A 35-year-old 80kg man was admitted in the Resuscitation Unit after a craniotomy and the placement of a double external ventricular drainage for a malignant midline glioma with obstructive hydrocephalus. After three weeks, when the patient presented septic symptoms (C-reactive protein of 23mg/dL, 4220leukocytes/mm3) the drainages were replaced. The cerebrospinal fluid (CSF) biochemistry was compatible with a bacterial central nervous system (CNS) infection (glucose<2mg/dL and proteins 550mg/dL). Broad spectrum antibiotic therapy was started with intravenous (IV) linezolid 600mg q12h and meropenem 2000mg q8h in a 4h extended infusion.
Five days later, a Class B carbapenemase (metallo-β-carbapenemase)-producing Enterobacter cloacae with intermediate susceptibility to meropenem with minimum inhibitory concentration (MIC) of 8mg/L and susceptible to colistin (MIC=0.20mg/L) was isolated in both CSF and blood cultures (drainages were not cultured). In addition, a Class A carbapenemase (KPC)-producing Klebsiella pneumoniae (colistin MIC≤2mg/L) and an extensively drug-resistant Pseudomonas aeruginosa (colistin MIC≤0.5mg/L; meropenem MIC=8mg/L) were isolated in blood cultures. These nosocomial microorganisms were not isolated in any other culture and they could be a consequence of an incorrect drainage manipulation in a COVID pandemic situation, with a higher prevalence of multi-drug resistant bacteria. Linezolid was stopped and intravenous colistimethate sodium (CMS) at a dose of 6 MIU q12h was added to meropenem due to the synergic effects of both antibiotics.1 In addition, intraventricular colistin (10mg q4h administered through each CSF drainage) was added to try to ensure therapeutic concentrations into the CSF, as described in exceptional cases.2,3
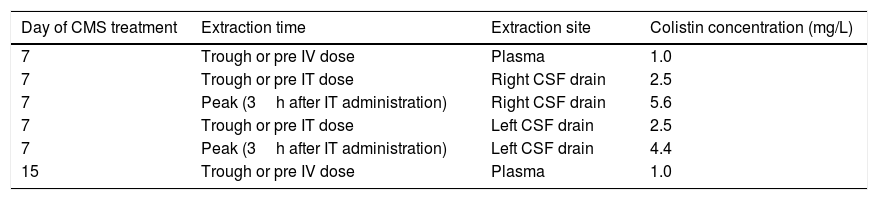
On day 7 of CMS treatment, total colistin sulphate levels in plasma, determined by high performance liquid chromatography, were infratherapeutic (Css<2mg/L). Due to severity of the infection, CSF levels of colistin were also determined and ranged between 2.5 and 5.6mg/L, a value 10 times higher than the colistin MIC. Both IV and intraventricular CMS doses were maintained. Eight days later plasma colistin concentration were still low (see Table 1). The intraventricular and IV CMS treatments were stopped after 15 and 30 days, respectively. Finally, after 63 days in the Resuscitation Unit, the patient could be discharged to a conventional hospital ward without any signs and symptoms of an active CNS infection.
Colistin levels in plasma and CSF.
| Day of CMS treatment | Extraction time | Extraction site | Colistin concentration (mg/L) |
|---|---|---|---|
| 7 | Trough or pre IV dose | Plasma | 1.0 |
| 7 | Trough or pre IT dose | Right CSF drain | 2.5 |
| 7 | Peak (3h after IT administration) | Right CSF drain | 5.6 |
| 7 | Trough or pre IT dose | Left CSF drain | 2.5 |
| 7 | Peak (3h after IT administration) | Left CSF drain | 4.4 |
| 15 | Trough or pre IV dose | Plasma | 1.0 |
Colistin-associated nephrotoxicity4 was not observed during CMS treatment being the estimated glomerular filtration rate greater than 120ml/min/1.73m2 during treatment. Neurotoxicity, a side effect caused by colistin,5 could not be assessed because patient's impaired status of consciousness caused by a diencephalic irritation during the previous surgery.
Meningoventriculitis caused by Enterobacter spp. is a rare infectious complication in neurosurgical patients but associated with a high morbidity and mortality.6 The treatment is often complex due to the isolation of bacterial strains resistant to multiple antibiotics, such as third-generation cephalosporins and even, as in the present case, to carbapenems. In these cases, colistin becomes one of the last available therapeutic options.
The achievement of adequate antibiotic concentrations at the infection site is essential in these difficult-to-treat infections. Although CNS penetration in patients with meningoventriculitis might be increased by 60% for some antimicrobials, in other cases intraventricular administration may be necessary to reach therapeutic levels.7
Colistin is an antimicrobial with a very complex pharmacokinetics. Therapeutic plasma colistin concentrations are difficult to achieve, even after the administration of very high CMS doses, especially in patients with conserved renal function.8 This is due to the fact that CMS is rapidly renally excreted before it can be hydrolyzed to colistin, the active compound.7 In addition, colistin penetration into the CSF after its IV administration has been reported to be very low and variable, ranging between 5% and 7% in some experiences and7 up to 25% in others.9
Our patient, with preserved renal function, presented suboptimal colistin plasma levels, even after the administration of a high CMS IV dose.8 The local intraventricular administration allowed to achieve optimal colistin levels in CSF (10 times above the MIC).7
In conclusion, when using colistin for the treatment of a CNS infection, local intraventricular administration could be necessary to reach optimal levels at the infection site, especially in the case of young patients with preserved renal function and infections caused by multi-drug-resistant Gram-negative bacteria.
In addition, therapeutic drug monitoring of colistin may be a useful strategy for optimizing the treatment of these complicated infections that can help to ensure an optimal exposure while reducing the risk of nephrotoxicity.