Vitamin D deficiency has been proposed to confer susceptibility to acquiring tuberculosis infection by impairing the innate immune response.
MethodsIn an exploratory study, we examined whether the levels of 25-hydroxyvitamin D3 (25(OH)D3) in serum, and cathelicidin – an antimicrobial peptide-induced under calcitriol – in the nasal fluid, would associate with the risk of acquiring tuberculosis infection.
ResultsWithin a prospective cohort of 231 tuberculosis household contacts tested with repeated interferon-gamma release assays, we serially analyzed all the uninfected contacts acquiring tuberculosis infection at follow-up (“converters”, n=18), and an age and sex-matched control group of contacts not acquiring tuberculosis infection (“non-converters”, n=36). The median levels of serum 25(OH)D3 did not differ between convertors and non-converters at baseline (14.9 vs. 13.2 ng/ml, p=0.41), nor at follow-up (19.0 vs 18.6ng/ml, p=0.83). Similarly, cathelicidin levels did not differ between both groups.
ConclusionThese data argue against a major role for hypovitaminosis D in tuberculosis infection susceptibility.
Se ha propuesto que la deficiencia de vitamina D, al afectar la respuesta inmunitaria innata, aumentaría la susceptibilidad de adquirir una infección por Mycobacterium tuberculosis.
MétodosEn un estudio exploratorio, examinamos si los niveles de 25-hidroxivitamina D3 (25(OH)D3) y de catelicidina (péptido antimicrobiano inducido bajo calcitriol) en suero y fluido nasal, respectivamente, se asocian con el riesgo de contraer una infección tuberculosa.
ResultadosEn una cohorte prospectiva de 231 contactos intradomiciliarios de tuberculosis en los que se realizaron ensayos de liberación de interferón-gamma en forma seriada, estudiamos a todos los contactos no infectados que adquirieron la infección al seguimiento («conversores», n=18), y a un grupo control pareado por edad y sexo que no adquirió la infección tuberculosa («no conversores», n=36). La mediana de los niveles séricos de 25(OH)D3 no difirió entre convestores y no conversores al inicio del estudio (14,9 vs. 13,2 ng/ml, p=0,41), ni al seguimiento (19,0 vs. 18,6 ng/ml, p=0,83). Igualmente, los niveles de catelicidina nasal no difirieron entre ambos grupos.
ConclusiónEstos resultados no apoyan la existencia de un papel significativo de la hipovitaminosis D en la susceptibilidad a la infección por tuberculosis.
Tuberculosis is one of the leading causes of death among infectious diseases, and undernutrition is long recognized as a major contributor of tuberculosis epidemics worldwide.1 Vitamin D (VD) deficiency is widespread given its low availability in standard diet, and has been associated with active tuberculosis in observational studies from different geographical areas.
In vitro, 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) – the active form of VD – boosts innate immunity and modulates bacterial antigen presentation.2 Upon Mycobacterium tuberculosis (Mtb) infection, 1α,25(OH)2D3 stimulates phagocytic cells, decreases tissue damage, and enhances expression of antimicrobial peptides such as β-defensin and cathelicidin.3 These peptides act as defense mechanisms against fungi and bacterial infections in the respiratory mucosa4 and cathelicidin promotes autophagy, an essential process for containment, degradation, and subsequent killing of Mtb.3
VD and its effectors levels may have a role in preventing Mtb acquisition in humans. Thus, we aimed to explore the association between serum VD levels and cathelicidin concentration in the upper respiratory epithelium, and the prospective risk of acquiring Mtb infection in tuberculosis contacts.
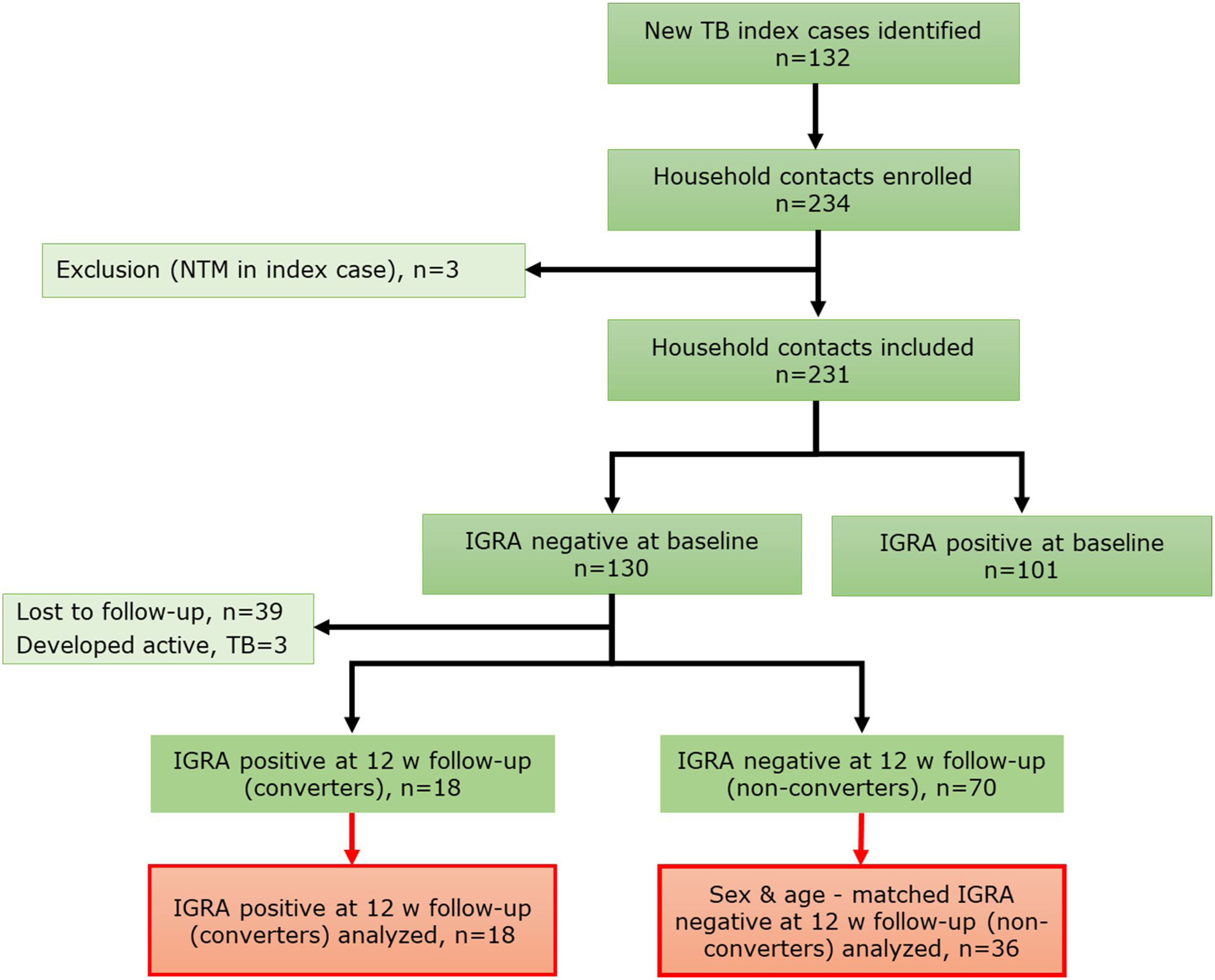
MethodsStudy designA prospective cohort study of household contacts (HHCs) (>15 years old) (n=231) of active pulmonary tuberculosis (PTB) cases was conducted between September 2017 and February 2020 in Santiago, Chile (Fig. 1). Contacts having active tuberculosis at baseline, prior tuberculosis, autoimmune diseases, pregnancy, HIV infection, use of immunosuppressants, and inhalation drugs were excluded. Demographic and clinical relevant information, including body mass index (BMI), vitamin supplementation and sunlight exposure estimated according to a previous publication,5 were recorded. All HHCs were tested with interferon-gamma release assay (IGRA) at baseline visit (V1) and again at 12 weeks (V2) follow-up if negative IGRA result at V1. Chest X-rays and symptom screening were done at V1, V2 and 24 week follow-up. Latent tuberculosis infection (LTBI) status was assessed by IGRA test (QuantiFERON®-TB Gold Plus (QFT), QIAGEN, Hilden, Germany). HHCs were classified as “converters” if IGRA result was negative at V1 and positive at V2, with both CD4 and CD4-CD8 tubes IFN-γ result>1UI/ml, and as “non-converters” if sustained negative IGRA test result at V1 and V2. In V1 and V2, blood and nasal lining fluid (NLF) samples were collected. Samples from all converters (n=18) and a randomly selected samples of age and sex-matched non-converters (n=36) were analyzed.
Ethical approval was obtained from the Institutional Review Board from Pontificia Universidad Católica de Chile. All participants provided written informed consent.
Nasal fluid collection and cathelicidin determinationNLF was collected introducing a Merocel pope ear wick (9mm×24mm) (Medtronic Inc., Minneapolis, USA) into a nostril for 2minutes (min) and stored at −80°C until processing.
NLF samples, thawed at 4°C for 10min, were incubated for 30min at 4°C with 300μl of elution buffer (1× PBS, 1% NP-40, 1% BSA). The Merocel sponge, placed inside a Corning Costar Spin-X® centrifuge tube filter (Sigma–Aldrich, Missouri, United States) mounted on a 1.5ml tube, was centrifuged at 16,000×g for 20min at 4°C. Cathelicidin levels were measured at a 1:50 dilution by ELISA (Hycult Biotech, Uden, The Netherlands) according to manufacturer's instructions. For absorbance values below the limit of detection (LOD) concentrations were considered as 0.05ng/ml for purpose analysis.
25(OH)D3 measurementSerum 25-hydroxyvitamin D3 (25(OH)D3) levels were determined by liquid chromatography with tandem mass spectrometry (LC–MS/MS) using the AB Sciex QTrap® 4500 tandem mass spectrometer (Foster City, California, United States) operated with atmospheric-pressure chemical ionization source. Measurements were performed at Biochemistry Laboratory of Red de Salud UC CHRISTUS. 25(OH)D3 status was defined as sufficient (>20ng/ml), insufficient (12–20ng/ml), or deficient (<12ng/ml) according to current consensus.6
Data analysisAnalyses, performed using GraphPad Prism v.7.0 (GraphPad Software, California, USA), were two-tailed and p-values<0.05 were considered statistically significant. Data were summarized with median and interquartile range (IQR). Comparisons between groups were performed using Mann–Whitney U test or Wilcoxon signed-rank test, for continuous variables, and Fisher's exact or Chi-squared test for categorical variables. Correlation was assess with Spearman nonparametric test. Sample size calculation was not done a priori given the exploratory nature of the study.
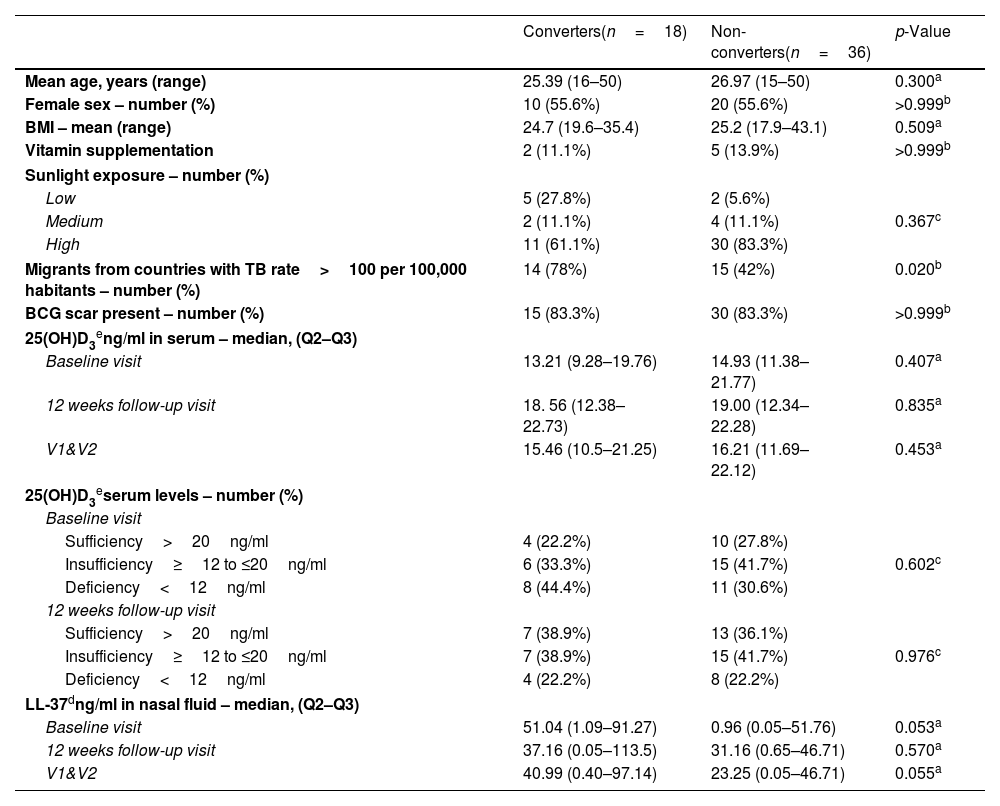
ResultsGeneral characteristics of enrolled participantsFrom a total of 231 HHCs, 130 (56.3%) had negative IGRA result at baseline (V1). Of these, 18 HHCs (13.8%) had IGRA conversion on follow-up (V2) and were included in this analysis. A total of 36 age and sex-matched non-IGRA converters (“non-converters”) were selected as the control group. Demographic and clinical characteristics are shown in Table 1.
Clinical and demographic characteristics of study participants.
| Converters(n=18) | Non-converters(n=36) | p-Value | |
|---|---|---|---|
| Mean age, years (range) | 25.39 (16–50) | 26.97 (15–50) | 0.300a |
| Female sex – number (%) | 10 (55.6%) | 20 (55.6%) | >0.999b |
| BMI – mean (range) | 24.7 (19.6–35.4) | 25.2 (17.9–43.1) | 0.509a |
| Vitamin supplementation | 2 (11.1%) | 5 (13.9%) | >0.999b |
| Sunlight exposure – number (%) | |||
| Low | 5 (27.8%) | 2 (5.6%) | |
| Medium | 2 (11.1%) | 4 (11.1%) | 0.367c |
| High | 11 (61.1%) | 30 (83.3%) | |
| Migrants from countries with TB rate>100 per 100,000 habitants – number (%) | 14 (78%) | 15 (42%) | 0.020b |
| BCG scar present – number (%) | 15 (83.3%) | 30 (83.3%) | >0.999b |
| 25(OH)D3eng/ml in serum – median, (Q2–Q3) | |||
| Baseline visit | 13.21 (9.28–19.76) | 14.93 (11.38–21.77) | 0.407a |
| 12 weeks follow-up visit | 18. 56 (12.38–22.73) | 19.00 (12.34–22.28) | 0.835a |
| V1&V2 | 15.46 (10.5–21.25) | 16.21 (11.69–22.12) | 0.453a |
| 25(OH)D3eserum levels – number (%) | |||
| Baseline visit | |||
| Sufficiency>20ng/ml | 4 (22.2%) | 10 (27.8%) | |
| Insufficiency≥12 to ≤20ng/ml | 6 (33.3%) | 15 (41.7%) | 0.602c |
| Deficiency<12ng/ml | 8 (44.4%) | 11 (30.6%) | |
| 12 weeks follow-up visit | |||
| Sufficiency>20ng/ml | 7 (38.9%) | 13 (36.1%) | |
| Insufficiency≥12 to ≤20ng/ml | 7 (38.9%) | 15 (41.7%) | 0.976c |
| Deficiency<12ng/ml | 4 (22.2%) | 8 (22.2%) | |
| LL-37dng/ml in nasal fluid – median, (Q2–Q3) | |||
| Baseline visit | 51.04 (1.09–91.27) | 0.96 (0.05–51.76) | 0.053a |
| 12 weeks follow-up visit | 37.16 (0.05–113.5) | 31.16 (0.65–46.71) | 0.570a |
| V1&V2 | 40.99 (0.40–97.14) | 23.25 (0.05–46.71) | 0.055a |
BMI=body mass index; V1&V2: Visit 1 (baseline) and Visit 2 (12 weeks follow-up).
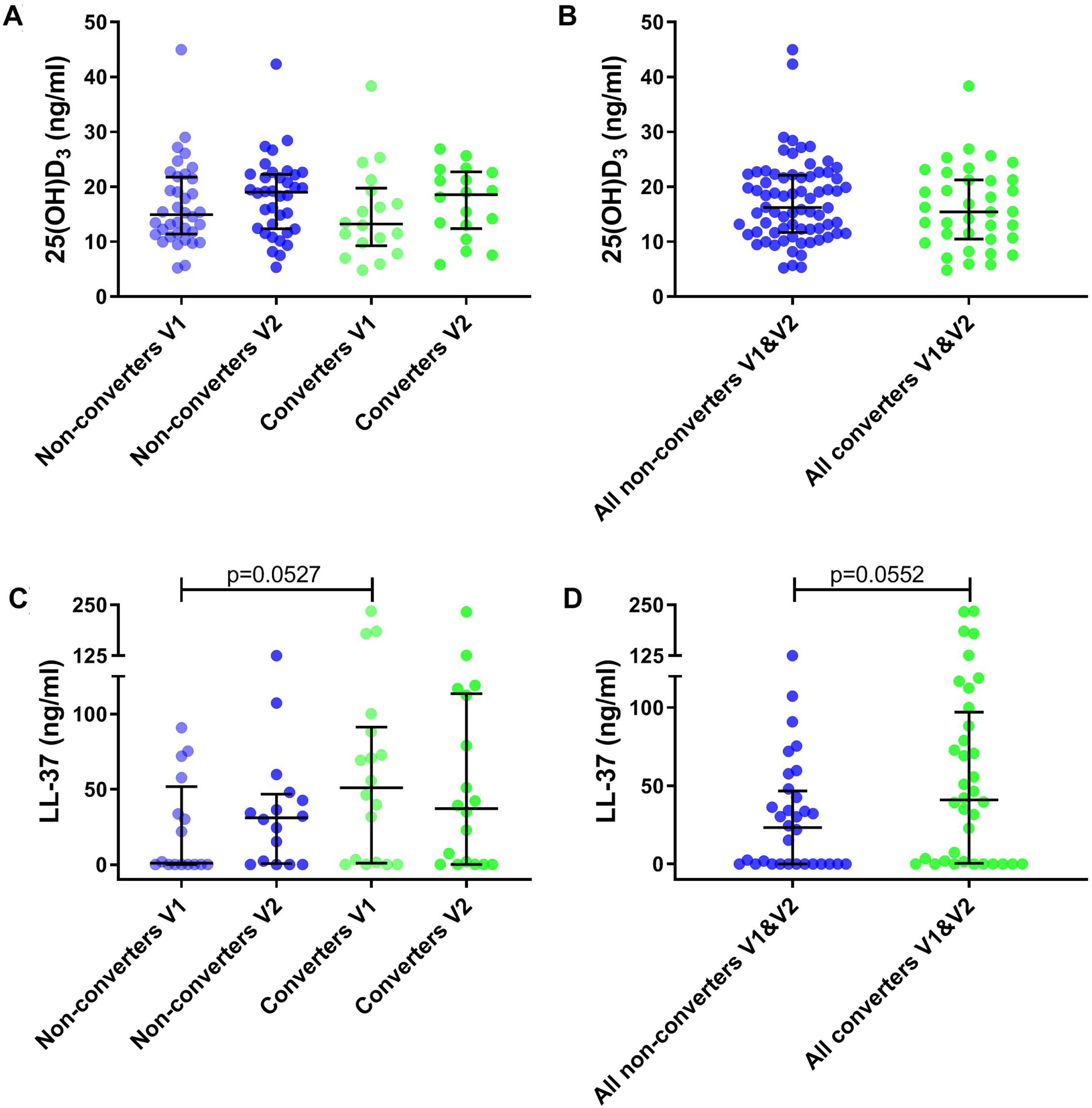
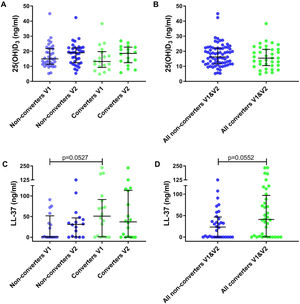
25(OH)D3 serum median level was 14.04ng/ml (IQR 10.86–21.32) for all participants. A total of 35.2% had VD insufficiency, 38.9% VD deficiency and 25.9% VD sufficiency. No significant differences were found between VD levels for converters and non-converters at V1 (13.21 vs 14.93ng/ml, p=0.41) nor at V2 (18.56 vs 19.00ng/ml, p=0.83) (Table 1 and Fig. 2A). Also, no significant differences were observed in converters (13.21 vs 18.56ng/ml, p=0.26) and non-converters (14.93 vs 19.00ng/ml, p=0.27) over time (V1 vs V2), nor according to sufficiency status (Table 1 and Fig. 2B) and seasonality (data not shown).
Metabolite levels in household contacts upon Mycobacterium tuberculosis exposure or recent infection. (A) Serum levels of 25(OH)D3 and (C) nasal fluid levels of LL-37 were measured in converters and non-converters at the baseline and follow-up visit. (B) Serum levels of 25(OH)D3 and (D) nasal fluid levels of LL-37 were measured in all converters (V1 and V2) and all non-converters (V1 and V2). The statistical significance was calculated using the Mann–Whitney U test using a two-tailed test. In plots A and C comparison were as follow: Non-converters V1 vs V2, Converters V1 vs V2, Non-converters V1 vs Converters V1, Non-converters V2 vs Converters V2.
We determined cathelicidin levels in 34 participants (18 converters and 16 non-converters) at V1 and V2. Cathelicidin concentrations for 12 participants in V1 (4 converters and 8 non-converters) and 9 in V2 (5 converters and 4 non-converters) were below the LOD. Cathelicidin levels did not differ in converters and non-converters at V1 (51.04 vs 0.96ng/ml, p=0.053) nor at V2 (37.16 vs 31.16ng/ml, p=0.57) (Table 1 and Fig. 2C). Also, no differences were observed over time (V1 vs V2) in converters (51.04 vs 37.16ng/ml, p=0.67) nor in non-converters (0.96 vs 31.16ng/ml, p=0.25) (Table 1 and Fig. 2D). Excluding samples with absorbance below the LOD did not modify our results.
Finally, no correlation was found between serum 25(OH)D3 and NLF cathelicidin levels for all participants (r=−0.012, p=0.925).
DiscussionIn the present study, 25(OH)D3 deficiency did not associate with the risk of acquiring Mtb infection in HHCs on follow-up. Although several studies describe VD deficiency is frequent in patients with active tuberculosis, only a few have focused on the prospective risk of active tuberculosis development or LTBI acquisition in contacts. Two prospective studies in Spain found VD deficiency was associated with risk of LTBI test conversion and active tuberculosis development in contacts.7,8 However, a case-control study found comparable VD levels between individuals with and without newly acquired LTBI, as well as between those progressing or not to active tuberculosis.9 Our results agree with the latter findings and those of a large randomized controlled trial in Mongolia where VD supplementation of VD deficient children did not result in lower risk of tuberculosis infection or disease.10
While no definitive association has been established between tuberculosis risk and VD status, extensive research has focused on its effector molecule cathelicidin.3 Patients with active PTB had similar cathelicidin plasma levels than uninfected individuals.11 However, peripheral blood cells of PTB cases had higher mRNA expression versus those of LTBI and uninfected individuals.12 Also, higher production was observed in polymorphonuclear cells of Mtb infected individuals,13 and in neutrophils, monocytes, alveolar macrophages, and human lung epithelial cells infected with Mtb in vitro.14 In this study, cathelicidin levels in nasal fluid did not differ in Mtb infected individuals; although local Mtb exposure might have been too short-lived15 to trigger cathelicidin expression in upper respiratory epithelial cells.
The main limitation in the present study is the small number of individuals included in the analysis given the small proportion of participants with new IGRA conversions in the all cohort. Thus, the study was probably underpowered to detect small differences between groups. However, the results were consistent when samples from different time-points were combined. It is also possible that due to the small sample size we did not find any association between VD and seasonality, a known factor to influence VD levels.16 Also, cathelicidin levels in nasal fluid may not necessarily represent those of intracellular or lower respiratory tract epithelium where Mtb exposure is higher.
In conclusion, neither serum 25(OH)D3 nor cathelicidin levels in nasal fluid were associated with susceptibility to acquiring tuberculosis infection in this study. Nonetheless, additional studies including a larger number of participants are needed to further the knowledge and determine the role of VD in newly infected individuals to contribute to this controversial topic.
FundingThis work was supported by ANID/CONICYTFONDECYT-1171570, 1211225 (ME), and 1191874 (RN).
Conflict of interestAll authors have declared no conflict of interest.
We thank Dr. Sandra Solari and Fidel Allende, from Clinical Laboratories of Red de Salud UC-CHRISTUS, for assistance in 25(OH)D3 measurements.