Although antiretroviral therapy (ART) for HIV/AIDS was introduced in 1987, improvement in disease progression and reduction in mortality at a population level was not observed until 1996, with the combination of three or more drugs. The objective was to estimate the clinical and economic benefit of ART in Spain in the 32-year period between 1987 and 2018.
MethodsA cost-benefit analysis was performed, using a second-order Monte Carlo simulation, from the societal (base case) and the National Health System (NHS) perspectives. New cases of HIV, AIDS and related deaths were obtained from the SINIVIH and UNAIDS registries, with population projections without ART using triple exponential smoothing. Expenditure on ART was obtained from the National AIDS Plan reports and market studies.
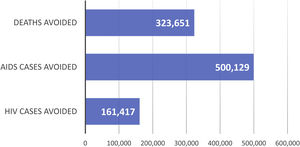
ResultsThe NHS invested 6185 million euros in 32 years. In that period, 323,651 AIDS-related deaths, 500,129 AIDS cases and 161,417 HIV cases were averted, with total savings of 41,997 million euros. The net benefit (net savings) is estimated at 35,812 million euros (societal) and 1032 million euros (NHS). For every euro invested in ART, a return on investment of € 6.79 and € 1.16 was obtained, respectively.
ConclusionsThe use of ART over 32 years prevented a large number of deaths and cases of AIDS and HIV, providing significant economic savings for the NHS. ART is an efficient intervention for the NHS.
Aunque el el tratamiento antirretroviral (TAR) del VIH/sida se introdujo en 1987, la mejora en la progresión de la enfermedad y reducción de la mortalidad poblacional no se observó sino hasta 1996, con la combinación de tres o más fármacos. El objetivo fue estimar el beneficio clínico y económico del TAR en España en el periodo de 32 años, comprendido entre 1987 y 2018.
MétodosSe realizó un análisis de coste-beneficio mediante la simulación de Monte Carlo de segundo orden, desde las perspectivas de la sociedad (caso base) y el Sistema Nacional de Salud (SNS). Los nuevos casos de VIH, sida y muertes relacionadas se obtuvieron de los registros SINIVIH y ONUSIDA, con proyecciones poblacionales sin TAR mediante suavizamiento exponencial triple. El gasto en TAR se obtuvo de informes del Plan Nacional del SIDA y estudios de mercado.
ResultadosEl SNS invirtió 6.185 millones de euros en 32 años. Durante este periodo se evitaron 323.651 muertes por sida, 500.129 casos de sida y 161.417 casos de VIH, con un ahorro total de 41.997 millones de euros. El beneficio neto (ahorros netos) se estima en 35.812 millones de euros (sociedad) y 1.032 millones de euros (SNS). Por cada euro invertido en TAR, se obtuvo un retorno de la inversión de 6,79 € y 1,16 €, respectivamente.
ConclusionesLa utilización de TAR durante 32 años ha evitado gran número de muertes y casos de sida y VIH, generando significativos ahorros económicos para el SNS. El TAR es una intervención eficiente para el SNS.
The introduction of zidovudine (AZT) in 1987, the first antiretroviral treatment (ART), provided modest control of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS)1. HIV, however, developed rapid resistance to this drug2. In fact, in Spain a significant impact of ART on disease progression and mortality reduction was not observed until 1996 with the combination of three or more drugs3,4. Since then, ART has led to a steady reduction in AIDS cases4 and related mortality in Spain3. It is estimated that 151,400 people with HIV are currently living in Spain, 87% of whom (131,775) are aware of their infection; that 97.3% of them are on treatment (128,216); and finally that 90.4% of these (115,900) achieved HIV viral suppression5,6, with a consequent reduction in the transmission of the disease.
According to a recent cost-benefit study7, globally ART averted 9.5 million deaths in the 1995–2015 period. The global net cost of ART in that period is estimated at US$ 301 billion, while the benefits of ART (as a result of HIV and AIDS cases and AIDS deaths averted) amounts to US$ 1053 billion. As a result, the net benefit of the health impact of ART amounts to US$ 752 billion7. In Spain, however, the cost-benefit of ART has not been assessed, according to a systematic review carried out by us. This study aims to estimate ART’s clinical and economic impact in Spain over a 32-year period (1987–2018).
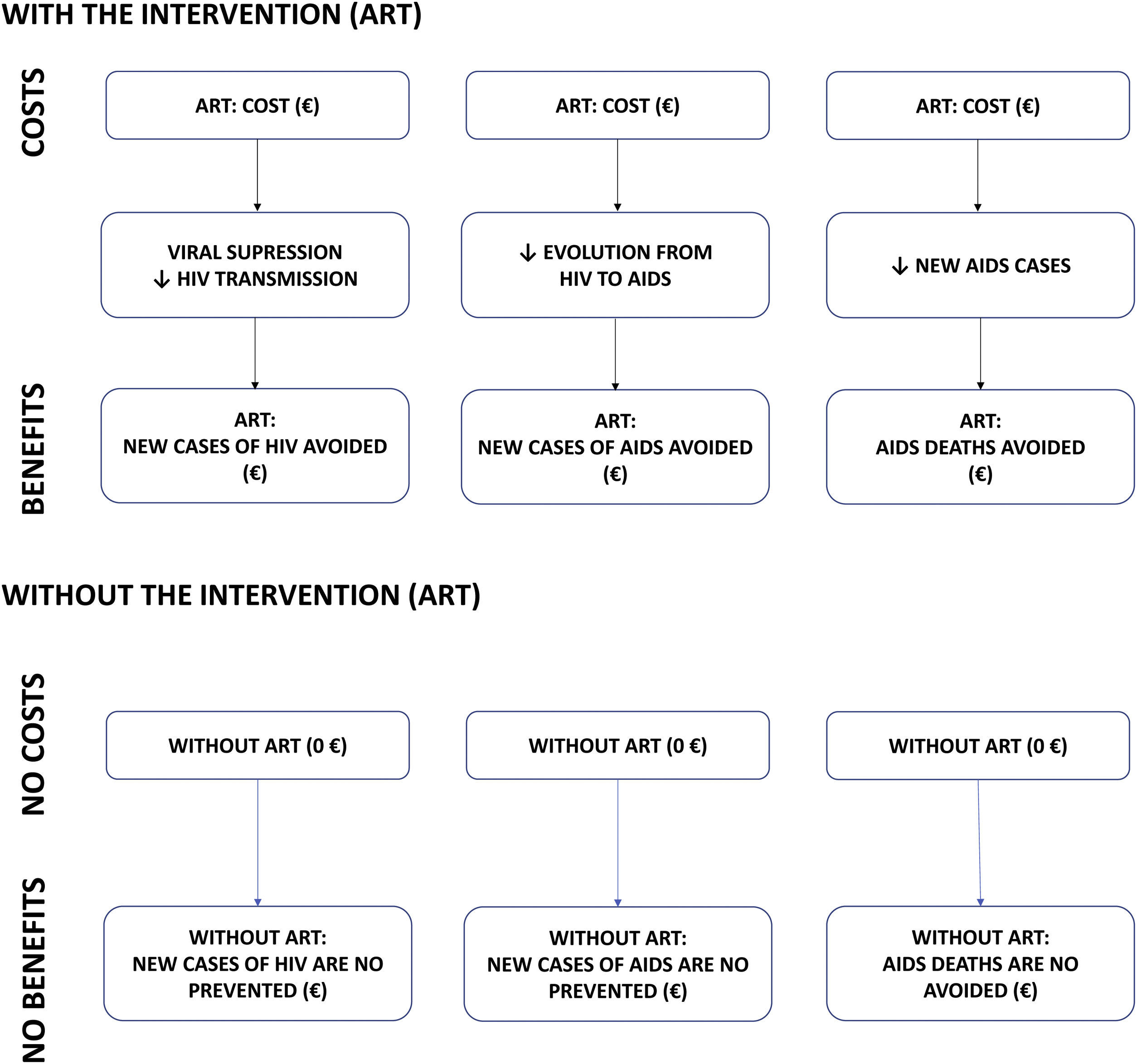
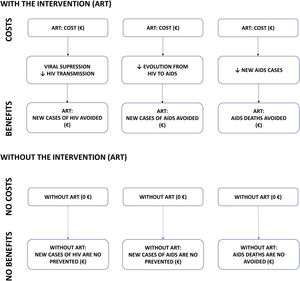
MethodsCost-benefit modelA cost-benefit analysis was conducted. Costs and health outcomes (benefits) are measured in monetary units in this type of analysis. In this study, the costs (2020 euros [€]) are the costs of purchasing ART and the benefits are the averted costs (i.e. savings) from the clinical outcomes of ART compared to a hypothetical scenario in which ART would not have been available: (i) new HIV infections averted; (ii) new AIDS cases averted; and (iii) AIDS deaths averted (Fig. 1). These clinical outcomes were assumed based on the following assumptions: (i) viral suppression by ART eliminates the transmissibility of HIV and, therefore, the appearance of new HIV cases8,9; (ii) avoiding the progression of HIV patients to AIDS would reduce new AIDS cases10,11; and (iii) reducing new AIDS cases would reduce AIDS-associated deaths7.
The cost-benefit analysis was modelled by a probabilistic analysis, using second-order Monte Carlo simulations, with 1000 simulations. This methodology made it possible to analyse the uncertainty of the model variables12–14, mainly the following: (i) the annual cost of ART in Spain; (ii) life expectancy in HIV patients, according to the period analysed; (iii) AIDS deaths averted; (iv) AIDS cases averted; and (v) new HIV cases averted. Continuous variables (costs, number of cases) were adjusted to gamma distributions12,13, based on the minimum and maximum values available or, alternatively, a variability of ±20% of the variable’s mean or available value.
General characteristics of the modelThe analysis was conducted according to a defined base case, agreed upon and validated by a panel of 4 experts in HIV management, including the average or most plausible (clinically or epidemiologically speaking) values of the variables. The base case defined established the following assumptions: annual discounted costs and benefits of 3%, societal perspective (including both direct health costs of HIV and AIDS cases and indirect costs – mainly employment – associated with the premature death of AIDS patients), the economic value of deaths averted based on the gross domestic product (GDP) per capita7,15–17 and considering additional life expectancy from the study by Gueler et al.18 Several sensitivity analyses were also performed, modifying some of the variables in the model to assess the robustness of the base case. In particular, the following variables were analysed: (i) annual discounting of costs and benefits: 0% and 6%; (ii) economic value of deaths averted: using the monetary value of statistical life (the monetary value of a statistical life is called the value, measured in monetary units, that society as a whole attributes to preventing any one of its members from dying; it can be calculated using the declared preferences method)19; and (iii) sensitivity analysis from the National Health System (NHS) perspective, considering only direct healthcare costs and without annual discounting, because health decision-makers are interested in the real budgetary impact without applying discounts20 of both ART costs and benefits, on the understanding that the NHS decision-maker (budget holder) is particularly interested in the actual costs to the healthcare system at any given time, not discounted21. Finally, two sub-analyses were carried out: (i) the estimation of the cost-benefit for the NHS attributable to the ART drugs marketed by Gilead Sciences in the period between 2002 and 2018, estimating the percentage of the total population based on patients treated annually according to sales data; and (ii) the analysis of three periods (years 1987–1996; 1997–2007 and 2008–2018) to analyse the evolution of the cost-benefit of ART over the 32-year time period.
Assumptions and sources of the economic modelThe main assumptions and sources of the economic model are summarised in Table S1 (Supplementary appendix). It was assumed that in a hypothetical scenario in which ART would not have been available, there would have been more cases of HIV infection, more AIDS cases and more AIDS deaths. The projection of new HIV cases, new AIDS cases and AIDS deaths in Spain in a scenario without ART was estimated from the cases described between 1981 and 1995, published by the United Nations Programme on HIV and AIDS (UNAIDS) and the Spectrum programme22,23 (Table 1). The projections were estimated using the triple exponential smoothing method24. New HIV diagnoses registered in Spain in the period 1987–2012 were also obtained from UNAIDS/Spectrum22,23 and those registered in the period 2013–2018 from the Information System on New HIV Diagnoses (SINIVIH) in Spain25 (Table 1).
AIDS deaths, AIDS cases and HIV cases projected (expected) without ART (mean values) and observed with ART.
| Years | AIDS deaths | AIDS cases | HIV cases | |||
|---|---|---|---|---|---|---|
| Expected* | Observed | Expected* | Observed | Expected* | Observed | |
| 1987 | 433 | 433 | 1095 | 1095 | 5726 | 3615 |
| 1988 | 800 | 800 | 2279 | 2279 | 6717 | 4184 |
| 1989 | 1378 | 1378 | 3173 | 3173 | 7716 | 4720 |
| 1990 | 2033 | 2033 | 3937 | 3937 | 8560 | 5149 |
| 1991 | 2657 | 2657 | 4579 | 4579 | 9269 | 5408 |
| 1992 | 3477 | 3477 | 5103 | 5103 | 9630 | 5460 |
| 1993 | 4227 | 4227 | 5527 | 5527 | 9745 | 5324 |
| 1994 | 5058 | 5058 | 7511 | 7511 | 9642 | 5056 |
| 1995 | 5857 | 5857 | 8760 | 7205 | 9465 | 4722 |
| 1996 | 6656 | 5749 | 10,013 | 6773 | 9257 | 4374 |
| 1997 | 7455 | 3019 | 11,265 | 4983 | 9087 | 4037 |
| 1998 | 8254 | 1878 | 12,517 | 3758 | 9007 | 3714 |
| 1999 | 9053 | 1844 | 13,769 | 3173 | 9017 | 3399 |
| 2000 | 9852 | 1717 | 15,022 | 2941 | 7667 | 2595 |
| 2001 | 10,651 | 1635 | 16,274 | 2536 | 7336 | 2171 |
| 2002 | 11,450 | 1614 | 17,526 | 2387 | 7569 | 1925 |
| 2003 | 12,249 | 1635 | 18,778 | 2334 | 7954 | 2018 |
| 2004 | 13,048 | 1554 | 20,031 | 2107 | 8427 | 2140 |
| 2005 | 13,847 | 1450 | 21,283 | 1889 | 8870 | 2257 |
| 2006 | 14,646 | 1315 | 22,535 | 1771 | 9283 | 2363 |
| 2007 | 15,445 | 1313 | 23,788 | 1660 | 9836 | 2456 |
| 2008 | 16,244 | 1215 | 25,040 | 1577 | 10,141 | 2528 |
| 2009 | 17,043 | 1079 | 26,292 | 1437 | 7655 | 74 |
| 2010 | 17,842 | 1020 | 27,544 | 1458 | 6334 | 539 |
| 2011 | 18,641 | 953 | 28,797 | 1293 | 6712 | 499 |
| 2012 | 19,440 | 880 | 30,049 | 1175 | 6375 | 273 |
| 2013 | 20,239 | 750 | 31,301 | 858 | 4914 | 139 |
| 2014 | 21,038 | 700 | 32,553 | 688 | 4970 | 81 |
| 2015 | 21,837 | 633 | 33,806 | 611 | 4704 | 0 |
| 2016 | 22,636 | 498 | 35,058 | 549 | 4702 | 887 |
| 2017 | 23,435 | 445 | 36,310 | 514 | 4414 | 807 |
| 2018 | 24,234 | 397 | 37,562 | 415 | 4408 | 780 |
| Total | 381,444 | 59,502 | 589,078 | 87,296 | 245,111 | 83,694 |
AIDS: Acquired Immune Deficiency Syndrome; ART: antiretroviral therapy; HIV: Human Immunodeficiency Virus.
*Triple Exponential Smoothing formulas: General equation:
St=αytIt−L+1−αSt−1+bt−1
Trend smoothing:
bt=ySt−St−1+1−γbt−1
Seasonal smoothing:
It=βytSt+1−βIt−L
Forecast:
Ft+m=St+mbtIt−L+m
where, α, β, γ are constants that takes the value from the range [0;1]; y is the observation value; S is the smoothed observation value; b is the trend rate; I is the seasonality index; F is the forecast for m periods ahead; t is an index denoting a time period.
The estimate of HIV viral suppression in Spain (90.4% [87.5%–92.8%]) was obtained from reports by the HIV and Risk Behavior Surveillance Unit of the National Epidemiology Centre3,5. Annual transmission rates (men who have sex with men [16.6%; 12.2%–22.3%], injection drug use [7.6%; 4.9%–11.0%], heterosexual [1.0%; 0.7%–1.3%], mother-to-child [22.6%; 17.0%–29.0%], blood products or transfusions [92.5%; 80.9%–96.1%]) were estimated from data in the systematic review by Patel et al.26
The annual cost of ART in Spain in the period 1987–1996 were calculated using the number of patients with HIV/AIDS collected by UNAIDS/Spectrum23 and the annual cost per patient of ART marketed each year27. The annual cost of ART in Spain (also considering the cost of generic drugs) in the periods 1997–2012 and 2013–2016 were obtained from reports of the National AIDS Plan4,28. The annual cost per ART in 2017–2018 were obtained from annual sales data in Spain29.
The annual cost per HIV-positive patient (1987–2018), assuming they had not been treated with ART, was estimated from the cost in 1995, according to the study by Antoñanzas et al.30 adjusted for the year-on-year CPI and considering a population of 29,062 HIV-prevalent patients in 1995, according to UNAIDS/Spectrum23 data. The annual cost of an AIDS patient without ART in the same period (1987–2018) was estimated using the same study, adjusted for the CPI23.
In the base case of the analysis, the monetary value of a life was estimated as the annual GDP per capita over the period 1987–2018, as in a previously published study7,16,17.
A conservative assumption was made to estimate the benefit of the ART marketed by Gilead Sciences, as 100% of the benefit was captured in the case of full treatment and 50% of the benefit in the case of treatment in combination with other non-Gilead drugs.
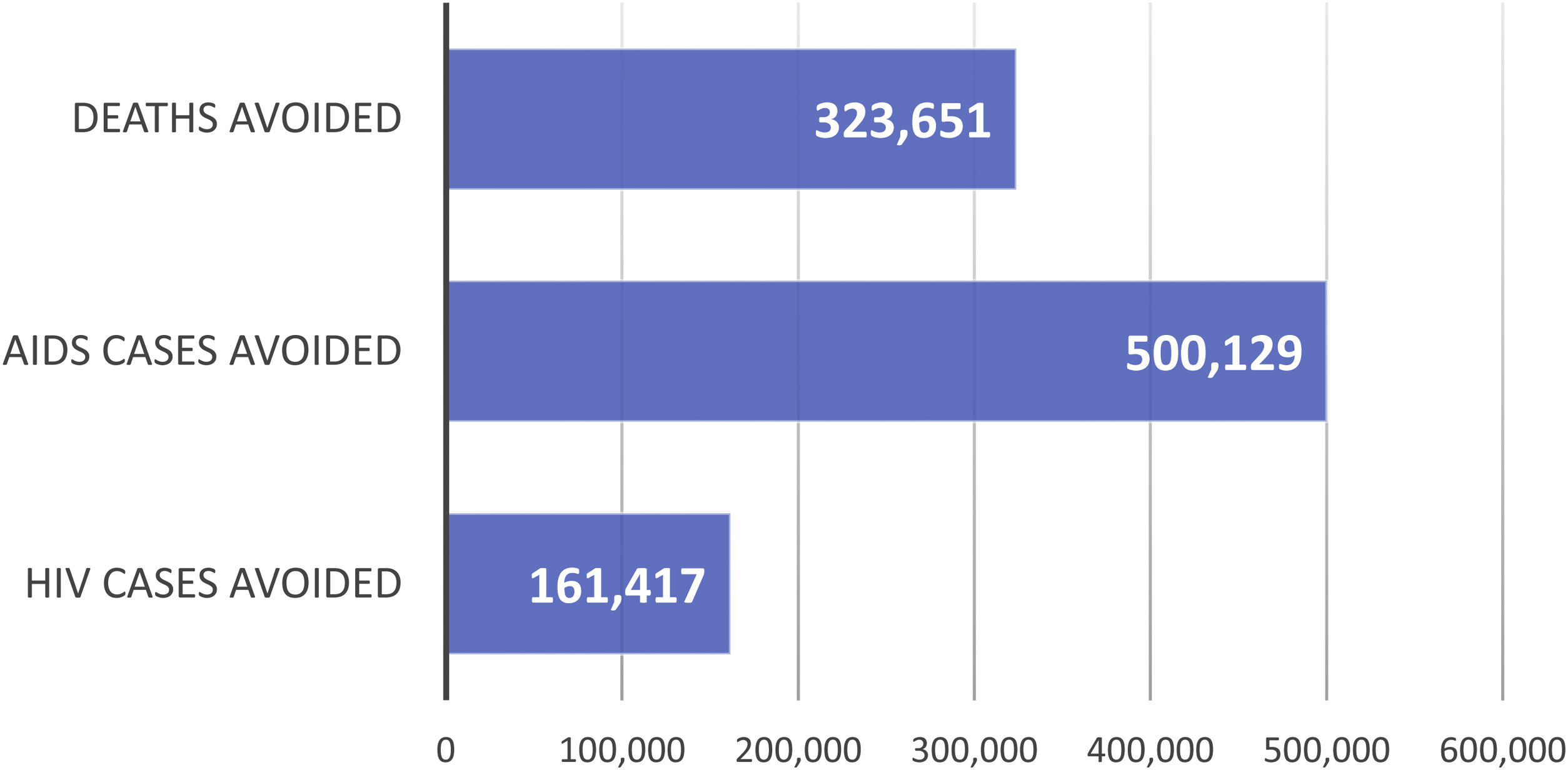
ResultsBase caseClinical benefitIt is estimated that, over the 32-year period analysed (1987–2018), ART averted 323,651 AIDS deaths (Fig. S1-Supplementary appendix; Fig. 2), 500,129 AIDS cases (Fig. S2-Supplementary appendix; Fig. 2) and 161,417 HIV cases (Fig. S3-Supplementary appendix; Fig. 2).
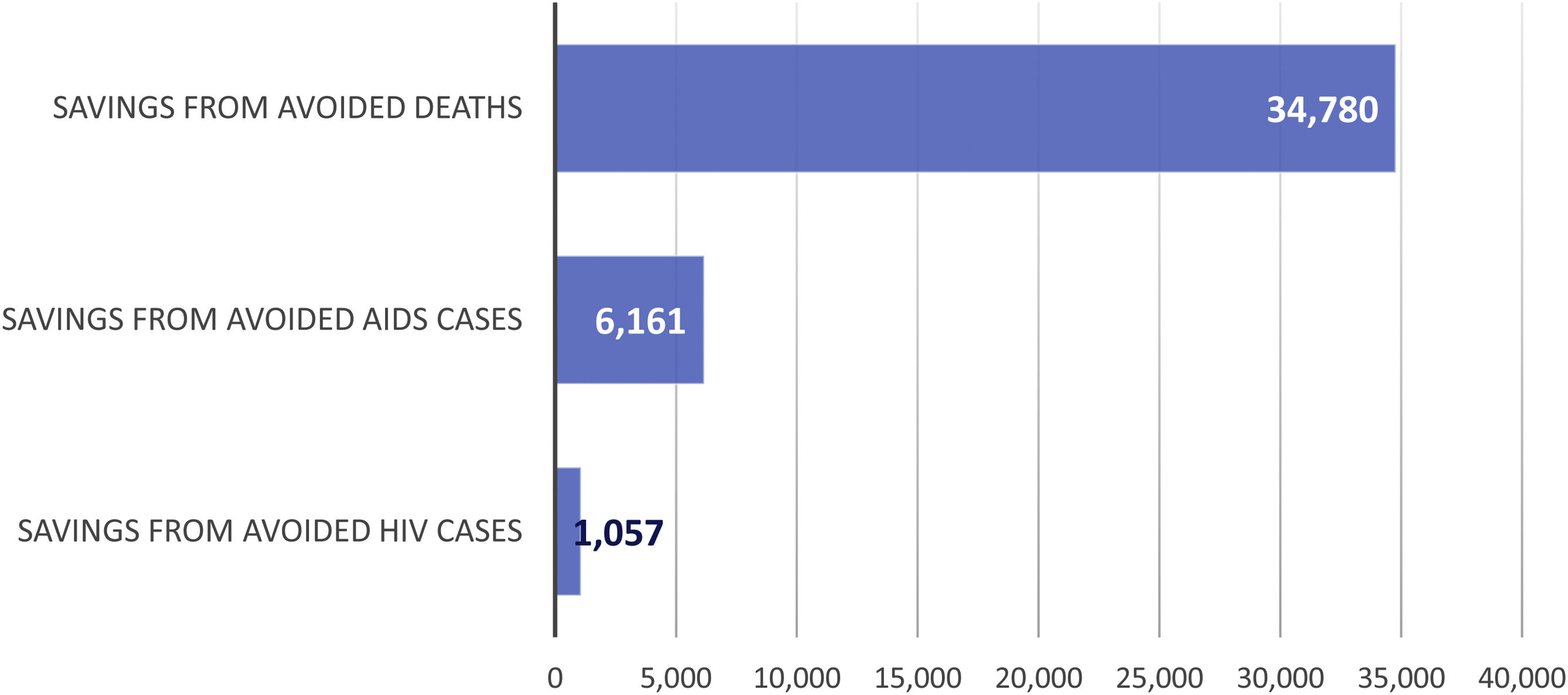
Economic impactThe economic impact of ART intervention is shown in Fig. 3 and Table 2. The cost of ART over the 32-year period amounts to 6185 (95%CI 5545; 6821) million euros. The benefit (savings) from averted deaths amounts to 34,780 (95%CI 23,346; 49,034) million euros; from averted AIDS cases to 6161 (95%CI 5511; 6844) million euros; and from averted HIV cases to 1057 (95%CI 1041; 1073) million euros. The total benefits (savings) associated with ART, for the given period, amounts to 35,812 (95%CI 24,353; 50,131) million euros (Table 2).
Results of the benefit/cost analysis of ART in Spain over a 32-year period (1987–2018) (million euros [€]). Base case. Societal perspective (3% annual discount).
ART over the 32-year period had a benefit/cost ratio of 6.79 (95%CI 5.39, 8.35). Thus, ART was benefit/cost (with a ratio of greater than 1) in Spain over the analysis period (Table 2).
Sensitivity analysisAs shown in Table 3 and Table S2-Supplementary appendix, ART was equally profitable when the analysis was conducted from NHS's perspective without annual discounting of costs and benefits or with discounts of 6%. Specifically, from the NHS’s perspective and without considering discounts, the cost of ART amounted to 11,809 million euros over the 32 years, and the benefits would amount to 14,015 million euros, resulting in a net benefit of 2206 million euros (Table 3). When the statistical lifetime’s monetary value was considered, the net benefit of the ART was €1,961,943 million over the 32 years (Table S2-Supplementary appendix).
Results of the benefit/cost analysis of ART in Spain over a 32-year period (1987–2018) (million euros [€]). NHS perspective (0% annual discount).
| Mean | 95% CI | |
|---|---|---|
| ART costs | 11,809 | 10,749; 13,067 |
| ART benefits | ||
| For deaths avoided | 12,243 | 10,922; 13,603 |
| For AIDS cases avoided | 1772 | 1746; 1796 |
| For HIV cases avoided | 14,015 | 12,668; 15,399 |
| ART Net benefit | 2206 | 1919; 2332 |
| Benefit/Cost indexa | 1.19 | 1.18; 1.18 |
The results of the two sub-analyses are in Sub-analysis-Supplementary appendix.
DiscussionAccording to the present study, ART to HIV-positive patients in Spain were highly profitable, both in deaths and AIDS and HIV cases averted, and in economic terms. According to the results obtained in our study, ART produced a benefit/cost ratio of 6.79, which is equivalent to that for every euro invested in ART, a societal return on investment of €6.79 was obtained. This societal return on investment is more favourable than that observed with other interventions analysed in our healthcare setting, namely €1.83 for multiple sclerosis31, €3.25 for heart failure32 and €5.04 for psoriasis33.
Among the strengths of the present study, the following should be highlighted: (i) actual epidemiological data with ART (deaths, new cases) were obtained from official sources and registries (UNAIDS, Spectrum, SINIVIH)22,23,25; (ii) all model assumptions were validated by Spanish experts in epidemiology and management of HIV/AIDS patients; and (iii) all study variables were analysed using a probabilistic model with a proven methodology, which allowed us to analyse the uncertainty of the model variables and to obtain the mean results of the net benefit and the benefit/cost ratio with their 95% confidence intervals14,34. This present benefit/cost analysis was performed following the guidelines and recommendations published in Spain35,36.
As in the global study by Forsythe et al.7, our nationwide study confirms that in the historical series ART has been a profitable health intervention, with the benefits (both health and economic) far outweighing the costs of acquiring ART. This cost-effectiveness has been greater since the use of highly active ART (HAART).
Although amply offset, according to this analysis, ART spending in Spain must be put into context. Our study estimate amounts to 6185 million euros applying the discount recommended by the economic evaluation guidelines and 11,809 million euros undiscounted over 32 years. By way of example, in 2019, it was 677 million euros. Spain’s NHS expenditure on pharmaceuticals and medical devices in 2019 was 23,638 million euros37. Therefore, annual expenditure attributable to ART constituted 2.86% of total NHS expenditure on pharmaceuticals and medical devices.
However, the most crucial aspect of ART is its impact on health outcomes in reducing HIV/AIDS incidence/mortality and maximizing patient health38. According to this study, in the period between 1987 and 2018, ART averted 323,651 AIDS deaths, 500,129 new AIDS cases and 161,417 new HIV cases.
Regarding the possible limitations of the study, first it should be borne in mind that a theoretical model has been used, which is, by definition, a simplified simulation of reality. Second, it was necessary (as is usual in this type of analysis) to make some assumptions in the model due to lack of data. The primary assumption was the projection of mortality and new HIV or AIDS cases under the hypothetical assumption that ART would not have been available in the period 1987–2018. These projections of expected deaths and cases were made using a proven method such as triple exponential smoothing24,39. This projection method is conservative compared to the linear least squares regression method, which would have yielded more cost-effective results for ART. The projection of deaths from AIDS and AIDS cases expected since 1996, was calculated from the trend observed between the years 1987–1995. Therefore, to carry out the projection, all the factors and prevention measures that determined the cases observed in the period 1987–1995 were taken into account. However, a limitation of the model could be the fact that the projections without ART did not explicitly consider the possible effect of the reinforcement of preventive measures that would have occurred in the event that ART had not been established in the period analyzed. However, an attempt has been made to compensate for this possible limitation by using a conservative calculation approach, based on triple exponential smoothing. On the other hand, the predictions made were contrasted with the behavior of HIV in countries with limited access to ART, as well as using the UNAIDS Spectrum predictive model.
ART has radically changed the impact of HIV infection from a lethal disease to a manageable chronic disease40. The UNAIDS target for 2030 is to have 95% of people living with HIV knowing their HIV status, 95% of people diagnosed with HIV receiving ongoing ART and 95% of these achieving viral suppression6,41. UNAIDS set this target because HIV treatment is critical to ending the AIDS epidemic and making HIV transmission a rare event, and that treatment prevents AIDS-related deaths, prevents new infections and saves money41. These assumptions have been analysed and confirmed in this study.
ConclusionsFor more than 32 years in Spain, investment in ART has limited the number of AIDS deaths and new cases of HIV and AIDS while providing (through maximised return on investment) significant economic savings for the NHS. ART’s cost-effectiveness has progressively increased over the 32-year period analysed, mainly due to ART’s increased efficiency and effectiveness.
Authors’ contributionsAll authors contributed to the design of the study, the interpretation of the data, the critical review of the publication and approved the final version of the manuscript for publication. Carlos Rubio Terrés and Darío Rubio Rodríguez made the economic model.
FundingThe study was funded, without any type of restriction, by Gilead Sciences, SL. Gilead Sciences, SL, had no role in the analysis of the data or in the preparation of the manuscript.
Conflicts of interestMJPE has done consulting work, received research grants, received session funding, and developed educational materials, now or in the last three years, for AbbVie, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. DPP has received research funding and/or consulting and/or conference fees from Viiv, Gilead, Janssen, and MSD. PV has received consulting and/or conference fees from Abbvie, ViiV Healthcare, Gilead Sciences, Janssen, and MSD. IJ has received training fees from ViiV Healthcare and consulting fees from Gilead. AC is an employee of Gilead Sciences, SL. DRR and CRT are consultants for Health Value, a company that received payments in connection with the study.
This study would not have been possible without the documentary support provided by SIDA STUDI (Víctor León, Head of the Center for Documentation and Pedagogical Resources).
Please cite this article as: Pérez-Elías MJ, Podzamczer Palter D, Ventayol Bosch P, Jarrín I, Castro A, Rubio-Rodríguez D, et al. Beneficio clínico y económico de 32 años de tratamiento antirretroviral de personas que viven con VIH en España: ¿ha sido una intervención eficiente? Enferm Infecc Microbiol Clin. 2022;40:550–556.






![Economic impact of ART (1987–2018) in Spain (million euros [€]). Societal perspective. AIDS: Acquired Immune Deficiency Syndrome; ART: antiretroviral therapy; HIV: Human Immunodeficiency Virus. Economic impact of ART (1987–2018) in Spain (million euros [€]). Societal perspective. AIDS: Acquired Immune Deficiency Syndrome; ART: antiretroviral therapy; HIV: Human Immunodeficiency Virus.](https://static.elsevier.es/multimedia/2529993X/0000004000000010/v2_202212170724/S2529993X21001313/v2_202212170724/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
