An outbreak of Serratia marcescens infections outbreak is described, as well as the epidemiological study that linked the outbreak to the use of 2% aqueous chlorhexidine antiseptic.
MethodIn late November 2014 an increasing incidence of S. marcescens isolates was detected in patients treated in the emergency department. It was considered a possible outbreak, and an epidemiological investigation was started.
ResultS. marcescens was isolated in 23 samples from 16 patients and in all new bottles of two lots of 2% aqueous chlorhexidine. The contaminated disinfectant was withdrawn, and the Spanish Drugs Agency was alerted (COS 2/2014). The epidemiological study showed that strains isolated from clinical samples and from chlorhexidine belonged to the same clone. No further isolates were obtained once the disinfectant was withdrawn.
ConclusionThe suspicion of an outbreak and the epidemiological study were essential to control the incidence.
Describimos un brote de infección por Serratia marcescens (S. marcescens) y la investigación epidemiológica que asoció el brote al uso de clorhexidina acuosa al 2% como antiséptico.
MétodoA finales del mes de noviembre del 2014 detectamos un aumento en la incidencia de infecciones por S. marcescens en pacientes atendidos en Urgencias, por lo que nos planteamos la posibilidad de estar ante un brote e iniciamos una investigación epidemiológica.
ResultadoSe aisló S. marcescens en 23 muestras de 16 pacientes y en todos los frascos nuevos de 2 lotes de clorhexidina acuosa al 2%. Se ordenó retirar el desinfectante y se alertó a la Agencia Española del Medicamento (COS 2/2014). El estudio epidemiológico demostró que los aislamientos de las muestras clínicas y las recuperadas de la clorhexidina pertenecían al mismo clon. Una vez retirados los lotes contaminados, no se produjeron más casos.
ConclusiónLa sospecha del brote y la investigación epidemiológica fueron fundamentales para controlar la incidencia.
Serratia marcescens (S. marcescens) is a Gram-negative bacillus belonging to the Enterobacteriaceae family. It can form part of the human microbiota but is also found in inanimate reservoirs poor in nutrients,1 including in hospitals, such as wash-hand basin taps,2 intravenous fluids3 and baby shampoo.4 These reservoirs can sometimes be the source of nosocomial outbreaks.
The Río Hortega University Hospital in Valladolid is a public 680-bed tertiary referral hospital serving a population of approximately 256,000. The incidence of S. marcescens bacteraemia here is very low; three episodes in 2013 and one up to the end of October 2014, all of nosocomial origin. Alarms were therefore raised in the last week of November 2014, when there was an increase in the incidence of S. marcescens isolates in blood cultures.
All the affected patients had been seen in Accident and Emergency (A&E), the isolates had the same antibiogram, and there was little or no relationship between the isolation and the patients’ initial symptoms. Over the ensuing days, S. marcescens continued to be isolated in samples not only from A&E patients, but also from patients in the ICU, where there was an outbreak involving 16 patients that lasted a month.
At the request of Microbiology, the hospital's Medical Management set up a multidisciplinary committee to coordinate an epidemiological and microbiological investigation into the outbreak, taking guidance from the literature on the subject.5–7 The results of the investigation were to enable measures which, in the end, brought the outbreak under control.
Material and methodsThe outbreak cases were defined as patients with isolation of S. marcescens in a clinical sample with a characteristic antibiogram since 21 November 2014, including both colonisation and infection. Colonisation was considered when there were no associated symptoms or specific treatment, and infection when there was isolation in patients with compatible symptoms in whom specific antibiotic treatment was prescribed.
Microbiological and epidemiological investigationWe contacted the company that supplied the blood culture bottles to find out if any of their clients had notified them of any cases of contamination.
At the first meeting of the specially set up multidisciplinary committee (3 December 2014, 7 cases) it was decided:
- 1.
To carry out an epidemiological study of the isolates in the clinical samples up to that point to confirm a common origin.
- 2.
To remove materials related to the taking of blood cultures from the departments involved (Adult and Paediatric A&E and ICU), replacing them with new material.
- 3.
To incubate six uninoculated blood culture bottles (blanks) from Paediatric A&E, eight from Adult A&E and eight from ICU.
At the next meeting on 9 December (nine cases), it was decided to start the search for the microorganism in the pharmaceutical products and even tools involved in the process of obtaining blood cultures.
Paediatric A&E was chosen because it was a small location that meant a workable volume for Microbiology and because the personnel responsible for the department had collected and kept all the material.
We decided to study: Bohmclorh® 2% aqueous chlorhexidine (6 bottles), povidone iodine (10 bottles), hand gel (2 bottles), hydrogen peroxide (2 bottles), alcohol (6 bottles), urea hand cream (1 bottle) and tourniquets.
All were spread both on brain-heart infusion broth, then transferred to blood agar after 24h, and direct on blood agar, with readings at 24h and 48h after aerobic incubation at 37°C.
After the results of this first search, more bottles of 2% aqueous chlorhexidine were collected and a sample of every lot available in the hospital (12 sealed containers in total) was spread on blood agar and incubated for 48h at 37°C in an aerobic atmosphere.
The clinical samples from the patients involved were processed using standard microbiological procedures.
The Vitek 2 system was used for identification and the antibiogram for all the isolates (bioMérieux®, France).
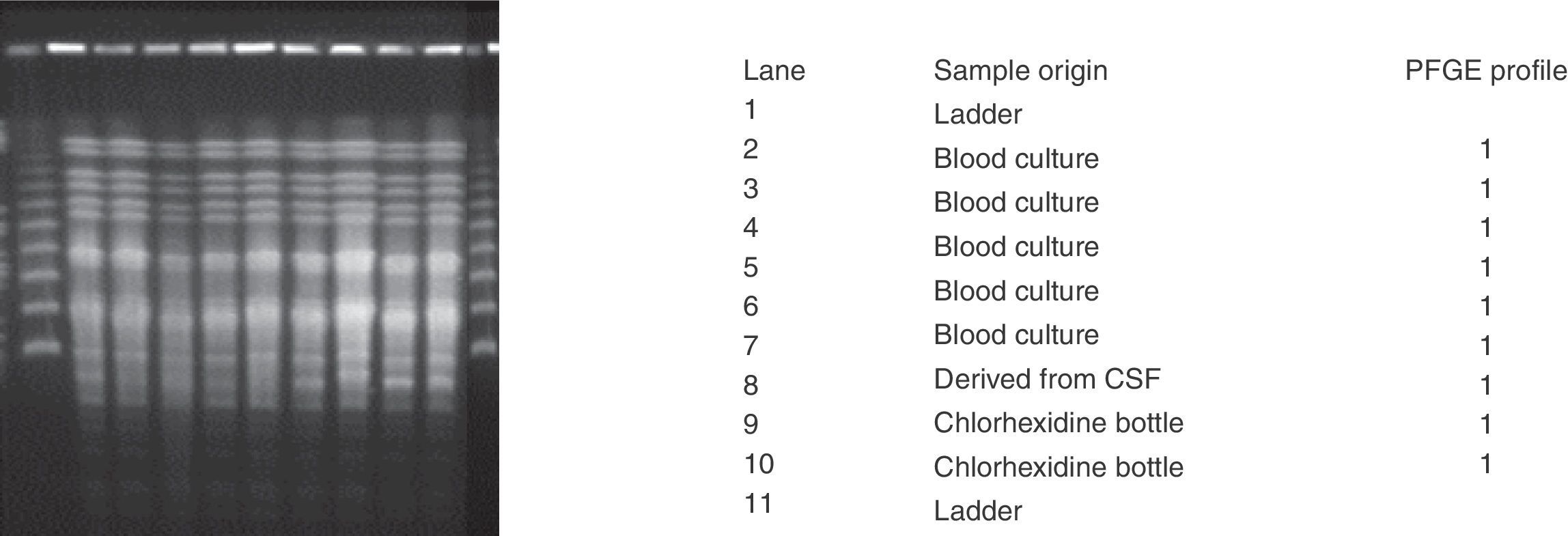
Dr Ana Vindel carried out the epidemiological study at the Spanish National Microbiology Centre using pulsed-field gel electrophoresis (PFGE) following digestion of the chromosomal DNA with XbaI. We sent three isolates from three intact bottles of 2% aqueous chlorhexidine, two from lot i-34 and one from lot i-35, and six isolates from different patients, five from blood cultures and one recovered from cerebrospinal fluid from a ventricular bypass.
ResultsFrom 21 November 2014, the Microbiology Laboratory began to detect an increased incidence of S. marcescens isolates recovered from blood cultures. The first three were from patients who had been seen in the paediatric and adult A&E Departments. The isolates had the same antibiogram, but the correlation of the microbiological findings with the patients’ symptoms was scant or non-existent.
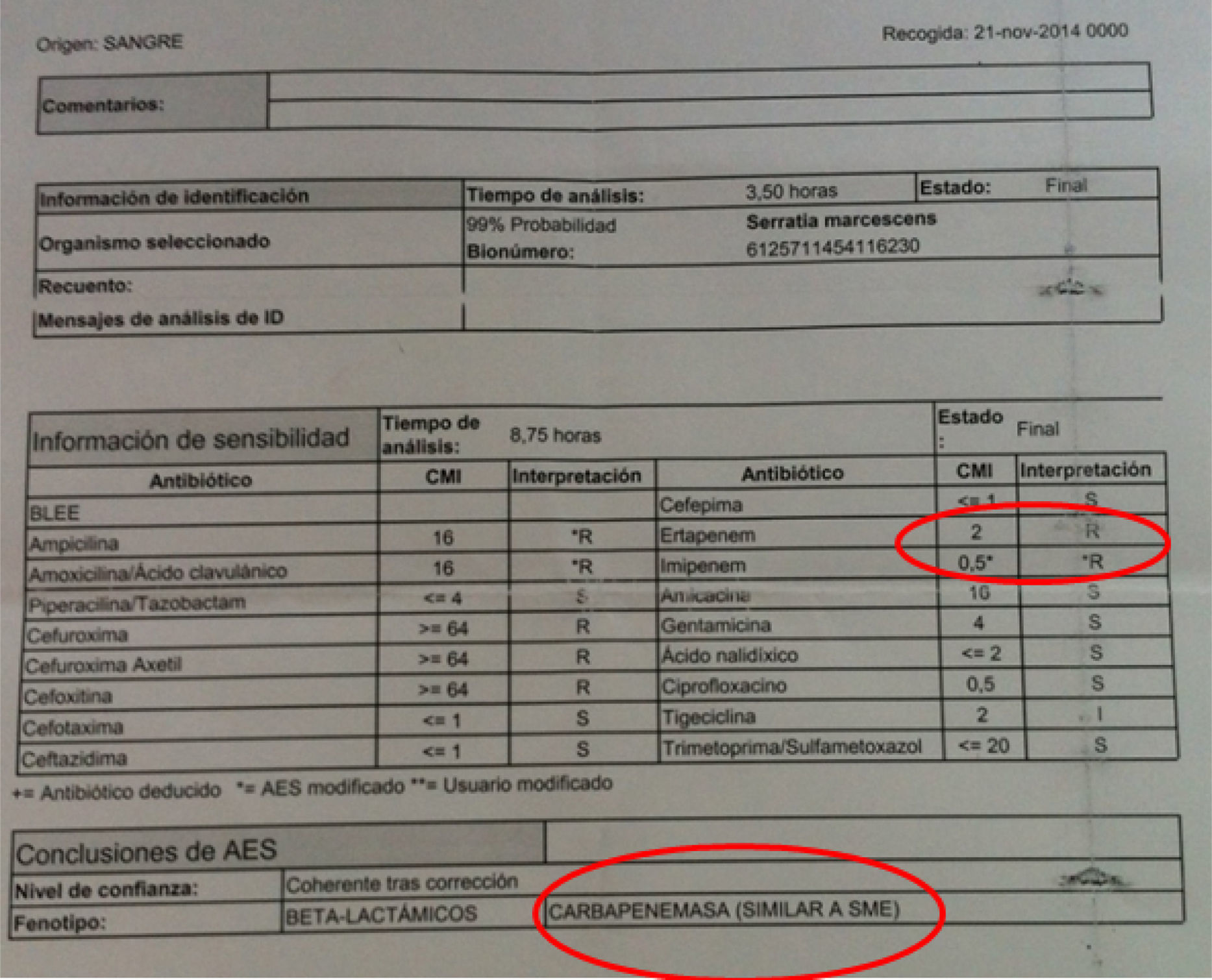
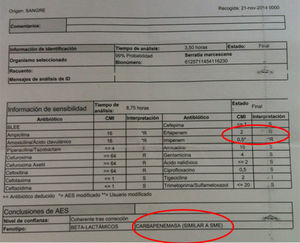
The antibiograms of these first three cases showed distinctive features; our automated system found an MIC of 2μg/ml for ertapenem, and 0.5μg/ml for imipenem, the result of which was that the expert system suggested it might be a strain that expresses a chromosomal class A carbapenemase, SME (Fig. 1). Study of the strains by E-test indicated that they were susceptible to ertapenem (MIC 0.25mg/l).
The distinctive features of these isolates were brought to the attention of the clinical departments involved so they could be carefully assessed. On 3 December, the hospital's Medical Management set up an interdisciplinary committee (paediatric and adult A&E departments, Microbiology, Preventive Medicine, Hospital Pharmacy, Nursing Directorate and Medical Management) to analyse the findings periodically and decide on the measures to be adopted.
Microbiological and epidemiological investigationOver the month that the outbreak lasted, S. marcescens was isolated in 23 samples from 16 patients. In 11 patients, the findings were considered as contaminations, but in the other five they were considered to be infections, meeting the Atlanta Centres for Disease Control and Prevention criteria for nosocomial infections.8 The five infections were: one nosocomial ventriculitis in a postoperative Neurosurgery patient who required a repeat intervention for replacement of cerebrospinal fluid drainage and antibiotic treatment with ciprofloxacin, meropenem and intrathecal amikacin; two cases of central venous catheter-related bacteraemia and one of primary bacteraemia (the three treated were with meropenem); and one secondary to pneumonia, treated with imipenem plus ciprofloxacin.
All patients made good progress after antibiotic treatment.
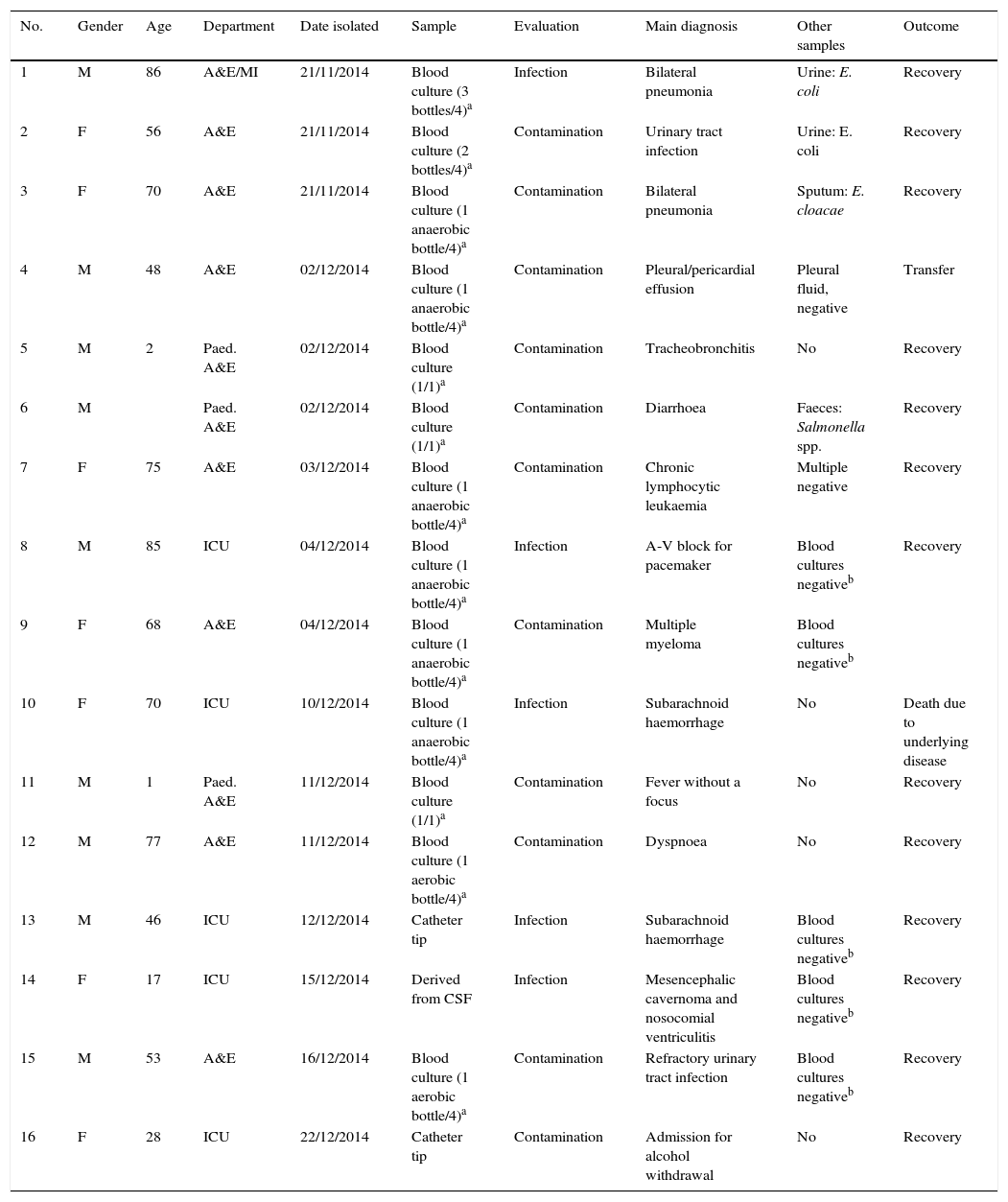
Patient demographics, isolation dates, processed samples and some clinical data are shown in Table 1.
Demographic and clinical characteristics of cases and evaluation of samples positive to Serratia marcescens.
| No. | Gender | Age | Department | Date isolated | Sample | Evaluation | Main diagnosis | Other samples | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 86 | A&E/MI | 21/11/2014 | Blood culture (3 bottles/4)a | Infection | Bilateral pneumonia | Urine: E. coli | Recovery |
| 2 | F | 56 | A&E | 21/11/2014 | Blood culture (2 bottles/4)a | Contamination | Urinary tract infection | Urine: E. coli | Recovery |
| 3 | F | 70 | A&E | 21/11/2014 | Blood culture (1 anaerobic bottle/4)a | Contamination | Bilateral pneumonia | Sputum: E. cloacae | Recovery |
| 4 | M | 48 | A&E | 02/12/2014 | Blood culture (1 anaerobic bottle/4)a | Contamination | Pleural/pericardial effusion | Pleural fluid, negative | Transfer |
| 5 | M | 2 | Paed. A&E | 02/12/2014 | Blood culture (1/1)a | Contamination | Tracheobronchitis | No | Recovery |
| 6 | M | Paed. A&E | 02/12/2014 | Blood culture (1/1)a | Contamination | Diarrhoea | Faeces: Salmonella spp. | Recovery | |
| 7 | F | 75 | A&E | 03/12/2014 | Blood culture (1 anaerobic bottle/4)a | Contamination | Chronic lymphocytic leukaemia | Multiple negative | Recovery |
| 8 | M | 85 | ICU | 04/12/2014 | Blood culture (1 anaerobic bottle/4)a | Infection | A-V block for pacemaker | Blood cultures negativeb | Recovery |
| 9 | F | 68 | A&E | 04/12/2014 | Blood culture (1 anaerobic bottle/4)a | Contamination | Multiple myeloma | Blood cultures negativeb | |
| 10 | F | 70 | ICU | 10/12/2014 | Blood culture (1 anaerobic bottle/4)a | Infection | Subarachnoid haemorrhage | No | Death due to underlying disease |
| 11 | M | 1 | Paed. A&E | 11/12/2014 | Blood culture (1/1)a | Contamination | Fever without a focus | No | Recovery |
| 12 | M | 77 | A&E | 11/12/2014 | Blood culture (1 aerobic bottle/4)a | Contamination | Dyspnoea | No | Recovery |
| 13 | M | 46 | ICU | 12/12/2014 | Catheter tip | Infection | Subarachnoid haemorrhage | Blood cultures negativeb | Recovery |
| 14 | F | 17 | ICU | 15/12/2014 | Derived from CSF | Infection | Mesencephalic cavernoma and nosocomial ventriculitis | Blood cultures negativeb | Recovery |
| 15 | M | 53 | A&E | 16/12/2014 | Blood culture (1 aerobic bottle/4)a | Contamination | Refractory urinary tract infection | Blood cultures negativeb | Recovery |
| 16 | F | 28 | ICU | 22/12/2014 | Catheter tip | Contamination | Admission for alcohol withdrawal | No | Recovery |
The supplier of the blood culture bottles ruled out contamination in the manufacturing process. Indeed, after five days of incubation, the uninoculated bottles were reported by the incubator as negative.
In terms of the microbiological search in products and tools, only cultures corresponding to chlorhexidine were positive.
Of the six bottles of 2% aqueous chlorhexidine collected from the Paediatric A&E Department corresponding to lots i-20, i-29 and i-34 and cultured with the rest of the tools and products, S. marcescens was isolated in the three bottles from lot i-34, one of them expressly opened for the culture.
After processing the 12 remaining bottles collected from all over the hospital following the first positive results, all sealed and belonging to lots i-22, i-25, i-26, i-34 and i-35, S. marcescens grew in the six bottles from lots i-34 and i-35.
When we sent the isolates from the 2% aqueous chlorhexidine to the Spanish National Microbiology Centre, we were informed that several hospitals in Spain were studying similar situations to ours. In addition, the Spanish National Microbiology Centre confirmed the MIC of these isolates to ertapenem at 0.25mg/l and ruled out the presence of carbapenemases, including SME.
Control of the outbreakAt the meeting on 11 December (12 cases), it was decided to withdraw Bohmclorh® 2% aqueous chlorhexidine from the entire hospital and replace it with another product.
That same day, the Hospital Pharmacy Service sent the alert to the Spanish Agency of Medicines and Medical Devices (Agencia Española de Medicamentos y Productos Sanitarios, AEMPS).
Over the following days, four more cases were detected. On further investigation, it was found that Bohmclorh® 2% aqueous chlorhexidine had not been completely withdrawn from the entire hospital.
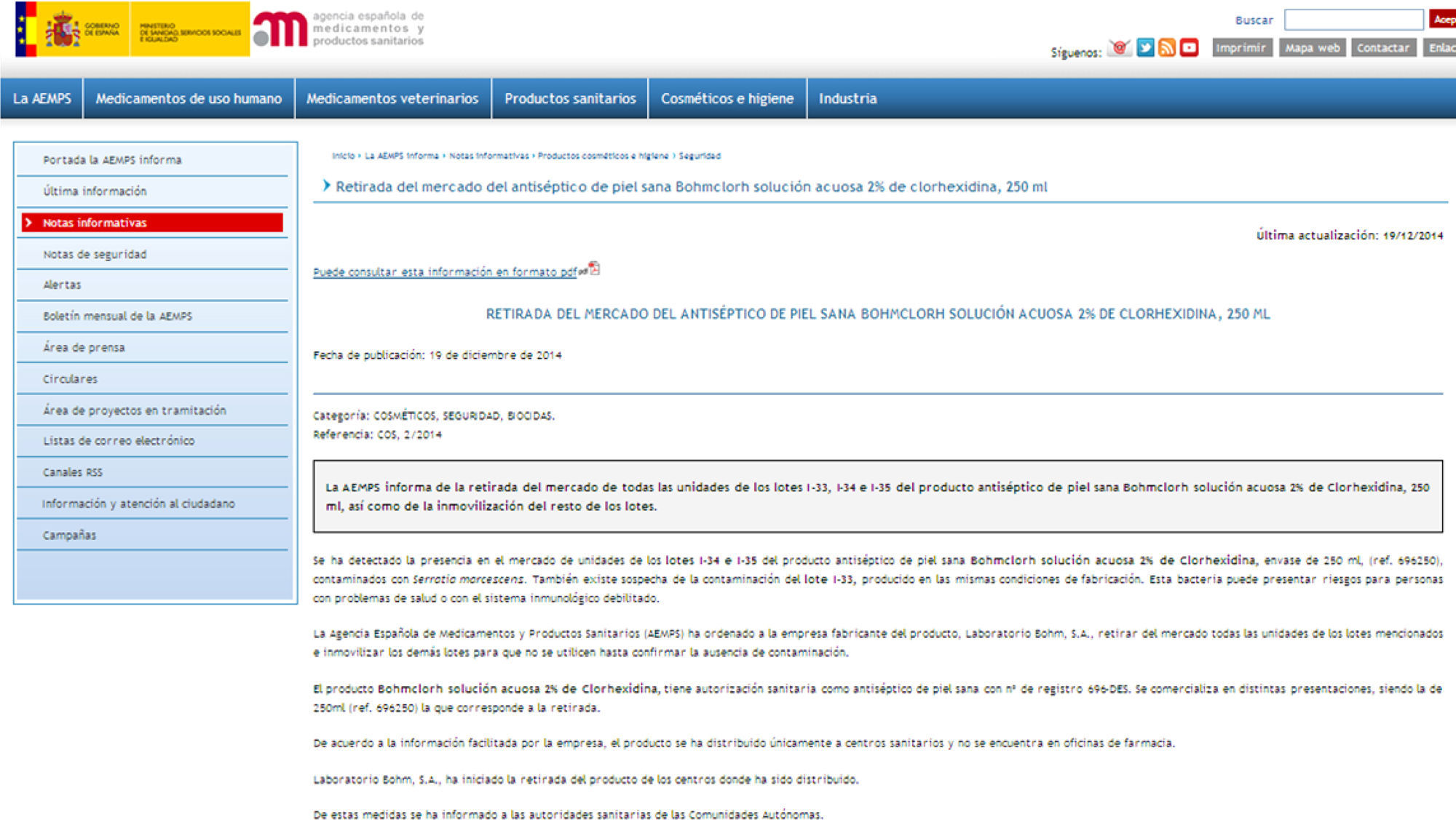
On 19 December, the AEMPS issued an alert (ref. COS 2/2014) (Fig. 2) reporting the withdrawal of lots i-34 and i-35 of the antiseptic product Bohmclorh® 2% chlorhexidine aqueous solution 250ml from the market because they were contaminated with S. marcescens.
On 23 December we received the results of the molecular epidemiology tests carried out at the Spanish National Microbiology Centre (Fig. 3), which showed that the strains in both the clinical samples and the chlorhexidine packaging belonged to the same clone.
On 30 December after a week with no cases, the outbreak was declared resolved.
DiscussionThe possibility of the emergence of infections, including epidemic outbreaks, resulting from contamination of materials used in healthcare is a known and accepted fact nowadays in the management of infectious diseases. This includes antiseptic solutions, which are not exempt from contamination by different resistant microorganisms. Among others, there have been reports of cases associated with povidone iodine, benzalkonium chloride and chlorhexidine.7,9–12 This article describes an outbreak of S. marcescens infection originating from Bohmclorh® 2% aqueous chlorhexidine solution, a disinfectant used in our hospital for cleaning the skin prior to the extraction of blood samples for blood culture or insertion or maintenance of vascular catheters.
The systematic review of microbiological isolates in the Microbiology Laboratory, especially those corresponding to sterile samples, and the low rate of S. marcescens bacteraemia in our hospital meant that we were quickly alerted to the possibility that we had an outbreak. The fact that the microorganism concerned was not a common cause of bacteraemia in the affected centre rapidly led us to suspect an outbreak and to investigate its origin. Some published studies report delays of up to a month in the detection of an outbreak as a consequence of inadequate monitoring of changes in the prevalence of certain microorganisms.7
Such reports should serve to underline the importance of keeping protocols for action and prevention of nosocomial infection, and the training and commitment of healthcare personnel, in terms of their compliance, continually up-to-date. In almost all outbreaks of this type, deficiencies have been discovered in the protocols for prevention and in compliance with them. Certain articles in the literature report errors in the chlorhexidine concentration used, i.e. lower than that recommended by the centre's protocols.7
Also important is good coordination among the departments involved and close monitoring of how the outbreak evolves. In our case, the intervention of the Hospital's Medical Management, which, after being informed about the suspected problem and its potential importance, immediately organised the setting up of an interdisciplinary committee to coordinate the epidemiological investigation and propose the measures to be adopted, made a decisive contribution to the control of the outbreak.
Systems must also be in place to allow rapid adoption and implementation of committee decisions by healthcare personnel. Since these outbreaks are often associated with materials and products involved in nursing care, it is important that nursing staff are also involved in monitoring the implementation of these measures. In fact, in our case, failures and delays in this area were what led to the appearance of new cases after the order to withdraw chlorhexidine.
However, even when the suspicion is acted on and the approach to these episodes is rapid and effective, it is very difficult, as in this case, to avoid an increase in costs for both the patient and institution deriving from antibiotic treatments and other diagnostic tests and therapeutic measures which sometimes also prolong the hospital stay.
Lastly, the role of referral centres which are often involved in the identification and control of these epidemic outbreaks is crucial, especially when, again as in this case, they affect several centres some geographical distance apart belonging to different health services. The participation of this type of centre is important for the coordination of efforts to identify the origin of the outbreak and in the dissemination of information to the affected centres. In our case, the role of the Spanish National Microbiology Centre (Dr Vindel) was instrumental in this regard, confirming the clonal origin of the outbreak and facilitating the exchange of information between the different affected centres, some of which have also recently published their experience.13–15
Conflicts of interestThe authors declare that they have no conflicts of interest and that this study has received no funding.
The authors are grateful for the invaluable contribution of Dr Ana Vindel of the National Microbiology Centre.
Please cite this article as: de Frutos M, López-Urrutia L, Domínguez-Gil M, Arias M, Muñoz-Bellido JL, Eiros JM, et al. Brote de Serratia marcescens producido por clorhexidina acuosa al 2% contaminada. Enferm Infecc Microbiol Clin. 2017;35:624–629.
Part of this work was presented at the 19th Congress of the SEIMC (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [Spanish Society of Infectious Diseases and Clinical Microbiology]), but it has not been presented to any other journal for review.