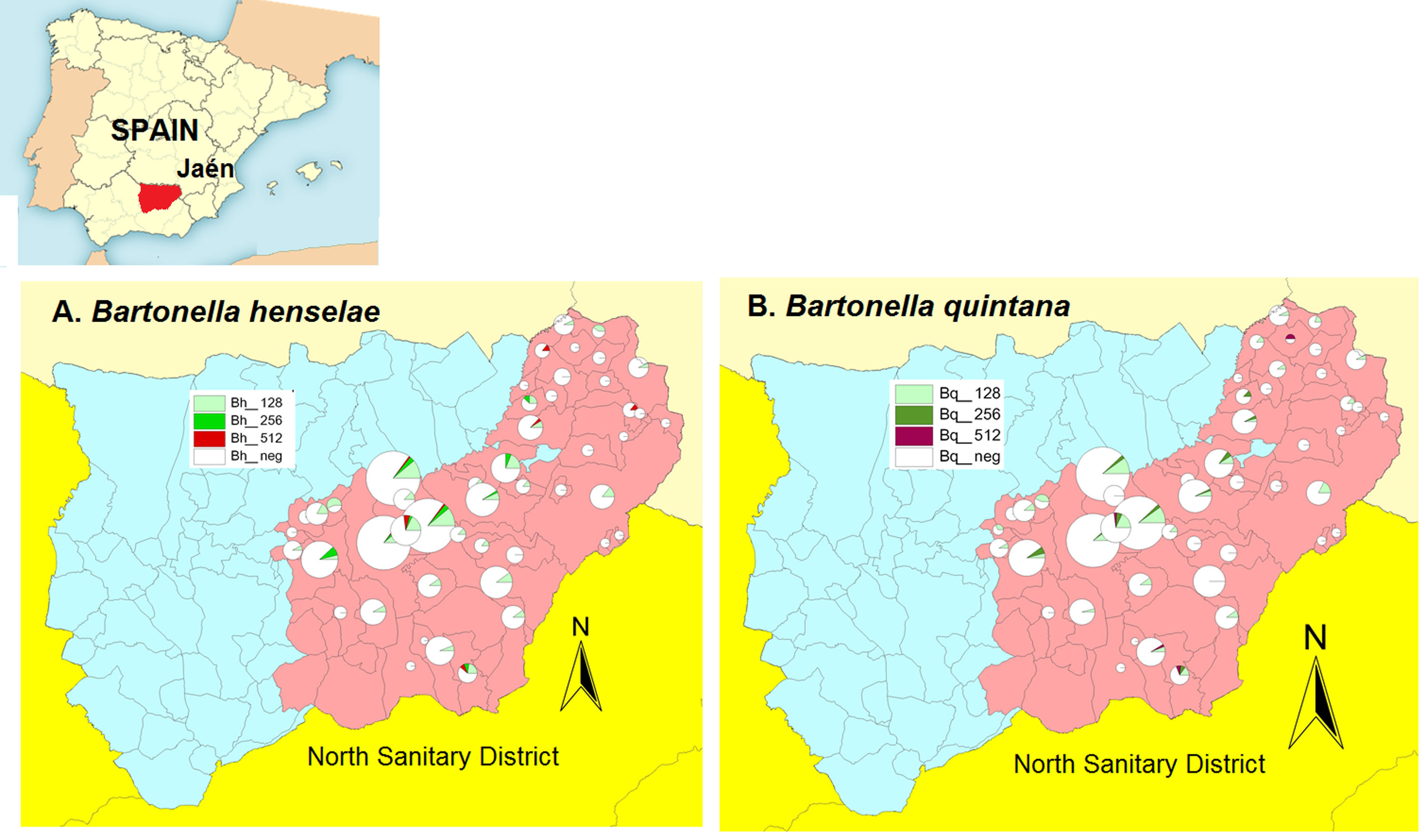
The aim of this paper is to evaluate the prevalence of past infection due to Bartonella spp. in Jaén province (Andalusia, Spain).
Methods: Serum samples from 605 healthy individuals (222 men, 383 women) from North Sanitary District of Jaén province (Andalusia) were tested for the presence of IgG antibodies against Bartonella by indirect immunofluorescence assay (IFA) using commercially available antigen for B. henselae and B. quintana (Focus Technologies, Cypress, CA). The average age in the sample was 52.4 years (55.2 for men, rank 3–106 years; 50.8 for women, rank 2–97 years), with a standard deviation of 21 years. The kit for detecting IgG antibodies uses Vero cells infected with either B. henselae or B. quintana. The serum samples were initially diluted 1/64 for the detection of IgG antibodies. Any serum sample found to be positive at the initial dilution was further titrated. Positive and negative controls were included in each test. Endpoint titters were obtained by serial dilution on positive specimens, with titers≥1/128 considered indicative of past infection to Bartonella spp.
For statistical analysis we considered gender and three age groups (less than 19, between 19 and 65, and 66 and more years old). Statistical analysis was performed using statistical tools of Microsoft Office Excel 2007. The Student's t-test was used to compare mean ages of subjects seropositive and seronegative for B. henselae and B. quintana. The chi-square test and Fisher's exact method for small samples were used for comparison of prevalence rates in the subgroups.2 Also we compared the results of the serological assays with the results of a previous serological study carried out with the same sera for investigating the seroprevalence against Rickettsiatyphi.3
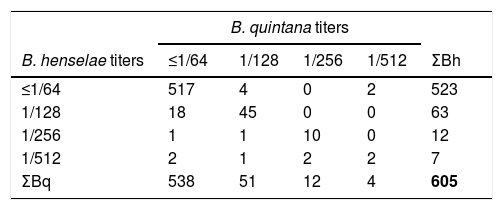
ResultsWe found a global prevalence of infection of 13.55% (82 positives) and 11.07% (67 positives) to B. henselae and B. quintana respectively, corresponding with 63 (51), 12 (12) and 7 (4) positive samples with titter 1/128, 1/256 and 1/512 respectively for B. henselae (and B. quintana) (Table 1). We do not find significant differences when we studied the distribution of positives in relation with age and gender. The number of positive serologies considering both B. henselae and B. quintana was 68 (11.18% over total population). Fig. 1 shows the distribution of the titles obtained in the municipalities of origin of the individuals studied, both for B. henselae and B. quintana.
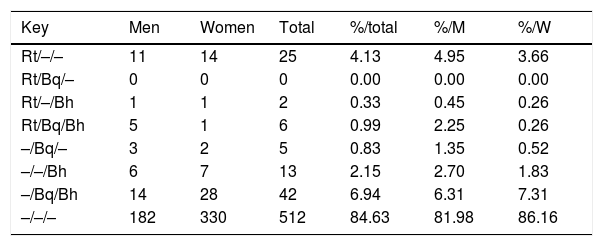
When we compared titles against Bartonella spp. and Rickettsia typhi over the same human serum samples3 over all positive serum (title≥1/128) for R. typhi, B. henselae and B. quintana, we observed that the 51.7% of positive sera react only with R. typhi, 23.86% of serums react against B. quintana and B. henselae, and 10.79% shows positive reaction against these three pathogens (Table 2).
Pattern of IgG antibody titers≥1/128 againts R. typhi, B. quintana and B. henselae (showed as Rt/Bq/Bh) in the study population (N=605).
| Key | Men | Women | Total | %/total | %/M | %/W |
|---|---|---|---|---|---|---|
| Rt/–/– | 11 | 14 | 25 | 4.13 | 4.95 | 3.66 |
| Rt/Bq/– | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Rt/–/Bh | 1 | 1 | 2 | 0.33 | 0.45 | 0.26 |
| Rt/Bq/Bh | 5 | 1 | 6 | 0.99 | 2.25 | 0.26 |
| –/Bq/– | 3 | 2 | 5 | 0.83 | 1.35 | 0.52 |
| –/–/Bh | 6 | 7 | 13 | 2.15 | 2.70 | 1.83 |
| –/Bq/Bh | 14 | 28 | 42 | 6.94 | 6.31 | 7.31 |
| –/–/– | 182 | 330 | 512 | 84.63 | 81.98 | 86.16 |
Our study confirms the widespread distribution of Bartonella sp. in Jaén province, as reflected by moderate prevalence of past infection due to Bartonella spp. agents in a representative sample of general population. Since in our study the titters of antibodies to B. henselae and B. quintana are very similar we cannot know the real prevalence of both microorganisms due to cross reactions among the different species of Bartonella. Only in 4 sera we have found difference more than 2 dilutions among the samples.
Several studies developed in Europe and America found comparable prevalence. In Greece, the observed seroprevalence for IgG antibodies were of 19.8% and 15% to B. henselae and to B. quintana, respectively.2 In a rural area of Brazil, da Costa et al.4 found a seroprevalence of 13.7% and 12.8% for B. henselae and B. quintana. Moreover, in a study conducted on blood donors shows that seropositivity rate of IgG antibodies against B. henselae range from of 3.3% (n=333) in Turkey,5 3.61% (n: 140), in New Zealand,6 4,4% (n: 498) in Sweden7 and 11.4% (n: 122) in Italy.8 In the first studies carry out in Spain (La Rioja) the prevalence of antibodies to B. henselae in cat owners, healthy population and HIV patients was respectively 28.9%, 5.9% and 17.3%.9,10 In a study carry out in Catalonia, B. henselae seroprevalence was signaled as 8.7% in a study carried out on a healthy population.11 Studies developed on veterinarian personnel shows high seroprevalence values than in blood donors or health people (33% for B. henselae and 10% for B. quintana; n=87).12 Seroprevalence to Bartonella quintana and Bartonella henselae among urban homeless and marginalized people in Europe and United States, range from 0–37.5% to 0–10.3% respectively.13
Multiple reactivity or cross-reactivity with other rickettsial antigens was frequently observed especially between closely related antigens such as B. henselae and B.quintana.14,15 For this test, Maurin et al.16 established a specificity of 87%, which can be improved using a cut-off titter of 128. In the case of B. henselae, a comparative study that evaluated different serological assays for diagnosis of cat-scratch disease comparing several IgM and IgG IFA test and ELISA test determined in the case of the test used in this study obtained a 98% of sensitivity and a 69% of specifity.17 Also is very common and well documented cross reactivity with Coxiella burnetii infections.14 Our results suggest an elevated frequency of asymptomatic carriers of antibodies against Bartonella spp. among healthy population of studied area. We can also confirm that most part of the infections by those Bartonella are subclinical or misdiagnosed. There are few published data regarding the seroprevalence in blood donors in our country, so determining if B. henselae is present in the blood of donors at the time of donation is very important, since this microorganism can survive up to 35 days in the red blood cells stored in a blood bank at 4°C.18
Our understanding of the transmission of B. quintana has changed in recent years. Although body louse is a well-known vector of trench fever, the growth kinetics of B. quintana in body lice has not been fully understood. It had been reported that multiplication rate. B. quintana started proliferation in body lice 4 days after ingestion and was constantly excreted in the feces for at least 3 weeks.19 Studies carried on body lice from homeless in San Francisco found a prevalence of B. quintana ranging from 12.3% to 33.3%.20 However, they also noted the appearance of this organism in head lice in the presence,21 but also in absence of body lice in the sampled individuals.20,22 All these questions have been solved when studies aimed at determining the genetic structure of populations based on analysis of mitochondrial genome sequences have been able to determine the existence of up to six distinct genetic clades.23,24 Despite having raised different hypotheses,23,25 the genetic basis and evolutionary relationships among body and head lice remain obscure.26 The change in the phylogenetic paradigm of lice also determines an adaptation of the epidemiological paradigm, so that it is currently considered that of both vector ecotypes (head and body lice) may be involved in the transmission of B.quintana.27,28 This is consistent with the results of several molecular studies detected B. quintana DNA in head lice worldwide, usually in people infested with both head and body lice,20,21,29 as well as those infected exclusively by head lice.30–32 Also it is possible that other vectors such as bed bugs or fleas could be involved in the transmission of B.quintana.33,34
Bartonella henselae has been signaled as the etiological agent of Cat scratch disease (CSD)35,36 in conjunction of other Bartonella species.37,38 The main transmission process enroll the scratch or, less likely, by the bite or lick of cats as well as arthropod (cat flea was generally signaled).39,40 CSD is characterized by local inflammation and significant enlargement of regional lymph nodes are usually seen within 10-14 days of scratching or biting by a cat. Systemic symptoms such as general discomfort and fever also develop, which can last several weeks. While most cases have a benign course and could resolve spontaneously, in some more severe cases various complications occur (endocarditis, encephalitis, meningitis, osteomyelitis and oculoglandular syndrome).41–45
Several Bartonella sp. different to B. hensaelae has been reported in continental Spain on potential vectors and mammal host, concerning rodents,46,47 lagomorphs,37,48 domestic and wild carnivore,49–52 chiropteran,53 ruminants54 and to veterinarian personnel working with domestic animals12 or sanitary workers.55 In this scenario we can consider that serologic test alone can determined the presence of antibodies against Bartonella spp., and no to the involved specie or species that should be interpreted in the adequate clinical and epidemiological context.
Anyway although new tools are available for diagnosing Bartonella infections such as molecular and culture ones12,55 it is necessary to know the prevalence of antibodies to Bartonella spp. in a determinate area since serology keep going the more used technique for diagnosing patients with Bartonella infections.
Conflicts of interestThe authors declare that they have no conflict of interest.
This project was partially supported by a gram from the Universidad de Jaen Research Program (Acción 16).