Streptococcus pneumoniae is an important cause of morbidity. Vaccination is the most effective measure to prevent it. The aim of this study is to analyse the evolution of invasive pneumococcal disease (IPD).
Material and methodsObservational study of IPD cases notified to the Epidemiological Surveillance Network of the Autonomous Community of Madrid between 2008 and 2015. The IPD case was defined as the disease caused by S. pneumoniae, with isolation and DNA or antigen detection, in samples from normally sterile sites. The isolated strains were sent to the Regional Public Health Laboratory for identification of the serotype. Serotypes were classified according to their inclusion in the 7-valent conjugate vaccine (PCV7), in the 13-valent vaccine, but not in the 7-valent vaccine (PCV13-additional) and not included in the 13-valent vaccine (non-PCV). The incidence rate ratios (IRRs) were calculated comparing the 2011–2012 and 2013–2015 periods with the 2008–2010 period.
Results4,307 cases were reported. 86.6% were serotyped. The IRR of IPD was 0.67 and 0.67 for all serotypes; 0.43 and 0.45 for PCV7 serotypes; 0.46 and 0.25 for PCV13-additional serotypes, and 1.01 and 1.32 for non-PCV13 serotypes in the 2011–2012 and 2013–2015 periods. The incidence of serotypes 8, 9N, 10A, 23B, 24F and serogroup 33 increased significantly in the 2013–2015 period. Serotypes 15B and 24F accounted for 24% of non-PCV13 cases in children under 5 years, serotypes 8 and 9N for 51% in the population aged 5–59 years and serotypes 8 and 22F for 25% in the population aged over 59 years.
ConclusionsThe incidence of serotypes not included in conjugate vaccines has increased, especially in children under 5 years, but the total incidence of IPD has decreased. It is important to continue with the epidemiological and microbiological surveillance programmes to assess the effect of vaccination on the incidence of IPD.
Streptococcus pneumoniae es una causa importante de morbilidad, y la vacuna es la medida más eficaz para prevenirla. El objetivo de este estudio es analizar la evolución de la enfermedad neumocócica invasora (ENI).
Material y métodosEstudio observacional de los casos de ENI residentes en la Comunidad de Madrid notificados a la Red de Vigilancia Epidemiológica entre los años 2008 y 2015. El caso de ENI se definió como la enfermedad producida por Streptococcus pneumoniae, con aislamiento, detección de ADN o detección de antígeno, en muestras procedentes de sitios normalmente estériles. Las cepas aisladas se enviaron al Laboratorio Regional de Salud Pública para la identificación del serotipo. Los serotipos se clasificaron según su inclusión en la vacuna heptavalente (VCN7), en la vacuna trecevalente pero no en la heptavalente (VCN13 adicional) y no incluidos en la VCN13 (no VCN). Se calcularon las razones de incidencia (RI) comparando los períodos 2011-2012 y 2013-2015 con el período 2008-2010.
ResultadosSe notificaron 4.307 casos. El 86,6% fueron serotipados. La RI de ENI para todos los serotipos fue de 0,67 y de 0,67; la RI para los serotipos VCN7 fue de 0,43 y de 0,45; la RI para los VCN13 adicional fue de 0,46 y de 0,25, y la RI para los no VCN fue de 1,01 y de 1,32 en los períodos 2011-2012 y 2013-2015. Los serotipos 8, 9N, 10A, 23B, 24F y el serogrupo 33 incrementaron su incidencia de manera significativa en el período 2013-2015. Los serotipos 15B y 24F supusieron el 24% de los casos no VCN13 en menores de 5 años, los serotipos 8 y 9N el 51% en población de 5 a 59 años y los serotipos 8 y 22F el 25% en mayores de 59 años.
ConclusionesLa incidencia de serotipos no incluidos en vacunas conjugadas ha aumentado, especialmente en menores de 5 años, pero la incidencia total de ENI ha disminuido. Es imprescindible continuar con los programas de vigilancia epidemiológica y microbiológica para valorar el efecto de la vacunación sobre la incidencia de la ENI.
Streptococcus pneumoniae is a significant cause of morbidity and mortality in both high-income and middle-income countries.1 It causes a broad spectrum of diseases; the invasive forms are the most serious.
The most effective measure to prevent invasive pneumococcal disease (IPD) is vaccination. Since 2006, the World Health Organization has recommended including conjugate vaccines in child immunisation programmes worldwide.2 The use of heptavalent conjugate vaccine (VCN7) has been shown to significantly reduce the incidence of cases caused by the serotypes that it contains.3,4 However, a decrease in cases caused by serotypes covered by conjugate vaccines could be accompanied by an increase in cases caused by other serotypes, thereby reducing the impact generated by including it in child immunisation programmes.3,5–8
In November 2006, the Autonomous Community of Madrid (ACM) included the pneumococcal conjugate vaccine against 7 serotypes (VCN7 with serotypes 4, 6B, 9V, 14, 18C, 19F and 23F)9 with a 3+1 regimen with doses at 2, 4, 6 and 18 months of age. In June 2010, VCN7 was replaced with pneumococcal conjugate vaccine against 13 serotypes (VCN13), which contains 6 serotypes in addition to those included in VCN7 (1, 3, 5, 6A, 7F and 19A), with a 2+1 regimen with doses at 2, 4 and 15 months of age. In July 2012, VCN13 was excluded from the child immunisation programme,10 but kept for risk groups. Since then, the vaccine has continued to be recommended by paediatric professionals individually with a 3+1 regimen with doses at 2, 4, 6 and 15 months. In 2015, the Interterritorial Council of the Spanish National Health Service approved a new child immunisation programme including the pneumococcal vaccine.11 In May 2015, the ACM updated its vaccination programme in accordance with the Spanish national recommendations12 to a 2+1 regimen with doses at 2, 4 and 12 months. Vaccination coverage exceeded 90% in those under 5 years of age.13
IPD has been a notifiable disease (ND) in the ACM since 2007.14 The objective of this study was to analyse trends in IPD between 2008 and 2015, as well as specific incidences by serotype.
Material and methodsCase definitionIPD was defined as disease caused by S. pneumoniae, with isolation, DNA detection or antigen detection in samples from normally sterile sites. The clinical form of presentation of the disease was not a determining factor with respect to case definition.
Study scopeThe study included all cases of IPD in residents of the ACM reported to the ND Surveillance System between 2008 and 2015. The ACM is one of the most heavily populated autonomous communities in Spain. Its Epidemiological Surveillance Network covers nearly 6.5 million people.
Clinical and laboratory dataStrains were obtained from clinical samples and isolated in microbiology laboratories at public and private hospitals in the ACM. Once a strain had been isolated, it was sent to the Regional Public Health Laboratory (LRSP) for serotype identification using the latex agglutination test (Pneumotest-Latex) and the Quellung reaction. The LRSP reported cases to the corresponding Area Public Health Services, where they were recorded in the database of the ND System for the region.
Individualised data were collected through a standardised ND form that included sociodemographic variables, clinical data and vaccine status. Clinical data were collected by consulting the information in the electronic medical record. If this information had to be broadened, the physicians who cared for the patients were asked to gather it. Vaccination data were extracted from the Registry of Vaccines of the ACM, which contains nominal information on the vaccination status of the entire population dating back to late 2004.
Data analysisThe cumulative annual incidence of IPD per 100,000 inhabitants was reported in terms of whether the group of serotypes was among those included in VCN7 (VCN7: 4, 6B, 9V, 14, 18C, 19F and 23F), was among the additional serotypes included in VCN13 (additional VCN13: 1, 3, 5, 6A, 7F and 19A) or was not among those included in any VCN (non-VCN). The annual incidence for each additional VCN13 serotype was also reported. To this end, the following age groups were taken into account: 0–4 years of age, 5–14 years of age, 15–39 years of age, 40–59 years of age and >59 years of age.
Follow-up time was divided into three periods, in accordance with the changes in the immunisation programme and the epidemiology of the disease: 2008–2010, 2011–2012 and 2013–2015. Changes over time in incidence were analysed by calculating cumulative incidence rates (IRs) and their levels of statistical significance were measured by using Poisson regression models and comparing cumulative incidences for the periods 2011–2012 and 2013–2015 to that for the period 2008–2010. This analysis was performed for the serotype groups described above and for the most common individual additional VCN13 and non-VCN serotypes. Broader age groups (0–4 years of age, 5–59 years of age and >59 years of age) were used to analyse individual serotypes.
The populations used were obtained from the Continuous Register of the Institute of Statistics of the ACM.15
ResultsA total of 4,307 cases of IPD were recorded in the ACM during the study period. Of these cases, 86.6% (n=3,730) were serotyped.
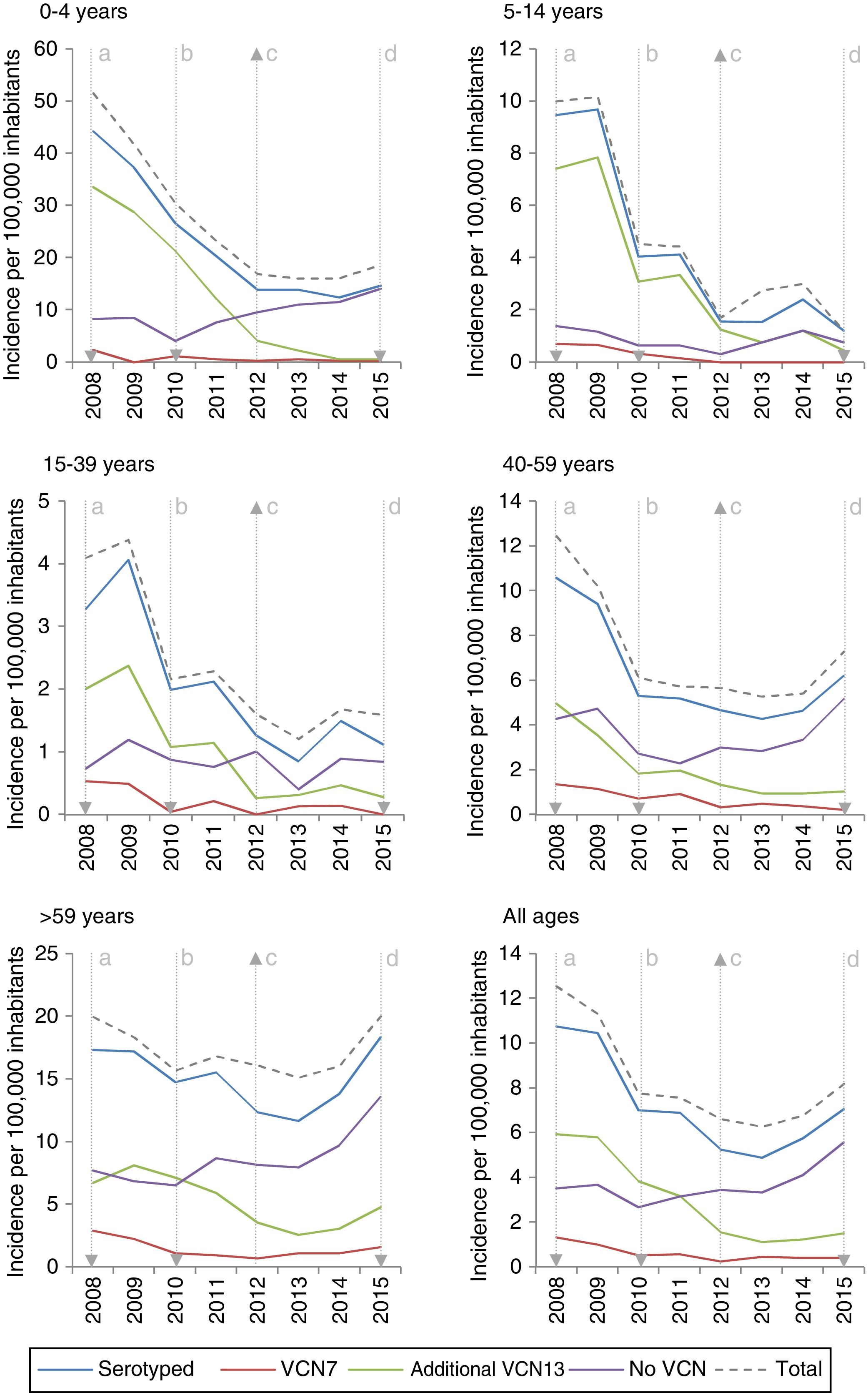
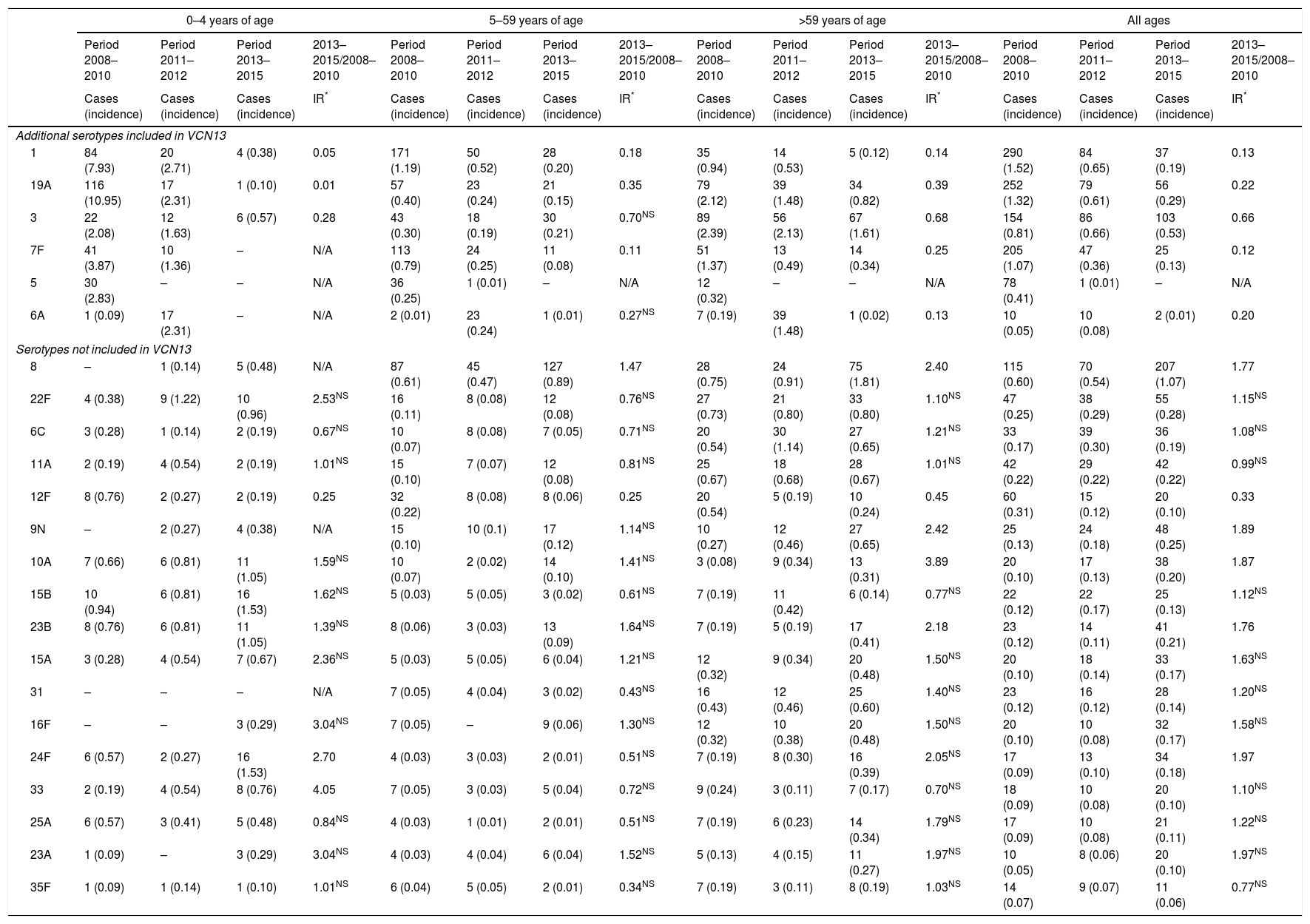
The incidence of VCN7 serotypes decreased from the start to the end of the period, from 1.32 in 2008 to 0.42 in 2015, especially in those 0–4 years of age and those 5–14 years of age. The incidence of the additional VCN13 serotypes decreased before VCN13 was introduced, from 5.93 in 2008 to 3.82 in 2010, whereas the incidence of non-VCN serotypes increased after VCN13 was introduced, from 2.66 in 2010 to 5.56 in 2015, especially in those 0–4 years of age and over 59 years of age (Fig. 1).
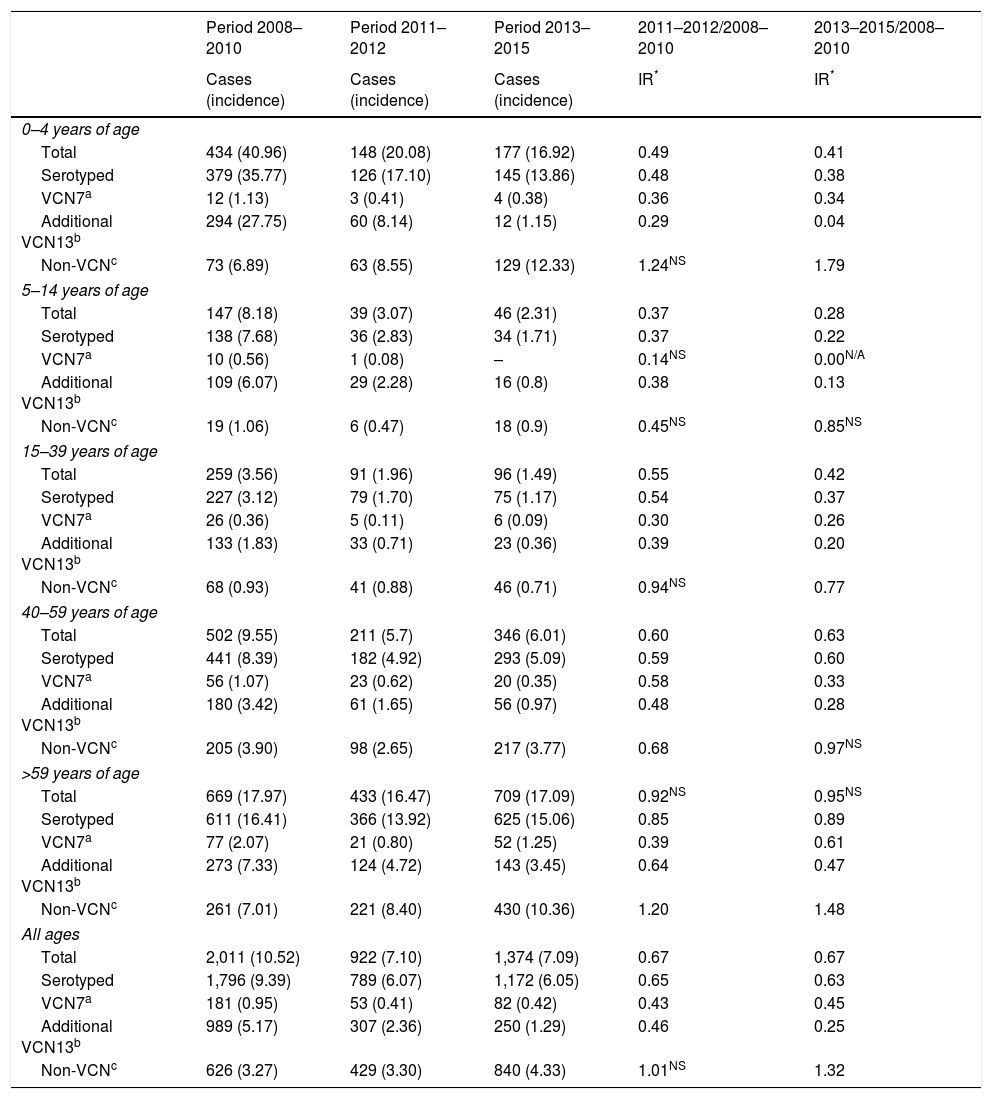
The incidence of IPD was 10.52 between 2008 and 2010. The IR showed a reduction in the incidence of the subsequent periods. The incidence was 0.67 in the period 2011–2012 (z=−9.89; p<0.01) and of the same magnitude in the period 2013–2015 (z=−11.28; p<0.01) (Table 1).
Changes over time in annual incidence per 100,000 inhabitants of invasive pneumococcal disease according to serotype by inclusion in conjugate vaccines and age group. Autonomous Community of Madrid. 2008–2015.
| Period 2008–2010 | Period 2011–2012 | Period 2013–2015 | 2011–2012/2008–2010 | 2013–2015/2008–2010 | |
|---|---|---|---|---|---|
| Cases (incidence) | Cases (incidence) | Cases (incidence) | IR* | IR* | |
| 0–4 years of age | |||||
| Total | 434 (40.96) | 148 (20.08) | 177 (16.92) | 0.49 | 0.41 |
| Serotyped | 379 (35.77) | 126 (17.10) | 145 (13.86) | 0.48 | 0.38 |
| VCN7a | 12 (1.13) | 3 (0.41) | 4 (0.38) | 0.36 | 0.34 |
| Additional VCN13b | 294 (27.75) | 60 (8.14) | 12 (1.15) | 0.29 | 0.04 |
| Non-VCNc | 73 (6.89) | 63 (8.55) | 129 (12.33) | 1.24NS | 1.79 |
| 5–14 years of age | |||||
| Total | 147 (8.18) | 39 (3.07) | 46 (2.31) | 0.37 | 0.28 |
| Serotyped | 138 (7.68) | 36 (2.83) | 34 (1.71) | 0.37 | 0.22 |
| VCN7a | 10 (0.56) | 1 (0.08) | – | 0.14NS | 0.00N/A |
| Additional VCN13b | 109 (6.07) | 29 (2.28) | 16 (0.8) | 0.38 | 0.13 |
| Non-VCNc | 19 (1.06) | 6 (0.47) | 18 (0.9) | 0.45NS | 0.85NS |
| 15–39 years of age | |||||
| Total | 259 (3.56) | 91 (1.96) | 96 (1.49) | 0.55 | 0.42 |
| Serotyped | 227 (3.12) | 79 (1.70) | 75 (1.17) | 0.54 | 0.37 |
| VCN7a | 26 (0.36) | 5 (0.11) | 6 (0.09) | 0.30 | 0.26 |
| Additional VCN13b | 133 (1.83) | 33 (0.71) | 23 (0.36) | 0.39 | 0.20 |
| Non-VCNc | 68 (0.93) | 41 (0.88) | 46 (0.71) | 0.94NS | 0.77 |
| 40–59 years of age | |||||
| Total | 502 (9.55) | 211 (5.7) | 346 (6.01) | 0.60 | 0.63 |
| Serotyped | 441 (8.39) | 182 (4.92) | 293 (5.09) | 0.59 | 0.60 |
| VCN7a | 56 (1.07) | 23 (0.62) | 20 (0.35) | 0.58 | 0.33 |
| Additional VCN13b | 180 (3.42) | 61 (1.65) | 56 (0.97) | 0.48 | 0.28 |
| Non-VCNc | 205 (3.90) | 98 (2.65) | 217 (3.77) | 0.68 | 0.97NS |
| >59 years of age | |||||
| Total | 669 (17.97) | 433 (16.47) | 709 (17.09) | 0.92NS | 0.95NS |
| Serotyped | 611 (16.41) | 366 (13.92) | 625 (15.06) | 0.85 | 0.89 |
| VCN7a | 77 (2.07) | 21 (0.80) | 52 (1.25) | 0.39 | 0.61 |
| Additional VCN13b | 273 (7.33) | 124 (4.72) | 143 (3.45) | 0.64 | 0.47 |
| Non-VCNc | 261 (7.01) | 221 (8.40) | 430 (10.36) | 1.20 | 1.48 |
| All ages | |||||
| Total | 2,011 (10.52) | 922 (7.10) | 1,374 (7.09) | 0.67 | 0.67 |
| Serotyped | 1,796 (9.39) | 789 (6.07) | 1,172 (6.05) | 0.65 | 0.63 |
| VCN7a | 181 (0.95) | 53 (0.41) | 82 (0.42) | 0.43 | 0.45 |
| Additional VCN13b | 989 (5.17) | 307 (2.36) | 250 (1.29) | 0.46 | 0.25 |
| Non-VCNc | 626 (3.27) | 429 (3.30) | 840 (4.33) | 1.01NS | 1.32 |
IR: incidence rate; N/A: p value not calculable; NS: changes not statistically significant (p≥0.05); –: no cases.
The incidence of VCN7 serotypes decreased in the period 2011–2012 and remained stable in the period 2013–2015, although in those >59 years of age the incidence increased in the period 2013–2015 without reaching the values in the period 2008–2010. The annual incidences for those >59 years of age were similar in 2008 (19.99) and in 2015 (20.01) despite a reduction in the intervening years (Fig. 1).
The incidence of the additional VCN13 serotypes deceased in both periods, especially in those 0–4 years of age. The VCN7 serotypes and the additional VCN13 serotypes accounted for 28% (332 out of 1,172) of the cases serotyped in 2013–2015, and their incidence was lower in those 0–4 years of age (1.53 cases per 100,000 inhabitants) than in those >59 years of age (4.70 cases per 100,000 inhabitants).
The incidence of non-VCN serotypes was seen to increase in the period 2013–2015 (IR=1.32; z=5.30; p<0.01). This increase was more marked in those 0–4 years of age (IR=1.79; z=3.97; p<0.01) and those >59 years of age (IR=1.48; z=4.98; p<0.01).
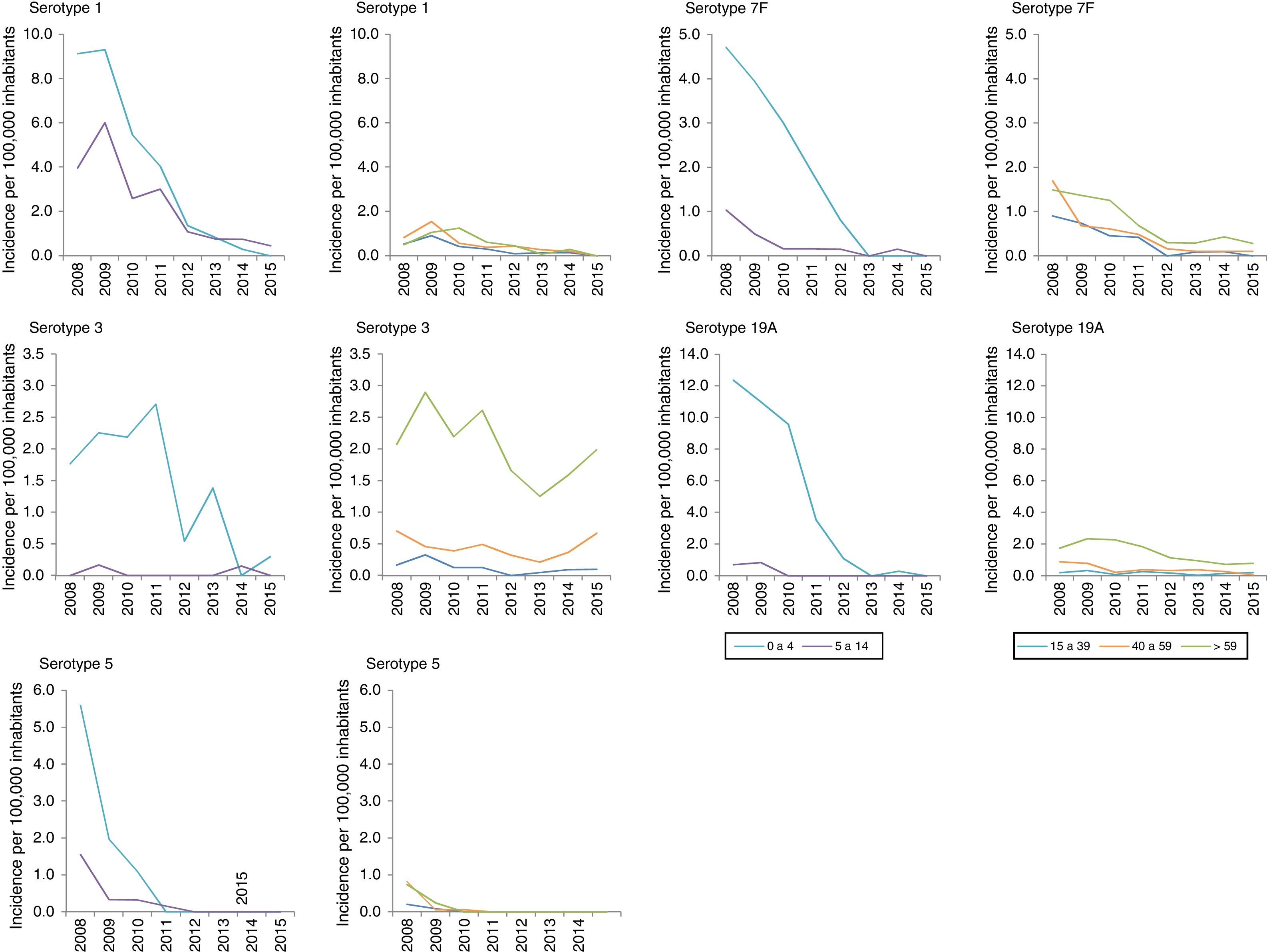
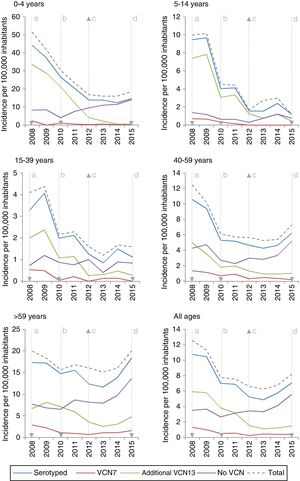
Among the additional VCN13 serotypes, serotype 1 was the one with the highest incidence in the period 2008–2010. The incidence of serotypes 1, 5, 6A, 7F and 19A declined in all age groups. This decline started before VCN7 was replaced with VCN13 for serotypes 5 and 7F. Serotype 3 showed an annual fluctuation (Fig. 2); its incidence decreased by 33% (IR=0.67; z=−3.27; p<0.01) in the period 2013–2015 (Table 2). Despite this, serotype 3 had the second-highest incidence (1.61 cases per 100,000 inhabitants) in those >59 years of age in the period 2013–2015. Among non-VCN serotypes, 8 had the highest incidence in the period 2013–2015, having been most significant in those >59 years of age (1.81 cases per 100,000 inhabitants).
Changes over time in invasive pneumococcal disease by period, serotype and age group. Autonomous Community of Madrid. 2008–2015.
| 0–4 years of age | 5–59 years of age | >59 years of age | All ages | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period 2008–2010 | Period 2011–2012 | Period 2013–2015 | 2013–2015/2008–2010 | Period 2008–2010 | Period 2011–2012 | Period 2013–2015 | 2013–2015/2008–2010 | Period 2008–2010 | Period 2011–2012 | Period 2013–2015 | 2013–2015/2008–2010 | Period 2008–2010 | Period 2011–2012 | Period 2013–2015 | 2013–2015/2008–2010 | |
| Cases (incidence) | Cases (incidence) | Cases (incidence) | IR* | Cases (incidence) | Cases (incidence) | Cases (incidence) | IR* | Cases (incidence) | Cases (incidence) | Cases (incidence) | IR* | Cases (incidence) | Cases (incidence) | Cases (incidence) | IR* | |
| Additional serotypes included in VCN13 | ||||||||||||||||
| 1 | 84 (7.93) | 20 (2.71) | 4 (0.38) | 0.05 | 171 (1.19) | 50 (0.52) | 28 (0.20) | 0.18 | 35 (0.94) | 14 (0.53) | 5 (0.12) | 0.14 | 290 (1.52) | 84 (0.65) | 37 (0.19) | 0.13 |
| 19A | 116 (10.95) | 17 (2.31) | 1 (0.10) | 0.01 | 57 (0.40) | 23 (0.24) | 21 (0.15) | 0.35 | 79 (2.12) | 39 (1.48) | 34 (0.82) | 0.39 | 252 (1.32) | 79 (0.61) | 56 (0.29) | 0.22 |
| 3 | 22 (2.08) | 12 (1.63) | 6 (0.57) | 0.28 | 43 (0.30) | 18 (0.19) | 30 (0.21) | 0.70NS | 89 (2.39) | 56 (2.13) | 67 (1.61) | 0.68 | 154 (0.81) | 86 (0.66) | 103 (0.53) | 0.66 |
| 7F | 41 (3.87) | 10 (1.36) | – | N/A | 113 (0.79) | 24 (0.25) | 11 (0.08) | 0.11 | 51 (1.37) | 13 (0.49) | 14 (0.34) | 0.25 | 205 (1.07) | 47 (0.36) | 25 (0.13) | 0.12 |
| 5 | 30 (2.83) | – | – | N/A | 36 (0.25) | 1 (0.01) | – | N/A | 12 (0.32) | – | – | N/A | 78 (0.41) | 1 (0.01) | – | N/A |
| 6A | 1 (0.09) | 17 (2.31) | – | N/A | 2 (0.01) | 23 (0.24) | 1 (0.01) | 0.27NS | 7 (0.19) | 39 (1.48) | 1 (0.02) | 0.13 | 10 (0.05) | 10 (0.08) | 2 (0.01) | 0.20 |
| Serotypes not included in VCN13 | ||||||||||||||||
| 8 | – | 1 (0.14) | 5 (0.48) | N/A | 87 (0.61) | 45 (0.47) | 127 (0.89) | 1.47 | 28 (0.75) | 24 (0.91) | 75 (1.81) | 2.40 | 115 (0.60) | 70 (0.54) | 207 (1.07) | 1.77 |
| 22F | 4 (0.38) | 9 (1.22) | 10 (0.96) | 2.53NS | 16 (0.11) | 8 (0.08) | 12 (0.08) | 0.76NS | 27 (0.73) | 21 (0.80) | 33 (0.80) | 1.10NS | 47 (0.25) | 38 (0.29) | 55 (0.28) | 1.15NS |
| 6C | 3 (0.28) | 1 (0.14) | 2 (0.19) | 0.67NS | 10 (0.07) | 8 (0.08) | 7 (0.05) | 0.71NS | 20 (0.54) | 30 (1.14) | 27 (0.65) | 1.21NS | 33 (0.17) | 39 (0.30) | 36 (0.19) | 1.08NS |
| 11A | 2 (0.19) | 4 (0.54) | 2 (0.19) | 1.01NS | 15 (0.10) | 7 (0.07) | 12 (0.08) | 0.81NS | 25 (0.67) | 18 (0.68) | 28 (0.67) | 1.01NS | 42 (0.22) | 29 (0.22) | 42 (0.22) | 0.99NS |
| 12F | 8 (0.76) | 2 (0.27) | 2 (0.19) | 0.25 | 32 (0.22) | 8 (0.08) | 8 (0.06) | 0.25 | 20 (0.54) | 5 (0.19) | 10 (0.24) | 0.45 | 60 (0.31) | 15 (0.12) | 20 (0.10) | 0.33 |
| 9N | – | 2 (0.27) | 4 (0.38) | N/A | 15 (0.10) | 10 (0.1) | 17 (0.12) | 1.14NS | 10 (0.27) | 12 (0.46) | 27 (0.65) | 2.42 | 25 (0.13) | 24 (0.18) | 48 (0.25) | 1.89 |
| 10A | 7 (0.66) | 6 (0.81) | 11 (1.05) | 1.59NS | 10 (0.07) | 2 (0.02) | 14 (0.10) | 1.41NS | 3 (0.08) | 9 (0.34) | 13 (0.31) | 3.89 | 20 (0.10) | 17 (0.13) | 38 (0.20) | 1.87 |
| 15B | 10 (0.94) | 6 (0.81) | 16 (1.53) | 1.62NS | 5 (0.03) | 5 (0.05) | 3 (0.02) | 0.61NS | 7 (0.19) | 11 (0.42) | 6 (0.14) | 0.77NS | 22 (0.12) | 22 (0.17) | 25 (0.13) | 1.12NS |
| 23B | 8 (0.76) | 6 (0.81) | 11 (1.05) | 1.39NS | 8 (0.06) | 3 (0.03) | 13 (0.09) | 1.64NS | 7 (0.19) | 5 (0.19) | 17 (0.41) | 2.18 | 23 (0.12) | 14 (0.11) | 41 (0.21) | 1.76 |
| 15A | 3 (0.28) | 4 (0.54) | 7 (0.67) | 2.36NS | 5 (0.03) | 5 (0.05) | 6 (0.04) | 1.21NS | 12 (0.32) | 9 (0.34) | 20 (0.48) | 1.50NS | 20 (0.10) | 18 (0.14) | 33 (0.17) | 1.63NS |
| 31 | – | – | – | N/A | 7 (0.05) | 4 (0.04) | 3 (0.02) | 0.43NS | 16 (0.43) | 12 (0.46) | 25 (0.60) | 1.40NS | 23 (0.12) | 16 (0.12) | 28 (0.14) | 1.20NS |
| 16F | – | – | 3 (0.29) | 3.04NS | 7 (0.05) | – | 9 (0.06) | 1.30NS | 12 (0.32) | 10 (0.38) | 20 (0.48) | 1.50NS | 20 (0.10) | 10 (0.08) | 32 (0.17) | 1.58NS |
| 24F | 6 (0.57) | 2 (0.27) | 16 (1.53) | 2.70 | 4 (0.03) | 3 (0.03) | 2 (0.01) | 0.51NS | 7 (0.19) | 8 (0.30) | 16 (0.39) | 2.05NS | 17 (0.09) | 13 (0.10) | 34 (0.18) | 1.97 |
| 33 | 2 (0.19) | 4 (0.54) | 8 (0.76) | 4.05 | 7 (0.05) | 3 (0.03) | 5 (0.04) | 0.72NS | 9 (0.24) | 3 (0.11) | 7 (0.17) | 0.70NS | 18 (0.09) | 10 (0.08) | 20 (0.10) | 1.10NS |
| 25A | 6 (0.57) | 3 (0.41) | 5 (0.48) | 0.84NS | 4 (0.03) | 1 (0.01) | 2 (0.01) | 0.51NS | 7 (0.19) | 6 (0.23) | 14 (0.34) | 1.79NS | 17 (0.09) | 10 (0.08) | 21 (0.11) | 1.22NS |
| 23A | 1 (0.09) | – | 3 (0.29) | 3.04NS | 4 (0.03) | 4 (0.04) | 6 (0.04) | 1.52NS | 5 (0.13) | 4 (0.15) | 11 (0.27) | 1.97NS | 10 (0.05) | 8 (0.06) | 20 (0.10) | 1.97NS |
| 35F | 1 (0.09) | 1 (0.14) | 1 (0.10) | 1.01NS | 6 (0.04) | 5 (0.05) | 2 (0.01) | 0.34NS | 7 (0.19) | 3 (0.11) | 8 (0.19) | 1.03NS | 14 (0.07) | 9 (0.07) | 11 (0.06) | 0.77NS |
IR: incidence rate (per 100,000 inhabitants); N/A: p value not calculable; NS: changes not statistically significant (p≥0.05); –: no cases.
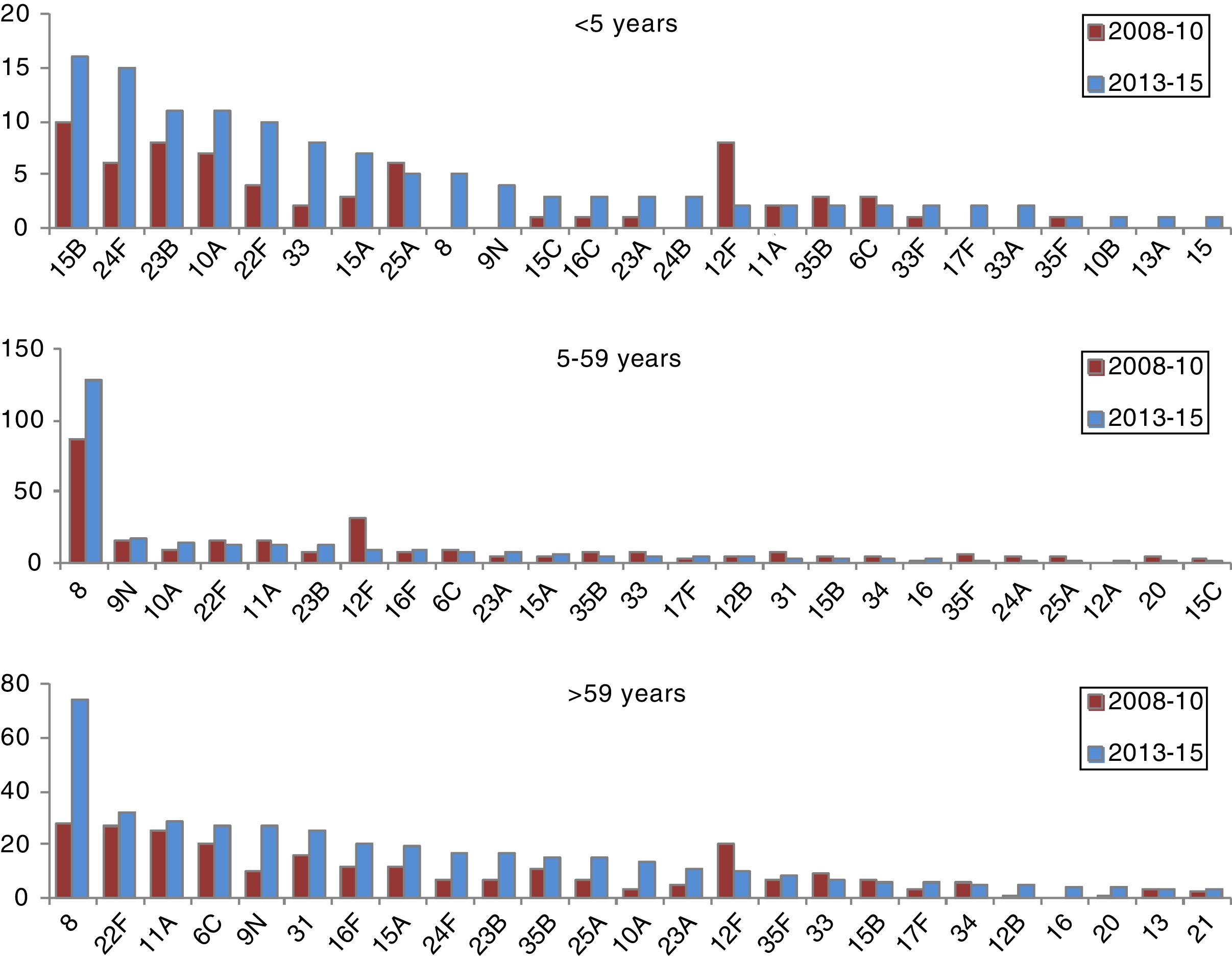
The incidence of cases due to serotypes 8, 9N, 10A, 23B and 24F and serogroup 33 showed a statistically significant increase in the period 2013–2015 in at least one age group (Table 2).
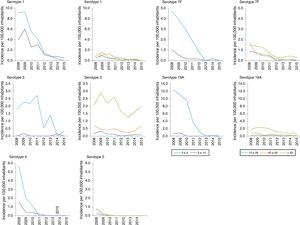
In those 0–4 years of age, the distribution of non-VCN serotypes was similar in the periods 2011–2012 and 2013–2015 (Fig. 3). The serotypes with the highest incidences during the period 2013–2015 in this age group were as follows: 15B (1.53 cases per 100,000 inhabitants), 24F (1.53), 10A (1.05), 23B (1.05) and 22F (0.96). Serotype 24F and serogroup 33 showed a significant increase in this group.
The non-VCN serotypes whose incidence showed a statistically significant increase were serotype 8 in those 5–59 years of age and serotypes 8, 9N, 10A and 23B in those >59 years of age.
DiscussionThe introduction of conjugate vaccines has had a major impact on the epidemiology of IPD. The incidence of vaccine serotypes has decreased, whereas the behaviour of serotypes not included in conjugate vaccines has proven difficult to interpret.
In those under 5 years of age, the incidence of the serotypes included in VCN13 in the period 2013–2015 was similar to that of England and Wales (1.43)16 and lower than that of Navarra.6,8,17 The differences in comparison to Navarra may have been due to the fact that its study ended in 2013, when the full effect of VCN13 had not yet been seen.
The decline in the incidence of the additional VCN13 serotypes started before the vaccine was introduced. This trend might have been due to the fact that the incidence of serotype 5 declined to the point of near-disappearance in 2009, having been reported as an epidemic serotype.18
Despite the use of VCN13 in children, serotype 3 is still common among adult patients; therefore, child vaccination does not appear to have an impact on IPD caused by serotype 3 in adults.19 This calls into question the capacity to generate a protective herd effect against this serotype.20
Before VCN13 was introduced, serotype 19A became one of the main aetiological agents for IPD in those under 5 years of age. This serotype is characterised by high rates of antibiotic resistance as well as high rates of clinical seriousness.7 The introduction of VCN13 reduced the incidence of this serotype to the point that it virtually disappeared.
The incidences of the serotypes covered by conjugate vaccines were seen to decline in all age groups, including cohorts that were not immunised. This supported the notion of a protective herd effect for most serotypes.21
The incidences of non-VCN serotypes declined before VCN13 was introduced and increased after it was introduced. This increase might have been due to changes in nasopharyngeal colonisation and occupation of the ecological niche freeing serotypes included in conjugate vaccines.22
It should be noted that IPD associated with serotype 8 increased. This serotype has been the most common serotype in recent years in the adult population in the ACM. A similar increase was recently reported in the United Kingdom.23 In Spain24, and elsewhere in Europe,25 it is an emerging serotype. Although no studies on nasal carriers have been conducted on a national level, data from Catalonia26 and Murcia27 have shown a low prevalence of serotype 8, which would indicate high invasiveness.
Between July 2012 and March 2015, the vaccine was off the systematic childhood vaccination schedule. The incidence of IPD during that period remained low. This might have been because it remained funded for risk groups and continued to be recommended by paediatricians with private access to it for the rest of the child population. Alternatively, the effects of the changes to the immunisation programme might have required a certain latency period before having repercussions for the epidemiology of the disease.
Even though the incidence of IPD decreased, cases caused by serotypes not included in conjugate vaccines are increasing, especially in those over 59 years of age. This replacement seen following the introduction of VCN728,29 has been reported in other studies with VCN13.16,22 However, the incidence of IPD in 2015 was less than the incidence of IPD in 2008; therefore, the replacement effect has not yet been established in our setting.
Although a protective herd effect of polysaccharide vaccine is being debated, this vaccine may influence the epidemiology of IPD in immunisation against serotypes in conjugate vaccines. Even so, the serotypes included in conjugate vaccines are more common in children, except serotype 3, which is more common in those over 59 years of age.
The IPD surveillance system was started in 2007. However, as no data were collected in the first few months and it was an incomplete year, it was excluded from the study. The main strength of this study was the fact that the ND of the ACM surveillance system actively and consistently collects population data and underwent no significant changes between 2008 and 2014. Another strength was the efforts made by the laboratories in managing samples for serotyping. The serotype was unknown in just 13.4% of cases. Our study reflected the epidemiology of IPD in a unique and uncommon situation: the withdrawal of the vaccine from the child immunisation programme at the expense of the government.
One weakness of this study was its lack of data prior to the introduction of VCN7. Information from that period would have enabled the reduction in the incidence to be better studied. In addition, the characteristics of this study precluded determination of whether the changes detected were due to the changes in the child immunisation programme or to other factors such as the secular trend of the disease, antibiotic pressure, changes in reporting and random chance.30 At least one of these factors might have been present in our study, since the reduction in the incidence of the cases caused by the additional serotypes included in VCN13 preceded the introduction of VCN13.
The site of isolation of strains may determine their serotyping. Samples obtained from pleural effusion cannot be detected by culture. In these situations, PCR could constitute an alternative to culture31 for serotype identification. Many of these cases of pleural effusion with negative culture are caused by serotype 1.32 In addition, due to the lytic enzymes of S. pneumoniae (autolysin and pneumolysin), some strains may lose their viability in repeated passes through culture and prevent serotyping.33 These situations explain some differences in incidence between serotyped cases and total cases. However, these discrepancies seem to have been due to random chance, as they followed a uniform distribution over the years of the study and among age groups.
In conclusion, our study showed a significant reduction in the incidence of IPD caused by the additional serotypes included in VCN13 in all age groups, thus supporting the notion of a protective herd effect. While overall incidence has decreased by 43%, the incidence of serotypes not included in conjugate vaccines has continuously increased, especially in those under 5 years of age. However, this increase has not yet offset the decline in the incidence of serotypes included in conjugate vaccines. Serotypes 8 and 22F accounted for 28.5% of serotypes not included in vaccines. In the future, research on vaccines with new serotypes could significantly contribute to public health interventions intended to manage IPD. In any case, active epidemiological and microbiological surveillance programmes must continue to assess the effect of vaccination on the incidence of invasive disease.34,35
FundingThis study was partly funded by SpIDnet (Evaluation of the impact of vaccination with conjugate vaccines on the epidemiology of invasive pneumococcal disease in Europe), a network funded by the European Centre for Disease Prevention and Control.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Latasa Zamalloa P, Sanz Moreno JC, Ordobás Gavín M, Barranco Ordoñez MD, Insúa Marisquerena E, Gil de Miguel Á, et al. Evolución de la enfermedad neumocócica invasora y sus serotipos en la Comunidad de Madrid. Enferm Infecc Microbiol Clin. 2018;36:612–620.