Leuconostoc spp. are facultatively anaerobic Gram-positive cocci involved in cases of hospital-acquired bacteremia, mainly in immunocompromised hosts. The available data is scarce due to its uncommon presentation.
MethodsWe describe all the episodes of Leuconostoc spp. bacteremia in a third level hospital in a 13-year period (2008–2021).
ResultsFour cases of clinically relevant bacteremia were detected. All cases were categorized as catheter-related. The following risk factors were found: previous glycopeptide therapy (75%), use of parenteral nutrition (100%) and cancer (75%). All isolates showed susceptibility to beta-lactams. Catheter removal was performed and wide spectrum antimicrobials were administered, with clinical response in all cases except one.
DiscussionApart from cancer and glycopeptide exposure, disruption of skin barrier and gastrointestinal conditions were identified as risk factors, as it was concordantly underlined in other case series. Susceptibility to beta-lactams is usually maintained. Catheter removal and administration of an active antibacterial therapy seem to be the best approach for Leuconostoc spp. catheter-related bacteremia.
Los microorganismos del género Leuconostoc son cocos grampositivos anaerobios facultativos, involucrados en casos de bacteriemia en pacientes hospitalizados, especialmente con factores de inmunosupresión. La literatura disponible es escasa por su baja frecuencia.
MétodosDescribimos todos los episodios de bacteriemia por Leuconostoc spp. en un hospital de tercer nivel en un periodo de 13 años (2008-2021).
ResultadosSe detectaron 4 aislamientos clínicamente significativos. Todos ellos fueron categorizados como bacteriemia relacionada con catéter. Se identificaron como factores de riesgo: la exposición previa a glucopéptidos (75%), nutrición parenteral (100%) y cáncer (75%). Todos los aislamientos presentaron sensibilidad a betalactámicos. Se procedió a retirada del catéter y se administraron antimicrobianos de amplio espectro con buena respuesta clínica, salvo en un caso.
DiscusiónAdemás del cáncer y la exposición a glucopéptidos, la disrupción de la barrera cutánea y las enfermedades gastrointestinales se identificaron como factores de riesgo, al igual que en otras series. La sensibilidad a betalactámicos suele mantenerse. La retirada del catéter y el uso de terapia antibiótica activa parece ser la mejor alternativa terapéutica para la bacteriemia relacionada con catéter por Leuconostoc spp.
Leuconostoc spp. (order Lactobacillales) are catalase-negative, alpha-hemolytic, facultatively anaerobic Gram-positive cocci usually arranged in pairs or chains and often found in fermenting vegetables, milk, dairy products, wines and meats. These bacteria exhibit high-level intrinsic resistance to glycopeptides due to the presence of peptidoglycan precursors of the cell wall terminating in d-alanyl-d-lactate rather than d-alanyl-d-alanine.1 Although once considered as non-pathogenic, since 1985 Leuconostoc spp. has been occasionally reported to be involved in hospital-acquired infections among immunocompromised patients2–4 or low birth weight premature neonates with underlying gastrointestinal conditions.5,6 One case of bacteremia was reported in an immunocompetent adult with ileum perforation.7 However, available data regarding risk factors, therapeutic approaches and outcomes is limited. We aimed to describe clinical features and outcome of all cases of Leuconostoc spp. bacteremia in our institution.
MethodsWe reviewed all cases of documented, clinically significant Leuconostoc spp. bacteremia diagnosed over a 13-year period (January 2008–December 2021) in a 1300-bed tertiary-care center in Madrid (Spain) with a reference population of 451,000 inhabitants in 2020. Cases were identified through the computerized database of the Department of Microbiology and defined by the isolation of Leuconostoc spp. in one or more blood cultures in the presence of symptoms and signs of systemic infection. Blood samples were processed using the BacT/Alert 3D® and BacT/Alert VIRTUO® systems (bioMérieux, Marcy-l’Étoile, France). All blood culture bottles were incubated for 5 days before being considered as negative. All isolates were identified with the VITEK® 2 system (bioMérieux) and, since 2014, with the MALDI-TOF MS Biotyper system (Bruker Daltonics Inc., Bremen, Germany). Antimicrobial susceptibility to penicillin, ceftriaxone, levofloxacin, vancomycin and cotrimoxazole was tested by the agar disk-diffusion method using Mueller-Hilton agar supplemented with sheep blood at 5% (bioMérieux) in accordance with the Clinical Laboratory Standards Institute (M45, CLSI). Since breakpoints of many antimicrobials are not available for Leuconostoc spp. in CLSI documents, we applied the Streptococcus pneumoniae breakpoints described in the 2006, 2010 and 2016 CLSI editions.
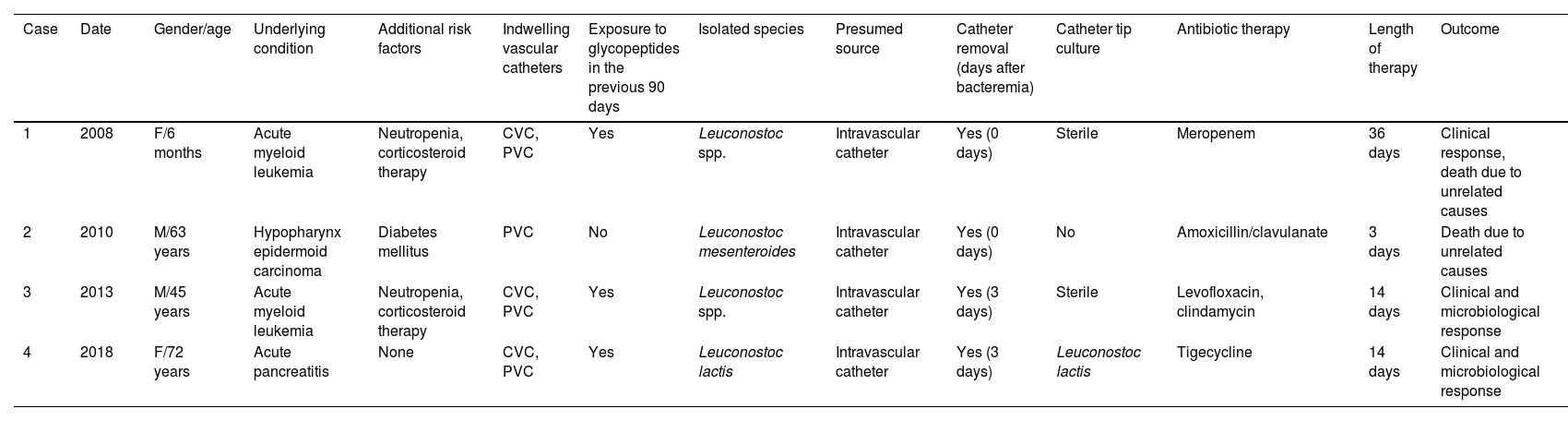
ResultsWe identified eight episodes of positive blood cultures for Leuconostoc spp. during the study period. Four were considered contaminants due the positivity of a single set of at least two drawn at the same time. Table 1 details the clinical and microbiological characteristics of the remaining four cases of true Leuconostoc spp. bacteremia. The clinical presentation fulfilled the definition of sepsis and included fever and elevation of acute phase reactants, although none of the episodes progressed to septic shock. Three of the patients exhibited at least one condition leading to immune compromise, including cancer (three cases), neutropenia (i.e. absolute neutrophil count<0.50×109cells/L) or corticosteroid therapy (two cases each). The diagnosis of acute myeloid leukemia was present in two patients. The four episodes were considered as likely related to the intravascular catheter due to the absence of an alternative origin of bacteremia and the rapid clearance following catheter withdrawal. Follow-up blood cultures were obtained and were sterile in all four cases. Polymicrobial infection was present in one case in which Staphylococcus capitis was concomitantly identified in blood cultures, a circumstance that would support the diagnosis of catheter-related bacteremia. Three of our patients had received vancomycin or teicoplanin within the 90 days preceding the onset of bacteremia. All isolates in our series showed susceptibility to penicillin, ceftriaxone and levofloxacin, whereas cotrimoxazole resistance was documented in one single case. All isolates were susceptible to beta-lactams, although the therapeutic regimens used were quite heterogeneous and included linezolid, levofloxacin, clindamycin and tigecycline. Catheter removal was performed in all four cases.
Clinical and microbiological characteristics, therapeutic regimens and outcome of four cases of Leuconostoc spp. bacteremia.
| Case | Date | Gender/age | Underlying condition | Additional risk factors | Indwelling vascular catheters | Exposure to glycopeptides in the previous 90 days | Isolated species | Presumed source | Catheter removal (days after bacteremia) | Catheter tip culture | Antibiotic therapy | Length of therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2008 | F/6 months | Acute myeloid leukemia | Neutropenia, corticosteroid therapy | CVC, PVC | Yes | Leuconostoc spp. | Intravascular catheter | Yes (0 days) | Sterile | Meropenem | 36 days | Clinical response, death due to unrelated causes |
| 2 | 2010 | M/63 years | Hypopharynx epidermoid carcinoma | Diabetes mellitus | PVC | No | Leuconostoc mesenteroides | Intravascular catheter | Yes (0 days) | No | Amoxicillin/clavulanate | 3 days | Death due to unrelated causes |
| 3 | 2013 | M/45 years | Acute myeloid leukemia | Neutropenia, corticosteroid therapy | CVC, PVC | Yes | Leuconostoc spp. | Intravascular catheter | Yes (3 days) | Sterile | Levofloxacin, clindamycin | 14 days | Clinical and microbiological response |
| 4 | 2018 | F/72 years | Acute pancreatitis | None | CVC, PVC | Yes | Leuconostoc lactis | Intravascular catheter | Yes (3 days) | Leuconostoc lactis | Tigecycline | 14 days | Clinical and microbiological response |
CVC: central venous catheter; F: female; M: male; PVC: peripheral venous catheter.
Previous studies have documented the increased susceptibility to Leuconostoc spp. bacteremia among patients with cancer, in which prolonged hospitalization periods and exposure to broad-spectrum antibiotics may play a role.2,8 Four out of nine cancer patients in a previous series reported also had a hematological malignancy, mainly acute lymphoblastic leukemia.3 In addition to the impairment of host immune response, the disruption of skin barrier function due to the presence of indwelling vascular catheters is another major contributor to Leuconostoc spp. infection.2,4 Parenteral nutrition was also identified as a risk factor in a case-control study performed in the setting of a hospital outbreak of L. mesenteroides subsp. mesenteroides bacteremia.2 The predominance of the vascular catheter as the presumed source of infection is concordant with the results reported by a recent Taiwanese cohort comprising 20 episodes, although none of the nine central venous catheter (CVC) tips cultured yielded Gram-positive cocci.4 Bacterial translocation from the gastrointestinal tract has been also proposed as a potential source among patients with malignancies.3 We should point out as a limitation the absence of a definite diagnosis of catheter-related bloodstream infection. CVC tip culture was negative in almost all cases of our series, except case #4. In addition, one single set of blood culture was obtained in each case, therefore differential time to positivity was not possible to perform. Antibiotic exposure in the preceding weeks has been identified as a frequent risk factor in previous studies, with glycopeptides and broad-spectrum beta-lactams being the agents most commonly reported.2–4
Misidentification of Leuconostoc spp. isolates is not uncommon since colonies may resemble viridans group streptococci or enterococci when cultured on sheep blood agar.9 The presence of alpha-hemolytic, catalase-negative Gram-positive cocci with high-level vancomycin resistance and negative for pyrrolidonyl aminopeptidase (PYR) and l-leucine aminopeptidase (LAP) production serve as criteria for genus-level differentiation.9 Initial misidentification with Weissella spp. was frequent due to its phenotypical and biochemical similarities, but currently definite identification can be achieved with MALDI-TOF.10,11
As exemplified by this and other series,4Leuconostoc spp. isolates were susceptible to beta-lactams. The optimal duration of therapy is not well established, although the criteria used for catheter-related bacteremia due to coagulase-negative staphylococci would be applicable. The prolonged course of meropenem therapy in case #1 may be explained by the lack of clinical stability and the uncertainty on the focus of infection. The successful use of tygecicline has been recently reported in a neutropenic patient.12 In line with most3,13 but not all previous series,4 outcomes were overall good, with no cases of persistent bacteremia or attributable mortality.
In conclusion, bacteremia due to Leuconostoc spp. is an uncommon but expected condition in patients with predisposing factors such as immune impairment, indwelling vascular catheters or previous exposure to vancomycin. Its relevance lies in the appropriate choice of therapy, given the difficulty to differentiate Leuconostoc spp. isolates from other Gram-positive cocci that are usually susceptible to glycopeptides. Once genus identification is confirmed, infection focus should be removed and antimicrobial treatment must be verified.
Funding sourcesR.R. hold a research training contract “Río Hortega” (CM19/00229) and M.F.R. a research contract “Miguel Servet” (CP 18/00073), both from the Spanish Ministry of Science, Instituto de Salud Carlos III.
Conflict of interestAll authors: The authors declare that they have no conflict of interest.