We studied the trend and seasonality of community-acquired Escherichia coli resistance and quantified its correlation with the previous use of certain antibiotics.
MethodsA time series study of resistant community-acquired E. coli isolates and their association with antibiotic use was conducted in a Primary Health Care Area from 2008 to 2012. A Poisson regression model was constructed to estimate the trend and seasonality of E. coli resistance.
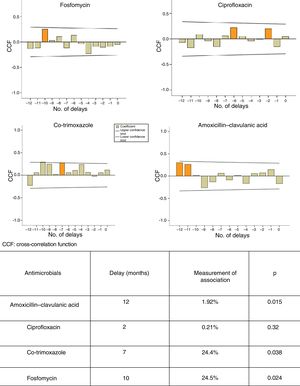
ResultsA significant increasing trend in mean E. coli resistance to cephalosporins, aminoglycosides and nitrofurantoin was observed. Seasonal resistance to ciprofloxacin and amoxicillin-clavulanic acid was significantly higher in autumn-winter. There was a delay of 7, 10 and 12 months between the use of cotrimoxazole (p<0.038), fosfomycin (p<0.024) and amoxicillin-clavulanic acid (p<0.015), respectively, and the occurrence of E. coli resistance.
ConclusionsAn average delay of 10 months between the previous use of amoxicillin-clavulanic acid, cotrimoxazole and fosfomycin and the appearance of resistant community-acquired E. coli strains was detected.
Estudiamos la tendencia y estacionalidad de las resistencias en Escherichia coli comunitario y se cuantifica su asociación con el uso previo de determinados antibióticos.
MétodosEstudio de series temporales de las resistencias de aislados comunitarios de E. coli y su relación con el consumo de antibióticos en un área de primaria durante 2008–2012. La tendencia y estacionalidad de las resistencias se estudiaron mediante regresión de Poisson.
ResultadosSe observó un aumento significativo de la resistencia promedio de E. coli a cefalosporinas, nitrofurantoína y aminoglucósidos. La estacionalidad de las resistencias fue significativa en otoño-invierno para amoxicilina-ácido clavulánico y ciprofloxacino. Observamos un retardo de 7, 10 y 12 meses entre el consumo de cotrimoxazol (p<0,038), fosfomicina (p<0,024) y amoxicilina-ácido clavulánico (p<0,015), respectivamente, y la aparición de resistencias.
ConclusionesDetectamos un retardo medio de 10 meses entre la utilización de amoxicilina-ácido clavulánico, cotrimoxazol y fosfomicina, y la aparición de cepas resistentes de E. coli comunitarios.
The increase in infections due to multidrug-resistant microorganisms, which is generating so much alarm due to their high morbidity and mortality as well as their high healthcare costs,1 has often been linked to the high selective pressure of the antimicrobials used in human health.2,3
So-called autoregressive integrated moving average (ARIMA) models are statistical tools for finding patterns to enable future estimates to be made. They have been applied to the management of nosocomial infection4 and more recently to the design of an efficient antimicrobial management programme following an outbreak of carbapenemase-producing bacteria.5 They also enable the relationships between the use of antibiotics and the development of resistance to be measured and estimate the delay to the development of increased resistance to an antibiotic following increased use of that antibiotic.6,7
The objectives of this study are to examine trends and seasonal variations in the resistance of community-acquired Escherichia coli isolates to certain antimicrobials and to measure the dynamic relationship between prior use of antibiotics and the development of resistance for a 5-year period using ARIMA models.
Materials and methodsThis was a retrospective study of time series of strains of E. coli isolated from patients from primary care (PC) in a healthcare area with 211,533 inhabitants, use of antibiotics and development of resistance between 2008 and 2012.
The antibiotic use data refer to the population studied and are expressed in terms of defined daily dose (DDD per 1000 inhabitants per day). They were provided by the Dirección General de Farmacia [Directorate General for Pharmacy] of the Autonomous Community. The study of susceptibility to ampicillin (AM), amoxicillin–clavulanic acid (AMC), cefoxitin (CFX), cefotaxime (CFT), cefuroxime (CFM), ciprofloxacin (CIP), co-trimoxazole (SXT), fosfomycin (F), nitrofurantoin (NF), gentamicin (G) and tobramycin (TO) was performed using the VITEK-2® system (bioMérieux, France) in the microbiology laboratory at the reference hospital. The data are expressed in terms of percentage of resistance (number of antibiotic-resistant E. coli isolates divided by total number of isolates). The month was used as a time aggregate both for use and for resistance.
Trends and seasonal variations in resistance were studied using a Poisson regression model where the percentage of resistance was used as a dependent variable. Trends were estimated using the variable representing the number of months elapsed in 5 years. Seasonal variations were estimated using the variable representing the month of the year, taking the month of January as a reference point. The result obtained was interpreted in terms of an incidence rate ratio (IRR) and its 95% confidence interval (95% CI). For trends, the IRR indicates the relative change in the series for each year elapsed. For seasonal variations, the IRR indicates the relative change in each month compared to the month taken as a reference point.
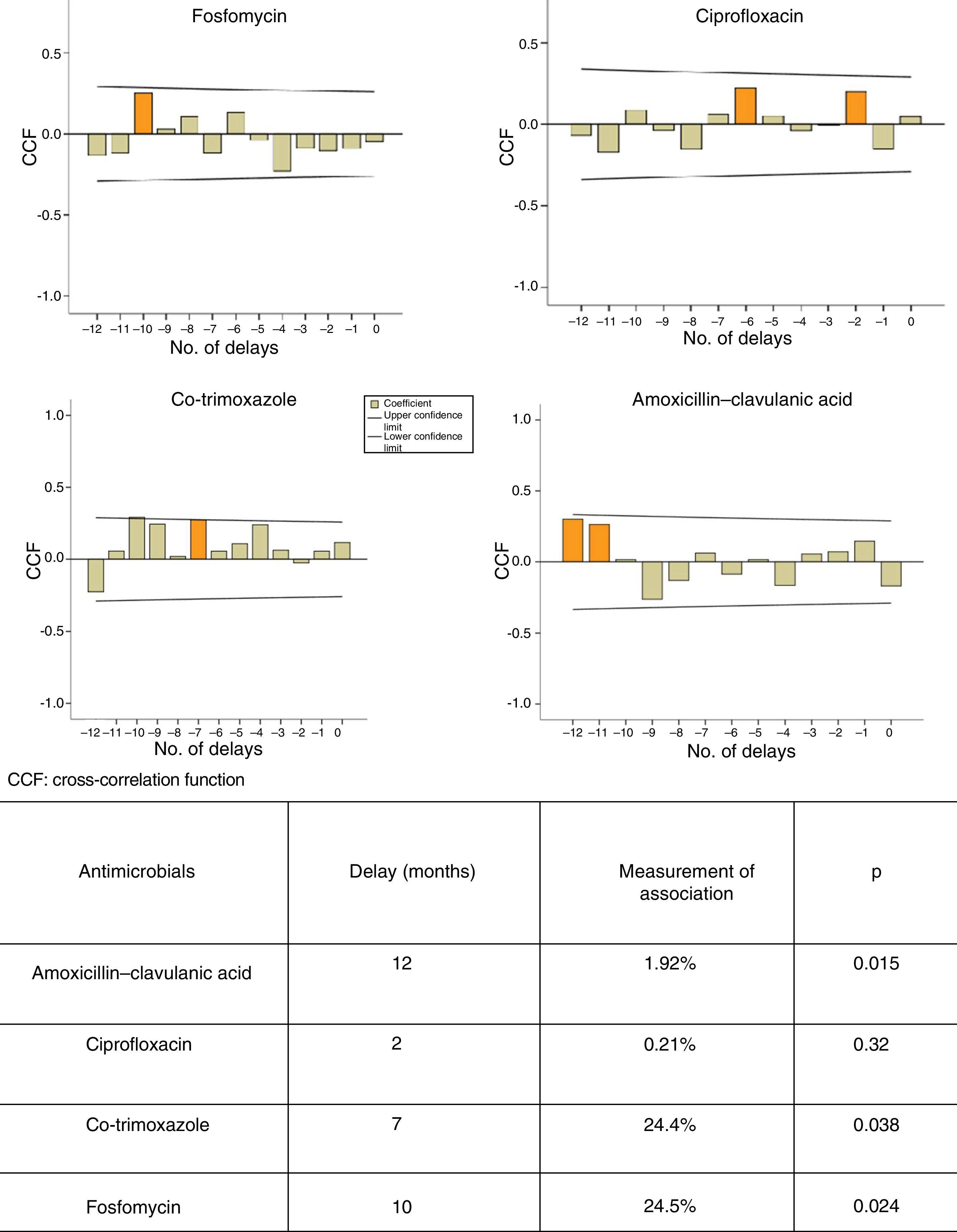
Different ARIMA models were built from time series according to the methodology reported by Box et al. in 19768 and adapted by López-Lozano et al. in 2000.6 First, graphs of cross-correlations were used to identify the delays in which the association between use and resistance was most significant. Next, transfer models were built to quantify the relationship between, on the one hand, the use of AMC, CIP, SXT and F and, on the other hand, the development of resistance in E. coli isolates.
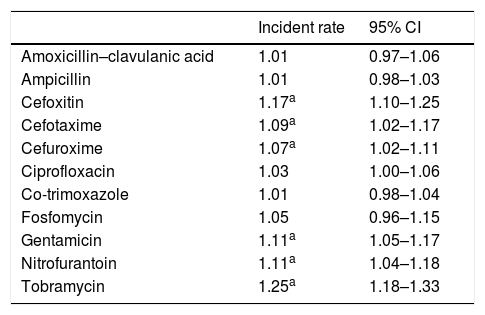
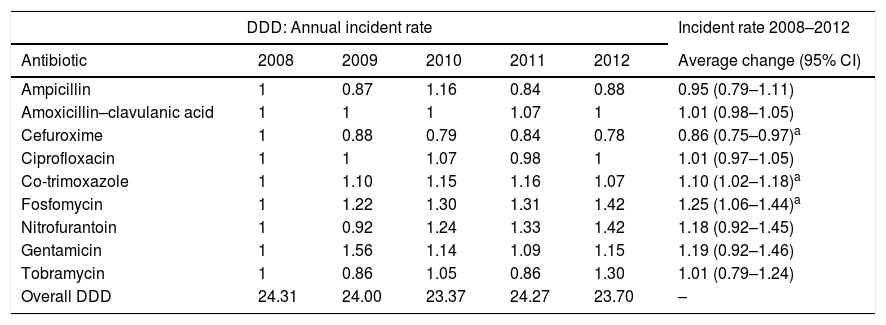
ResultsA total of 9326 community-acquired E. coli isolates were studied. The average change in the percentage of resistance and the average change in the use of antibiotics are shown in Tables 1 and 2, respectively. We found that the use of fosfomycin, co-trimoxazole and nitrofurantoin gradually increased, whereas the use of ciprofloxacin remained stable throughout the period studied. An ascending trend was observed in resistance to the cephalosporins studied, with an average increase in resistance of 7%, 9% and 17% for CFM, CFT and CFX, respectively. Average resistance to aminoglycosides (AMGs) also increased significantly: 11% for G and 25% for TO, as well as 11% for NF. There were two peak increases in AMC resistance, one in 2009 (IRR: 1.25; 95% CI: 1.06–1.47; p=0.008) and the other in 2011 (IRR: 3.55; 95% CI: 1.14–1.58; p<0.001). CIP resistance increased as of 2010 (IRR: 1.22; 95% CI: 1.06–1.40; p=0.006).
Average change in percentage of resistance (2008–2012).
| Incident rate | 95% CI | |
|---|---|---|
| Amoxicillin–clavulanic acid | 1.01 | 0.97–1.06 |
| Ampicillin | 1.01 | 0.98–1.03 |
| Cefoxitin | 1.17a | 1.10–1.25 |
| Cefotaxime | 1.09a | 1.02–1.17 |
| Cefuroxime | 1.07a | 1.02–1.11 |
| Ciprofloxacin | 1.03 | 1.00–1.06 |
| Co-trimoxazole | 1.01 | 0.98–1.04 |
| Fosfomycin | 1.05 | 0.96–1.15 |
| Gentamicin | 1.11a | 1.05–1.17 |
| Nitrofurantoin | 1.11a | 1.04–1.18 |
| Tobramycin | 1.25a | 1.18–1.33 |
Average change in antibiotic consumption (2008–2012).
| DDD: Annual incident rate | Incident rate 2008–2012 | |||||
|---|---|---|---|---|---|---|
| Antibiotic | 2008 | 2009 | 2010 | 2011 | 2012 | Average change (95% CI) |
| Ampicillin | 1 | 0.87 | 1.16 | 0.84 | 0.88 | 0.95 (0.79–1.11) |
| Amoxicillin–clavulanic acid | 1 | 1 | 1 | 1.07 | 1 | 1.01 (0.98–1.05) |
| Cefuroxime | 1 | 0.88 | 0.79 | 0.84 | 0.78 | 0.86 (0.75–0.97)a |
| Ciprofloxacin | 1 | 1 | 1.07 | 0.98 | 1 | 1.01 (0.97–1.05) |
| Co-trimoxazole | 1 | 1.10 | 1.15 | 1.16 | 1.07 | 1.10 (1.02–1.18)a |
| Fosfomycin | 1 | 1.22 | 1.30 | 1.31 | 1.42 | 1.25 (1.06–1.44)a |
| Nitrofurantoin | 1 | 0.92 | 1.24 | 1.33 | 1.42 | 1.18 (0.92–1.45) |
| Gentamicin | 1 | 1.56 | 1.14 | 1.09 | 1.15 | 1.19 (0.92–1.46) |
| Tobramycin | 1 | 0.86 | 1.05 | 0.86 | 1.30 | 1.01 (0.79–1.24) |
| Overall DDD | 24.31 | 24.00 | 23.37 | 24.27 | 23.70 | – |
Overall DDD: total DDD for therapeutic group J01 (antibacterials for systemic use) at the health centres in our setting by year of study.
Regarding seasonal variations, resistance to AMC and CIP increased significantly in autumn and winter—CIP in December (IRR: 1.27; 95% CI: 1.03–1.57; p=0.025) and AMC in September (IRR: 1.30; 95% CI: 1.01–1.68; p=0.039), October (IRR: 1.41; 95% CI: 1.10–1.81; p=0.007) and December (IRR: 2.19; 95% CI: 1.03–1.70; p=0.029).
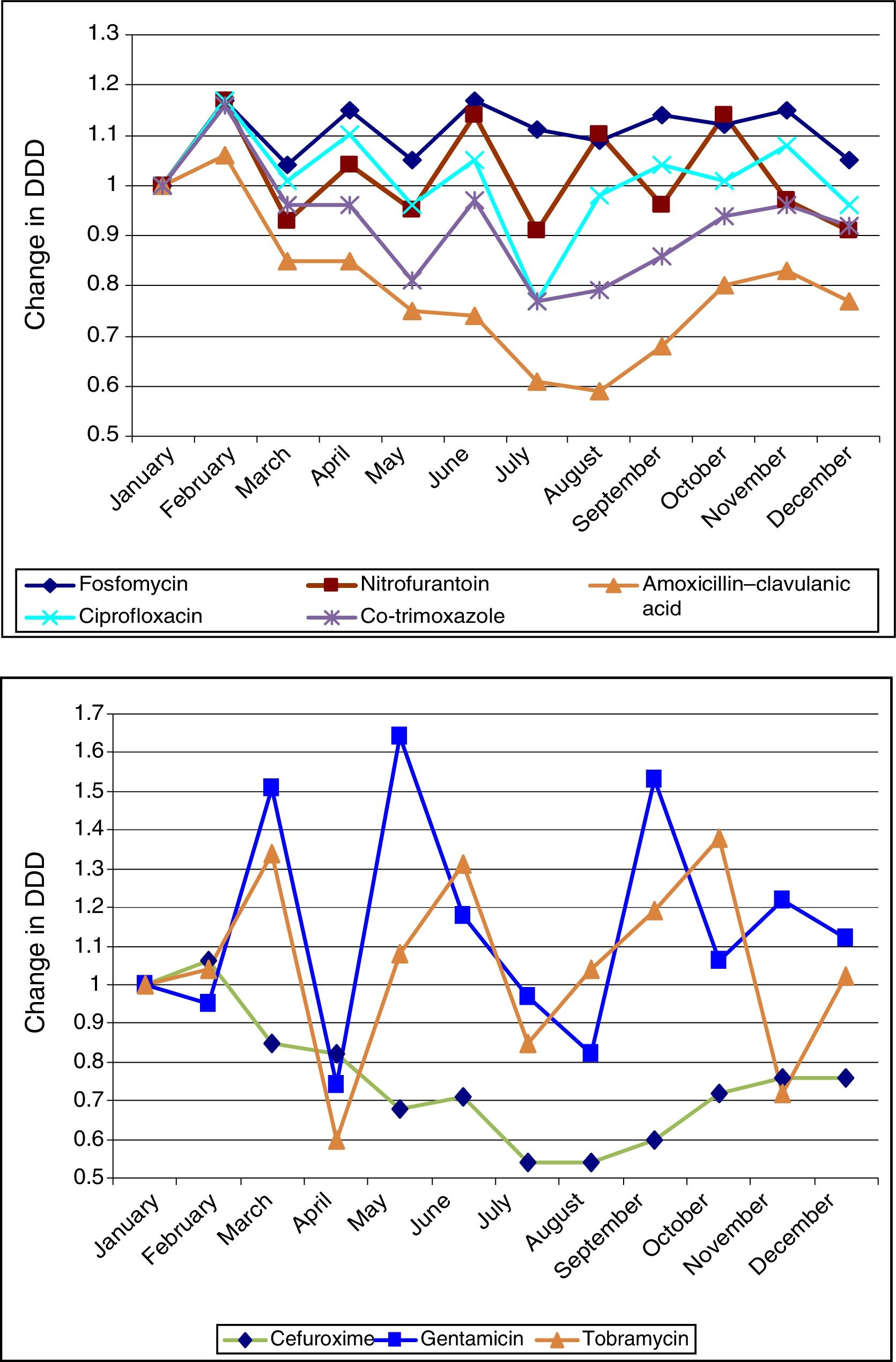
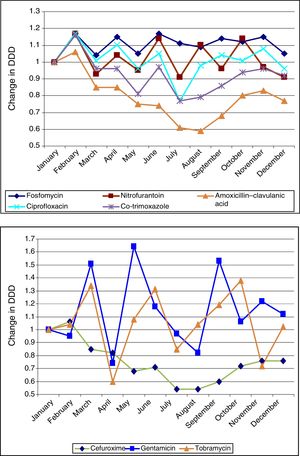
Figure 1 shows the ARIMA models for AMC, CIP, SXT and F. A positive relationship is seen between the prescription of these antimicrobials and the development of resistance in community-acquired E. coli, with a delay ranging from 2 to 12 months. This relationship was significant for AMC, SXT and F. Fig. 2 shows seasonal variations in average use.
Seasonal variations in average monthly use of the antibiotics studied (2008–2012). The antibiotics in the above graph are those that are most commonly used in primary care (fosfomycin, nitrofurantoin, amoxicillin–clavulanic acid, ciprofloxacin and co-trimoxazole). They show more uniform seasonal variations than those in the below graph (cefuroxime, gentamicin and tobramycin).
Our study has once again shown the solid relationship between the use of antimicrobials and the development of resistance for a microorganism as prevalent as E. coli. Analysis of time series enables prediction of the future behaviour of the series studied based on past behaviour. On this basis we sought to predict the development of resistance in community-acquired E. coli isolates based on series of resistance and use of CIP, AMC, SXT and F obtained in the past five years, as well as to estimate the delay in its presentation.
The ascending trend in the resistance of E. coli to cephalosporins, NF and AMG found in our study is consistent with European Antimicrobial Resistance Surveillance System report data.9 This could be related to the dissemination of broad-spectrum beta-lactamases in the community, growing since the 1980s,10 and often presenting co-resistance to other antibiotics such as fluoroquinolones and AMGs. Another possible cause is acquisition of cephalosporins in E. coli from animals.11 Peak increases in AMC resistance could be related to greater seasonal use of this and other antibiotics due to the influenza A epidemic which started in Spain in April 2009, leading to higher rates of morbidity and superinfection.
Several studies have used ARIMA models to determine the relationship between the use of antibiotics and the development of resistance.6,7,12,13 Hsueh et al. found a significant relationship between increased resistance of E. coli to CFT and CIP and increased use of beta-lactam antibiotics, carbapenems, FQs and AMGs.12 A French study has shown a significant association between the use of fluoroquinolones and increased resistance to ofloxacin and CIP in E. coli strains of urinary origin in hospitalised patients, with delays of less than six months.7 According to our study, each increase by one unit in the use of SXT, F, and AMC is expected to result in an increase in resistance with delays of seven, 10 and 12 months, respectively. We believe that widespread awareness of these data could guide physicians in empirical antibiotic prescription.
Our mean extrahospital antibiotic use in terms of DDD (23.93) was higher than the Spanish mean (20.30) and even higher than the European mean (21.16) in the same period.14 As antimicrobial use is far less managed in a community setting than in a specialised setting, using these predictive models in our setting would offer the option of active intervention to reduce the consequences of this deficiency in reasonable prescription of antibiotics and come closer to the target of the Optimisation Programmes for antibiotics in PC. The implementation of these programmes is a high priority in most Spanish communities.15 Therefore, it would be a good idea to conduct multi-centre studies evaluating the validity of the association and the delays found, as well as to broaden the study to other combinations of microorganisms and antimicrobials.
This study suffers from the limitations particular to retrospective studies: it has a higher risk of information and selection biases. Another limitation is its lack of application of DDDs to the child population.
In conclusion, we found a positive relationship between the use of the antimicrobials analysed and the development of resistance in community-acquired E. coli. This relationship was significant for AMC, SXT and F. The delay in the development of these forms of resistance ranged from seven to 12 months. Thus ARIMA models would enable forecasts to be established to make more reasonable use of antibiotics in a community setting.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank Dr José María Tenías Burillo for his unconditional support.
Please cite this article as: Asencio Egea MÁ, Huertas Vaquero M, Carranza González R, Herráez Carrera Ó, Redondo González O, Arias Arias Á. Tendencia y estacionalidad de las resistencias de Escherichia coli comunitarios y su relación dinámica con el consumo de antimicrobianos mediante modelos ARIMA. Enferm Infecc Microbiol Clin. 2018;36:502–506.