We report the case of a 68-year-old male with a medical history of diabetes mellitus type 2, arterial hypertension, severe obstructive sleep apnea and ischemic cerebrovascular disease who was admitted to our hospital for elective cardiac surgery, due to multivessel coronary artery disease after a non-ST-elevation myocardial infarction.
During the first day of hospitalization the patient had two episodes of fever above 38°C with shivering and petechial lesions in lower limbs. He denied headache, seizure, caught, chest or abdominal pain. The patient could not recall any contact with animals recently. Only C reactive protein (85.2mg/L), fibrinogen (544mg/dL) and procalcitonin (0.4ng/mL) were slightly elevated.
Catheter-related phlebitis, in the right hand, was observed. To rule out catheter-related bacteremia, four samples of 10mL of blood obtained from the peripheral venous catheter and venopunction were placed into 2 pair of blood culture bottles (BD Bactec® Plus Aerobic/F and Plus Anaerobic/F) and were processed in the BD Bactec® Fx System (BD Diagnostics). Additionally, the peripheral catheter was removed.
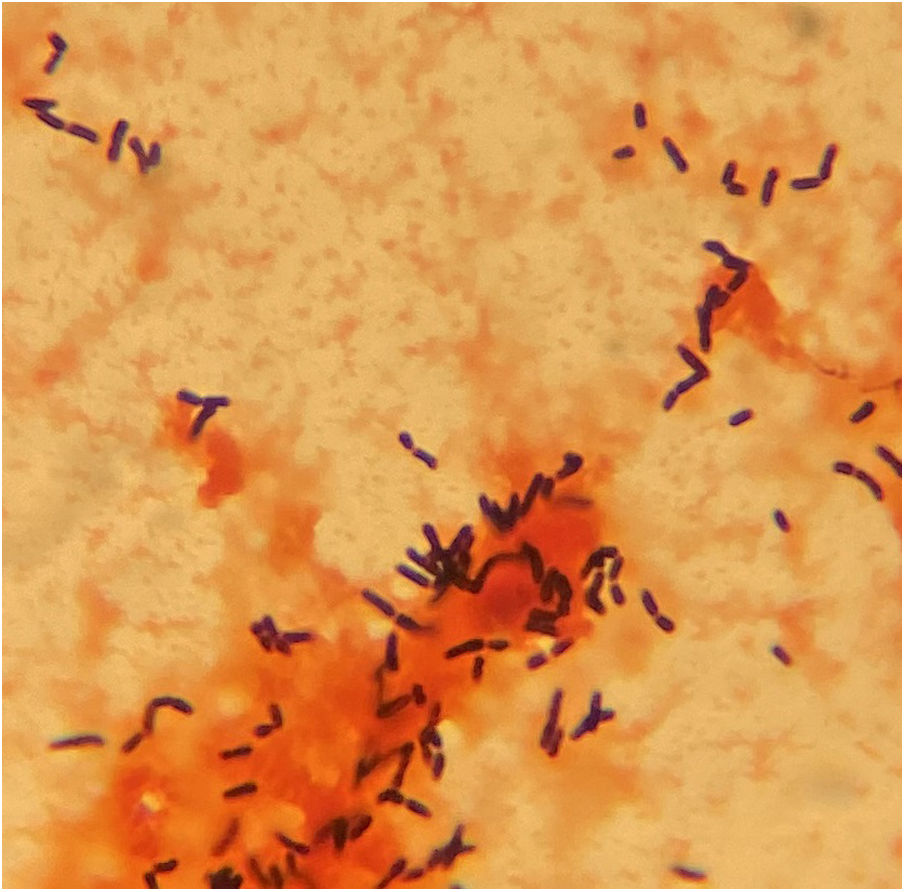
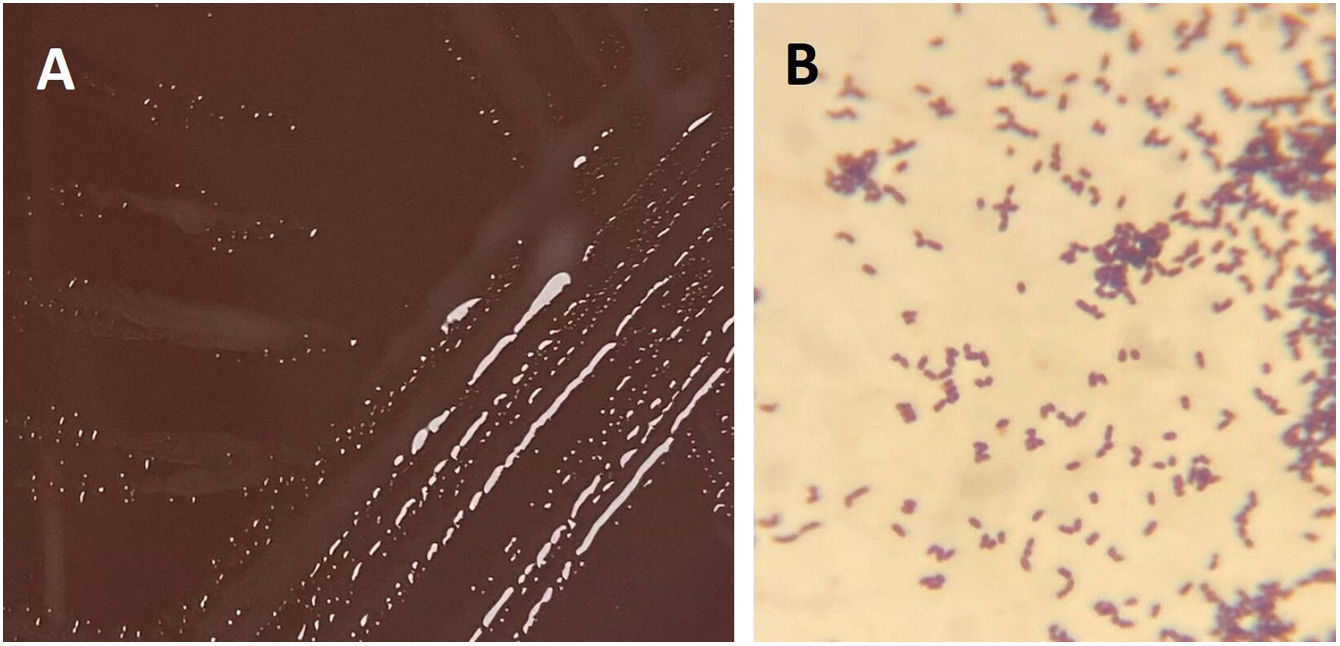
After 30 and 35h of incubation, the two aerobic bottles from the catheter and venopunction flagged positive, respectively. Gram stain revealed Gram-positive bacilli (Fig. 1) that changed to Gram-positive coccobacilli and cocci after 24 and 72h of incubation in solid media (5–7% CO2 atmosphere at 37°C in BD BBL™ Chocolate II Agar). In this agar, round, small (<2mm), smooth, semitransparent, mucoid, coalescing, and shiny colonies were observed, which became larger and salmon-pink in color with age but not before 4 days of incubation.
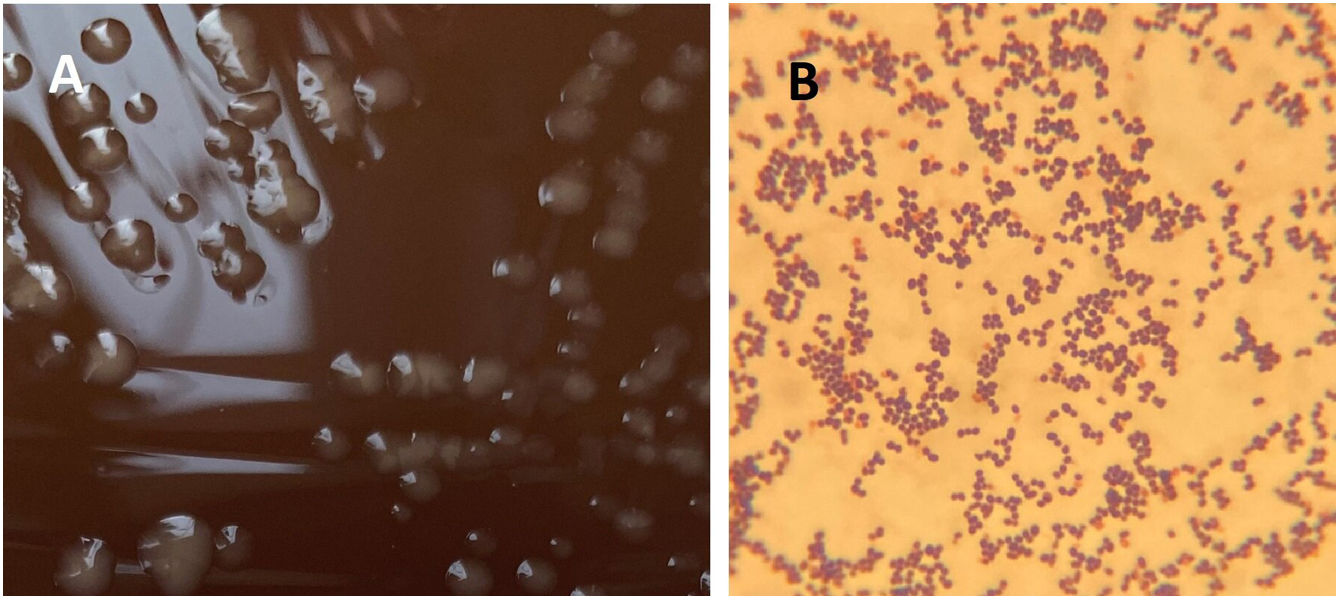
Diagnosis and evolutionIdentification using MALDI-TOF (Bruker® Daltonics GmbH) directly from the blood culture (triton-formic acid-ethanol extraction) yielded Rhodococcus hoagii as the first proposed identification with a low score of 1.30, and repetition from colonies after 24h of incubation did not produce any result. Based on Gram stain morphology, bacilli in liquid media, coccobacilli and cocci after 24 and 72h of incubation in solid media, respectively (Figs. 2B and 3B), and colonies appearance (Figs. 2A and 3A), a presumptive diagnosis of Rhodococcus spp. was provided.
A third set of blood cultures, obtained 6 days after removal of the catheter, achieved the growth of the same microorganism after 5 days of incubation. In this case, a definitive diagnosis was possible with a higher score (1.82) using MALDI-TOF from colonies. During hospital admission serial transthoracic echocardiographies were negative for endocarditis. Surgery was performed one day before this third set flagged positive.
Further species identification was confirmed by the partial sequencing of the 16S ribosomal RNA gene. The purified amplicons were sequenced and the result obtained was compared using BLAST, demonstrating 99% similarity to R. hoagii. The antimicrobial susceptibility testing was determined by MIC test strips (Liofilchem, Inc., Italy) and the results were interpreted according to PK-PD breakpoints defined by EUCAST (www.eucast.org) as follows: imipenem (MIC 2mg/L, susceptible), levofloxacin (MIC 0.75mg/L, susceptible increased exposure), and linezolid (MIC 3mg/L, resistant). After the identification, antibiotic therapy with ciprofloxacin 500mg every 12h was added for 7 days before surgery and 14 days after surgery.
Rhodococcus spp. is a pleomorphic Gram-positive coccobacillus, varying the percentage of presentation of the coccoid or bacillary form according to the conditions of growth and the phase in which it is. This zoonosis-producing microorganism causes several diseases like granulomatous pneumonia and lung abscess in foals less than six months old. It sporadically infects other mammals including cats, dogs and pigs, in which produces submandibular adenitis, and is being increasingly reported as a cause of infection in immunocompromised humans. Because diphtheroids are regarded as part of normal respiratory microbiota, the importance of R. hoagii as a pulmonary pathogen may not be fully appreciated and its prevalence may be underestimated.1
In humans, R. hoagii is the main representative species related to pathological processes. It is an intracellular pathogen that infects macrophages and polymorphonuclear cells, especially in patients with alterations of the cellular immunity, immunosuppressive treatment, hematological neoplasm and, in general, any type of immunodeficiency2 causing respiratory infection, skin infection, abscesses, peritonitis, and bacteremia. Infection is possible, although rare, in immunocompetent persons, with different clinical presentations like respiratory disease and cerebellar abscess. In a systematic review including 531 infections caused by R. hoagii,1 no bacteremia related to peripheral venous catheter infections was described. Despite this, catheter-related bacteremia by R. hoagii, although rare, should be considered.3 In terms of treatment, it is important to emphasize that the condition of intracellular pathogen gives R. hoagii some special characteristics such as the need for prolonged treatments with combined antibiotic regimens, especially in immunocompromised patients.1
It is important to recognize the characteristic appearance of these microorganism colonies and its variable morphology in the Gram stain in order to make an adequate early diagnosis, since it may not be adequately identified by MALDI-TOF because the cell wall of R. hoagii stops the complete uptake of energy when fired upon by the laser.4 A Ziehl-Neelsen stain (preferably staining modification that decolorizes slides with 0.5–1% sulfuric acid solution) and biochemical tests as positive catalase and negative oxidase could be helpful in the presence of a low MALDI-TOF score. Its identity can be confirmed by species-specific and cholesterol oxidase (choE) PCR using genomic DNA as template.5
Conflict of interestThe authors declare that they have no conflict of interest.