We read with interest the letter by Marco et al.1 concerning the low sensitivity of rapid antigen tests (RAT) to screen for SARS-CoV-2 infection.
The authors reported an outbreak of SARS-CoV-2 in a prison. Having diagnosed three cases by RAT, they screened a total of 81 inmates, with a SARS-CoV-2 infection incidence of 11% (9/81) by RAT. Between three and five days later, the 72 negative cases were screened again by real-time PCR (rt-PCR), 37% of which (27/72) tested positive. The authors labelled the previous RAT results as false negatives, concluding that rt-PCR should be the SARS-CoV-2 screening technique of choice given the low sensitivity of RATs.
It is vital to understand certain important points concerning RAT screening strategies, such as how to correctly interpret the results, when their use is indicated and their advantages over rt-PCR.
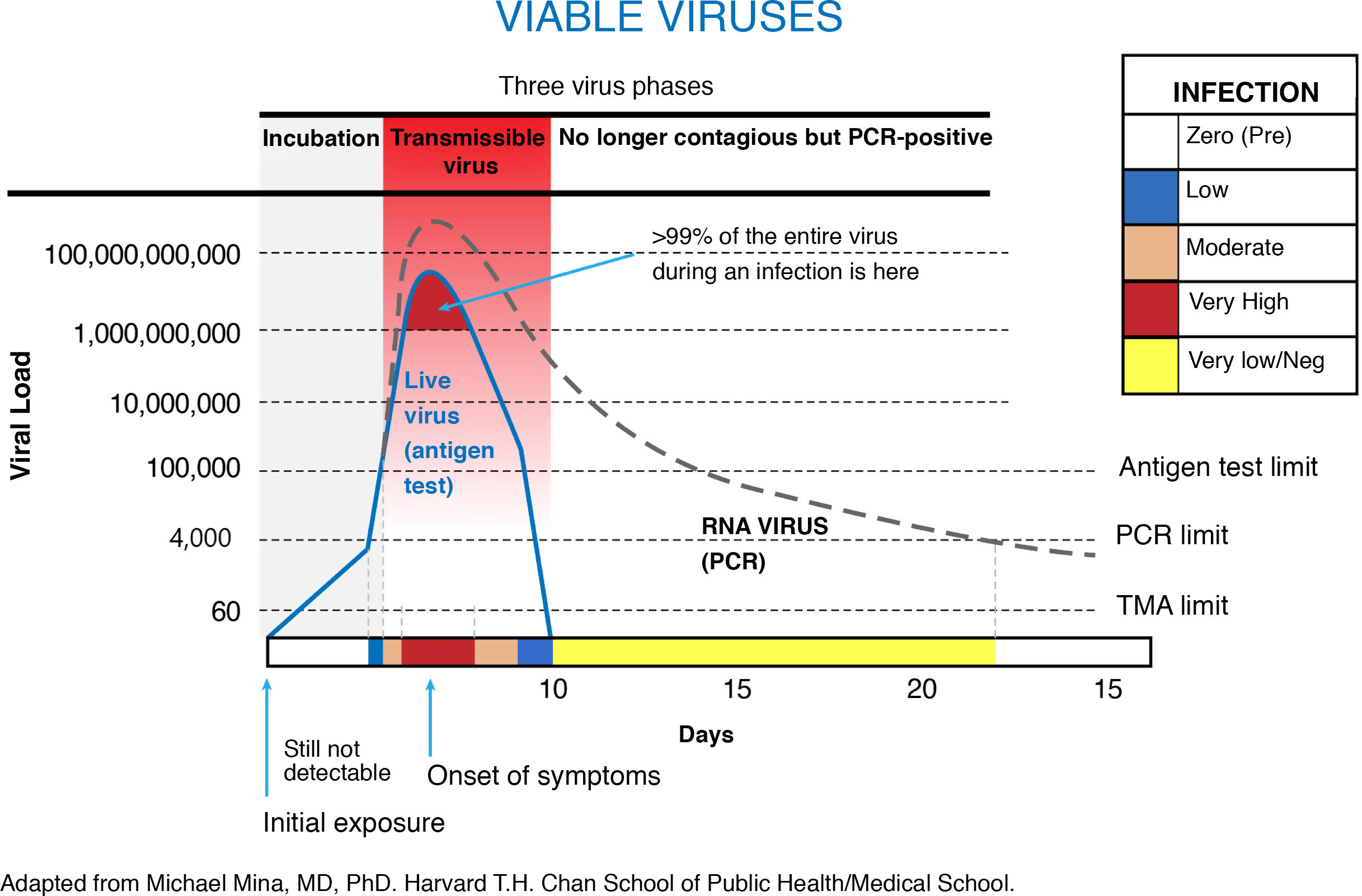
RATs have a high sensitivity for detecting individuals with a high viral load who could potentially transmit the virus (symptomatic and asymptomatic)2. This sensitivity is dependent on the viral load of the patient, which could be related to the ‘cycle threshold’ (Ct) of rt-PCR, an indirect marker of viral load in a infected subject. RATs are effective tools for diagnosing infected subjects with Cts <25, which are correlated with viruses that grow in cell cultures and are transmissible3,4, having shown sensitivity rates approaching 100%2. As a result, RATs may often be negative from the 5τη day of symptoms onset (or 10τη day since exposure) and universally in subjects with a low viral load.
The rt-PCR testing conducted between three and five days after the initial screening yielded a positivity rate of 37% in subjects with a prior negative RAT. SARS-CoV-2 nucleic acid amplification testing (NAAT) with rt-PCR or TMA (transcription-mediated amplification) detects positive results several days (in the case of rt-PCR) or weeks (in the case of TMA) after the abatement of symptoms, when subjects are no longer infectious.
As such, RAT and rt-PCR detect different stages of the disease. RATs yield positive results over a shorter period of time during the acute phase of infection.
An analysis of the SARS-CoV-2 viral kinetics (Fig. 1) reveals that high-frequency rapid antigen test screening strategies are just as effective in detecting infectious individuals as a low-frequency rt-PCR testing regimen5. The difference lies in the characteristics of each test: unlike rt-PCR, RATs are “versatile” tests that can be used anywhere, are inexpensive and return results in 15 min. Applying a RAT screening strategy could identify the same number of infectious individuals as rt-PCR.
Returning to the report by Marco et al., it would be useful to know the Cts of the rt-PCR-positive samples three and five days after the initial test, primarily to ascertain the patients' actual risk of virus transmission. Cts greater than 25–30 represent a low risk of virus transmission and a high probability of a negative RAT result, regardless of whether the subject is symptomatic or asymptomatic. A negative RAT followed by a positive rt-PCR in already isolated subjects essentially suggests that the Ct is elevated and the final phase of infection or a resolved infection is being detected.
This is particularly true of RAT, an ultrasensitive technique that detects up to 60 copies of SARS-CoV-2 (as opposed to 3,000–5,000 copies for rt-PCR)6. RAT is currently the most commonly used technique in mass screening strategies because samples can be pooled in the laboratory and because positive results can be returned for up to eight weeks after infection.
In conclusion, we believe that RAT and NAAT (rt-PCR or TMA) detect different phenomena. If you want a simple way to identify subjects with the potential to transmit SARS-CoV-2, RATs are the ideal tool. However, if you would like to screen a cross-sectional cohort that identifies the largest possible number of subjects infected (current and recent), NAAT would be the technique that detects the most number of cases.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Revollo Barriga B, Llibre Codina JM. Test rápidos antigénicos o PCR en tiempo real para SARS-CoV-2, ¿qué test usar y por qué? Enferm Infecc Microbiol Clin. 2021;39:531–532.