BCGitis is a rare complication after intravesical administration of Bacillus Calmette-Guérin for high-grade superficial bladder cancer and carcinoma in situ. May cause vascular involvement.
We present 2 cases and a review of the literature of the case reports pubished on the 10 years prior to April of 2022, when this proyect was finished, which described a case of aortoiliac mycotic aneurysm after receiving this treatment.
Of the 51 cases included (49 revised and 2 original), 100% were men, 82% were older than 65 years. The median latency period was 15 months (IQR 18). The most frequent location was the abdominal aorta, rupture occurred in 45,1% of patients. The most frequent symptom was abdominal or lumbar pain (61%), followed by general syndrome (49%). In 39,2% cases, it was associated with retroperitoneal abscesess. Attributable mortality was 13,6%.
BCGitis should be included in the differential diagnosis in patients who have received BCG therapy and present vascular involvement, even years after being treated.
La bcgítis es una complicación infrecuente del tratamiento intravesical con Bacillus Calmette-Guérin para el cáncer superficial de vejiga de alto grado y el carcinoma in situ. Puede causar afectación vascular.
Presentamos 2 casos y una revisión de la literatura de series de casos publicadas en los 10 años previos a la finalización de este trabajo en arbril de 2022, que describiesen un caso de aneurisma micótico aortoilíaco tras recibir este tratamiento.
De los 51 casos incluidos (49 revisados y 2 originales), el 100% eran hombres, 82% tenían más de 65 años. La mediana del período de latencia fue de 15 meses (RIQ 18). La localización más frecuente fue la aorta abdominal, documentándose rotura en 45,1%. El síntoma más frecuente fue dolor abdominal o lumbar (61%), seguido de síndrome general (49%). Asoció absceso retroperitoneal un 39,2%. La mortalidad atribuible fue de 13,6%.
La bcgítis debería incluirse como diagnóstico diferencial de pacientes que hayan recibido terapia con BCG y presenten afectación vascular, incluso años tras el tratamiento.
BCGitis, or disseminated Bacillus Calmette-Guérin (BCG) disease, is a rare infection caused by an attenuated variant of Mycobacterium bovis (M. bovis), with an estimated incidence of between 1% and 5%.1,2 It occurs as a complication of the treatment of superficial bladder neoplasia with intravesical instillations of BCG. The most common involvement is genitourinary, followed by pulmonary, hepatic and, in fourth place, vascular involvement. Depending on the site of involvement, it usually presents early (<3 months after BCG administration), as it tends to occur in liver and lung disease, or late (>3 months), as in the muscular and vascular form.1 At this level it can give rise to mycotic aneurysms in the aortoiliac segment, which make up the group with the highest mortality (up to 16%).3 Its late presentation and non-specific symptoms make diagnosis difficult.
We present two cases with vascular involvement and perform a systematic review of the literature on the matter.
MethodCase AA 77-year-old male, ex-smoker, with a history of arterial hypertension, dyslipidaemia and diabetes. Diagnosed at another hospital for urothelial carcinoma in 2011, he was treated with BCG instillations until 2014. At the start of treatment, he presented a sternal lytic lesion accompanied by perihilar lymph nodes with increased uptake on positron emission tomography (PET), an aetiological diagnosis was not obtained and the patient received treatment with anti-inflammatory drugs.
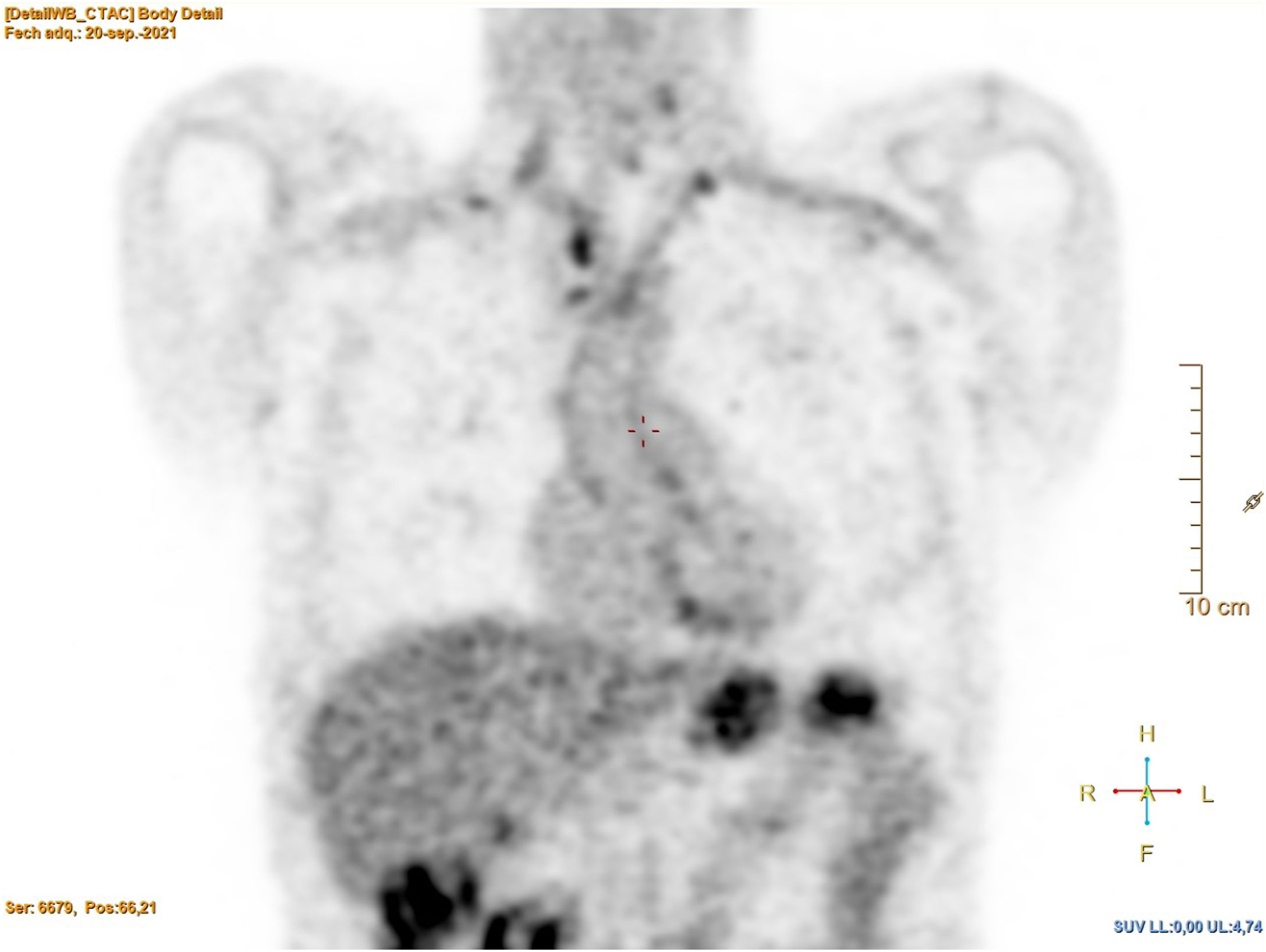
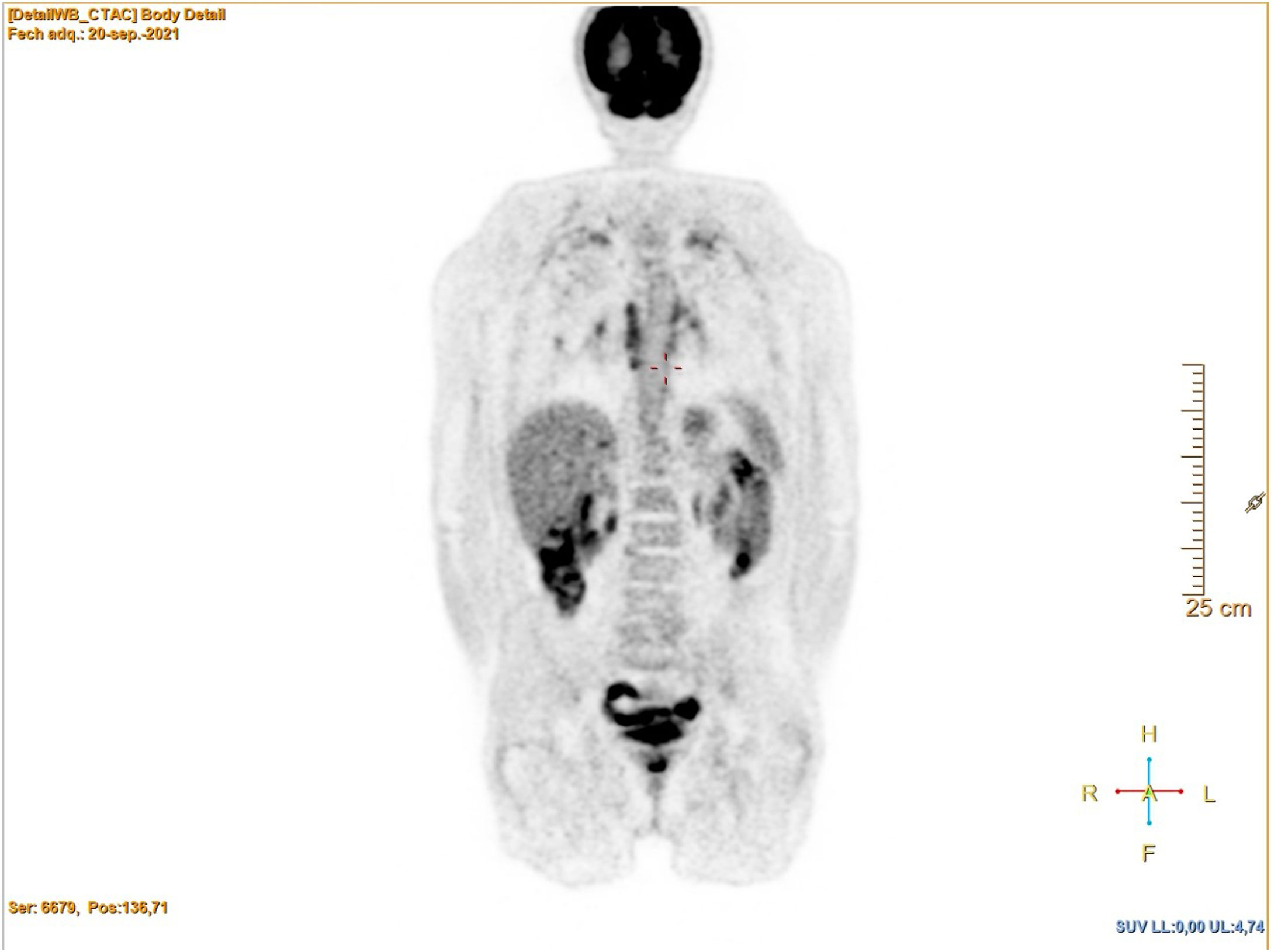
He sought care in 2021 for a general syndrome lasting months, with significant weight loss (20 kg in six months), with no other symptoms. The physical examination was normal. Laboratory tests only revealed anaemia (Hb 10.4 g/dl) and elevated C-reactive protein (CRP 30.1 mg/l). A computed tomography (CT) scan was performed that showed scar-like lesions in both lung apices, slightly enlarged mediastinal and hilar nodes, with a nonspecific appearance, and a 33-mm abdominal aneurysm. PET revealed hypermetabolism in the wall of the great vessels, in the hilar and mediastinal and lateral cervical lymph nodes, as well as multiple foci in both lung fields (Figs. 1 and 2).
Bronchoscopy with biopsy of the mediastinal lymph nodes was performed, in which lymphocyte cellularity was observed, without the presence of granulomas or malignant cells. A urine culture for mycobacteria was performed in which M. bovis, BCG strain, was isolated. Treatment with isoniazid + rifampicin + ethambutol was prescribed for the first two months, followed by isoniazid + rifampicin the following months, with good response. In the check-up in the clinic at six months, the patient was asymptomatic, with weight gain and normal test results.
Case BA 73-year-old male, ex-smoker, with a history of obesity, arterial hypertension, dyslipidaemia, COPD, old stroke and 49-mm abdominal aortic aneurysm. Diagnosed with bladder neoplasia in 2016, he was treated with six intravesical instillations of BCG, which were suspended due to poor tolerance (last 20 months before this episode).
He sought care in 2018 for fever of a week's duration, associated with hyporexia, asthenia, exertional dyspnoea and low back pain. Neither the examination nor the laboratory tests revealed any pathological data. A CT was performed in which images suggestive of a mycotic aneurysm were observed, but the blood and urine cultures did not obtain isolates.
One month later, he was admitted with intestinal occlusion due to adhesions, and was operated on. In the postoperative period, he presented abdominal pain, so a CT scan was performed that showed contained rupture of the aneurysm, and an endovascular prosthesis was placed. The patient's subsequent progress was poor, with multiple complications: acute ischaemia of the right lower limb, pneumothorax, atrial fibrillation with rapid ventricular response and heart failure, and bilateral cavitating pneumonia related to invasive ventilation. Within the aetiological study, cultures for mycobacteria were collected, demonstrating acid-alcohol-fast bacilli in urine. Antibiotic coverage was modified (amikacin + meropenem + linezolid + isoniazid + rifampicin), but the patient died three days later, 41 days after the first surgical intervention. After his death, urine culture results were received, isolating M. bovis BCG strain.
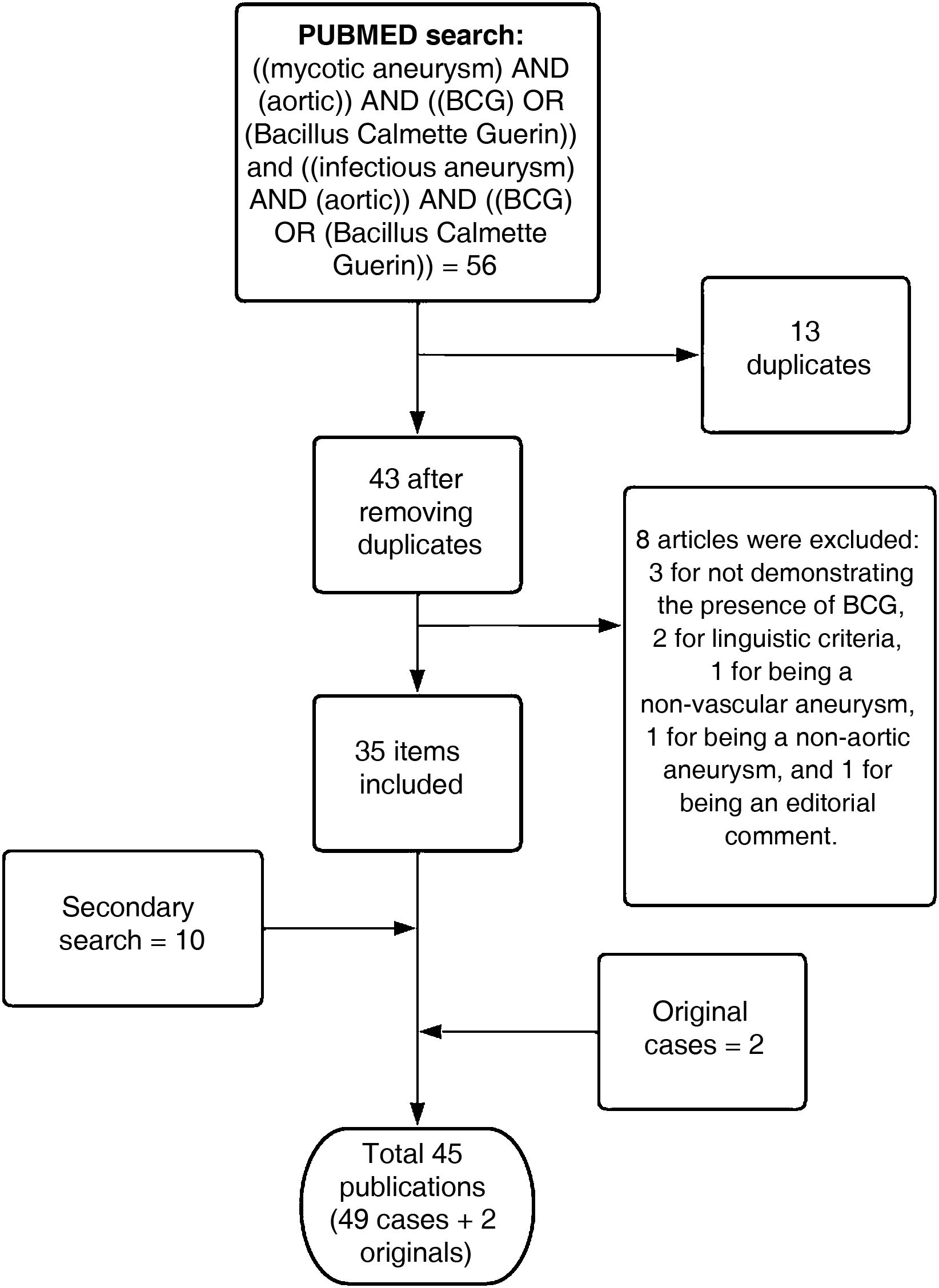
Literature reviewA bibliographic search was carried out in PubMed of articles published, in English and Spanish, between 2012 and April 2022. Subsequently, a secondary search was carried out from the articles found. The search terms and summary of the selection process are described in Fig. 3.4–48
The data obtained were included in a spreadsheet for analysis. Quantitative variables were expressed in numbers and percentages. Quantitative variables were expressed as mean (standard deviation) or median (interquartile range) values.
ResultsWe included our two cases and 49 cases from the literature obtained from case series published between 2012 and April 2022. All of the 51 cases included were male. Regarding the geographical distribution of the cases, 23 (45.1%) occurred in the Central Western European region and 20 (39.2%) in North America. A total of 78.4% of the patients were over 65 years of age, with a mean age of 72.4 years (SD 7.6). In addition to the history of urothelial carcinoma shared by all the patients, data on other comorbidities have been collected, appearing reflected in 30 of the 51 cases. Of these, 18 (60%) had hypertension, 10 (33.3%) dyslipidaemia, seven (23.3%) ischaemic heart disease, six (20.7%) COPD, and five (16.7%) diabetes. Fifteen (50%) of the 30 cases in which this information was collected were smokers (current or past). The latency period between the last instillation with BCG and the diagnosis of BCGitis was collected in 46 of the 51 cases, with an interval between 0 and 270 months and a median of 15 months (IQR 18).
Regarding the aneurysmal lesion, the presence of a vascular lesion in the aortoiliac segment had been previously detected in 12 of the 51 patients (23.5%). In 11 of them (91.7%), the vascular infection occurred on the pre-existing lesion (whether an aneurysm or the graft used in its repair). Of the 39 cases without prior vascular injury, 28 (71.8%) developed a mycotic aneurysm in the abdominal aorta, eight (20.5%) in the thoracic aorta, and six (15.4%) in the common iliac artery. In all, 7.8% of the patients developed aneurysms in more than one location within this segment. Massive or contained rupture of the aneurysmal lesions occurred in 23 patients (45.1%).
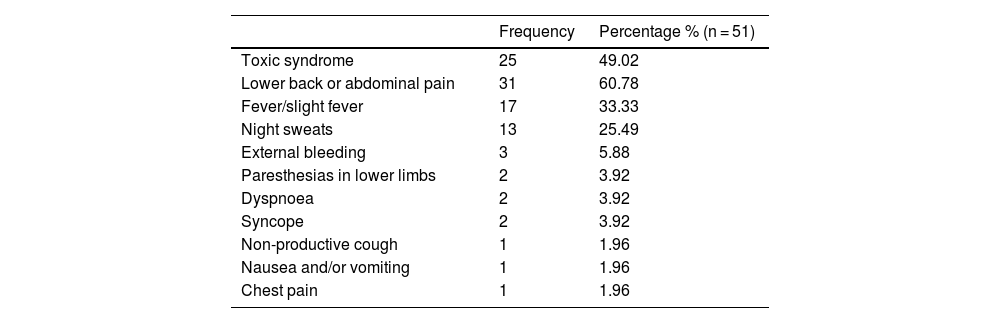
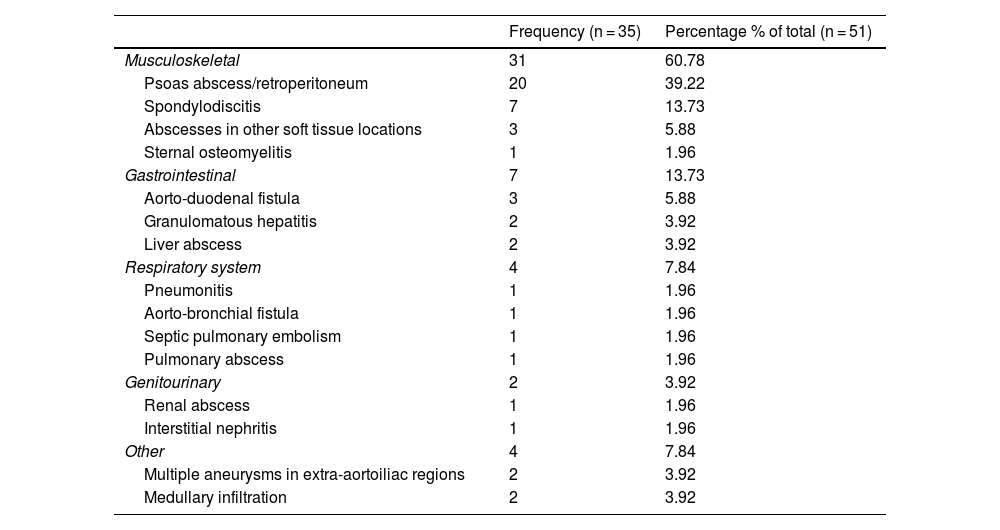
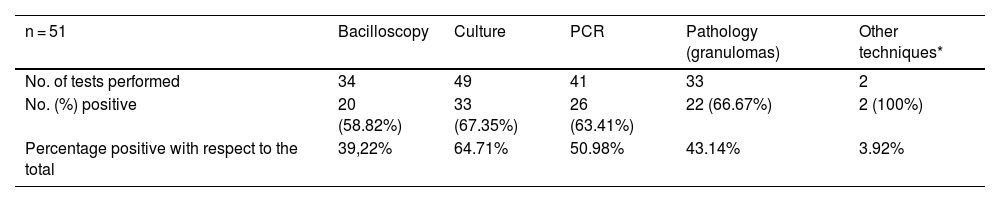
The clinical picture presented by the patients is shown in Table 1. Of the cases shown, 16 (33.4%) did not present another source of infection apart from the vascular lesion. The distribution of extravascular locations in the 35 patients (68.6%) that did present them is shown in Table 2. The distribution of the diagnostic method used is shown in Table 3.
Symptoms present at diagnosis.
| Frequency | Percentage % (n = 51) | |
|---|---|---|
| Toxic syndrome | 25 | 49.02 |
| Lower back or abdominal pain | 31 | 60.78 |
| Fever/slight fever | 17 | 33.33 |
| Night sweats | 13 | 25.49 |
| External bleeding | 3 | 5.88 |
| Paresthesias in lower limbs | 2 | 3.92 |
| Dyspnoea | 2 | 3.92 |
| Syncope | 2 | 3.92 |
| Non-productive cough | 1 | 1.96 |
| Nausea and/or vomiting | 1 | 1.96 |
| Chest pain | 1 | 1.96 |
Sites of infection outside the aortoiliac segment.
| Frequency (n = 35) | Percentage % of total (n = 51) | |
|---|---|---|
| Musculoskeletal | 31 | 60.78 |
| Psoas abscess/retroperitoneum | 20 | 39.22 |
| Spondylodiscitis | 7 | 13.73 |
| Abscesses in other soft tissue locations | 3 | 5.88 |
| Sternal osteomyelitis | 1 | 1.96 |
| Gastrointestinal | 7 | 13.73 |
| Aorto-duodenal fistula | 3 | 5.88 |
| Granulomatous hepatitis | 2 | 3.92 |
| Liver abscess | 2 | 3.92 |
| Respiratory system | 4 | 7.84 |
| Pneumonitis | 1 | 1.96 |
| Aorto-bronchial fistula | 1 | 1.96 |
| Septic pulmonary embolism | 1 | 1.96 |
| Pulmonary abscess | 1 | 1.96 |
| Genitourinary | 2 | 3.92 |
| Renal abscess | 1 | 1.96 |
| Interstitial nephritis | 1 | 1.96 |
| Other | 4 | 7.84 |
| Multiple aneurysms in extra-aortoiliac regions | 2 | 3.92 |
| Medullary infiltration | 2 | 3.92 |
Distribution of diagnostic tests performed and their results.
| n = 51 | Bacilloscopy | Culture | PCR | Pathology (granulomas) | Other techniques* |
|---|---|---|---|---|---|
| No. of tests performed | 34 | 49 | 41 | 33 | 2 |
| No. (%) positive | 20 (58.82%) | 33 (67.35%) | 26 (63.41%) | 22 (66.67%) | 2 (100%) |
| Percentage positive with respect to the total | 39,22% | 64.71% | 50.98% | 43.14% | 3.92% |
Patient follow-up was included in 44 of the 51 reviewed articles. Of the patients followed up, 36 (81.8%) responded favourably to the prescribed treatment. Six patients (13.6%) died due to BGGitis, while another two cases died of unrelated causes. The data collected is listed in Annex 1, available in the online version of this publication.
DiscussionIn most cases, BCGitis occurs as a complication of intravesical treatment with BCG instillation for urothelial carcinoma of the bladder. Vascular involvement has an incidence between 1% and 6.7%.3,49 In our review, the cases were concentrated in Central Western Europe and North America, which could be due to the higher incidence of bladder cancer in the Caucasian population,50, although recent studies state that Central Western Europe (specifically Spain51) and North America are followed by North Africa and Western Asia,2 with the data belonging to the last two possibly having been masked due to the lack of publications in these regions in this regard. All the cases reviewed and the two cases belonging to our hospital were male, as previously described.52 This trend could be explained by several hypotheses: first, smoking is the main risk factor for bladder neoplasia (up to 50% of cases).53 In our review, half of the patients were or had been smokers. Although the frequency of smoking in females is on the rise, males have traditionally been more exposed to the toxin, which could justify the differences found. However, several studies have determined that the risk of bladder neoplasia associated with smoking is the same in both sexes,53 and that an upward trend is already observed in the incidence of this pathology in the female sex.54 Therefore, it is possible that the prevalence of BCGitis in women will increase in the coming years. Another factor that can make this pathology more common in men is the BCG instillation technique. It is a more traumatic instillation in the male sex due to the morphology of the male urethra,54 which could facilitate bacteraemia and favour vascular involvement.52
The age of our two patients (73 and 77 years) was close to the mean age found in the bibliographic review (72.4 years), as well as in other reviews (mean 71.8 years).1
In our series, the median latency period after the last BCG instillation was 15 months (IQR 18), close to what has already been described, with series with medians of 13 months (IQR 19).52 We must bear in mind that vascular involvement presents with late symptoms, and that we can find reviews of other clinical forms of BCGitis with medians of 169.5 days from the last instillation.1 Of our two cases, case B presented a latency period consistent with the series, about 12 months, whereas case A was not diagnosed until 84 months after his last treatment.
The abdominal aorta is the most frequent location of non-infectious aneurysms in the aortoiliac segment.55 Multiple factors come into play in the development of these aneurysmal lesions, such as male gender, advanced age, Caucasian race, smoking, hypertension, and dyslipidaemia,56–60 and their diagnosis is incidental in most cases. In the cases reviewed in this series, the most frequent location is also the aortoiliac segment (71.8%), followed by the thoracic aorta (20.5%), and 91.6% of the patients with known aneurysms presented the infection on the pre-existing lesion, so it is likely that in some of the other cases there was also a previous undiagnosed aneurysmal lesion. A fundamental characteristic that differentiates mycotic aneurysms from other aortic aneurysms is their increased risk of rupture.61 This is due to the action that local inflammation has on the activation of enzymes that destroy collagen and elastin, increasing the fragility of the wall.62 The percentage of patients in the collected series with rupture of the aneurysm was 45.1%, as in case B, and the percentage of ruptures found in the bibliography was 35%.63 In another review carried out that included 74 cases of BCG vascular infections, a rupture rate of 42% was described, although we must bear in mind that locations outside the aortoiliac segment were included.64
Regarding the clinical signs, the most common was toxic syndrome, present in 49% of the reviewed patients and in both our cases. Fever and night sweats were also very common. This symptomatology also presents a high incidence in the reviewed cohort study.1 This set of signs and symptoms are those expected in a patient with a significant systemic disease, which is why in many cases it would suggest neoplastic processes, inflammatory processes or more prevalent infections. Therefore, the absence of specific data that guides the aetiology of the condition could be a cause for diagnostic delay. Lower back or abdominal pain were also frequently described symptoms, a characteristic that is shared with mycotic aneurysms caused by other pathogens.61 A specific characteristic of BCG vascular infections is the high frequency with which they are associated with musculoskeletal or soft tissue involvement (up to 60.8% of the total in our review). The most frequent form of presentation within this category was abscess in the psoas muscle (39.2% of the total). Other reviews describe musculoskeletal involvement in 27% of cases and specifically the psoas muscle in 22%.1 The fact that they included other aneurysms in other locations could be one of the reasons why lower frequencies are obtained, given that in this case local spread due to contiguity to the retroperitoneal space seems to be the most plausible hypothesis.
The most effective technique for demonstrating the presence of the bacillus was the culture of various samples (in many cases intraoperative samples of the resected material or punctures of the content of an abscess), though even then, its sensitivity was 67.3%. compared to 63.41% obtained when performing the PCR technique. In other reviews, culture is described as the most frequently used diagnostic method.49 Although histological diagnosis is described as the exclusive diagnostic method in some cases,64 in most of them it was used to support the previously mentioned microbiological tests. In our two cases, the sample used was urine. However, this was not especially used in the other patients (only in two of the cases in our review, and only in one of them as an exclusive sample). The lack of research related to the type of sample and the method used prevents us from comparing these findings to find out if urine culture (with the great benefit of the innocuousness of its collection) could be considered a good option compared to other more invasive samples.
Finally, during the follow-up of the patients, 81.8% responded favourably to empirical antibiotic treatment to treat the M. bovis infection, consisting of isoniazid, rifampicin and ethambutol (this bacillus is intrinsically resistant to pyrazinamide, therefore it is excluded from the conventional tuberculostatic regimen), accompanied, when necessary, by surgical repair of the vascular defect. This review found a mortality of 13.6%, close to the rest of the literature, between 10% and 16%.2,3
In conclusion, mycotic aneurysms from BCGitis are a rare complication of intravesical BCG instillation treatment for superficial bladder cancer. It should be included in the differential diagnosis of patients with a history of BCG instillations in the past, especially those with general syndrome and lower back or abdominal pain. The most common location is the abdominal aorta and it may be associated with a psoas abscess. It has a significant risk of rupture, but with proper treatment the prognosis is usually favourable.
Ethical responsibilitiesThis project has been developed without external financing.
The cases collected are included in a specific registry authorised by the Santiago-Lugo Research Ethics Committee.
Conflicts of interestThe authors declare that they have no conflicts of interest.