Ceftolozane/tazobactam has shown excellent activity against Pseudomonas aeruginosa, but this drug is not always included in commercial panels. The aim of the study was to evaluate the performance of 2 gradient strips (BioMérieux and Liofilchem) and a commercial microdilution panel (Sensititre, EURGNCOL panel) using this combination against carbapenem-resistant P. aeruginosa isolates.
MethodsThree commercial methods were tested with 41 metallo-beta-lactamase-producing and 59 non-carbapenemase-producing P. aeruginosa isolates. Broth microdilution was used as reference.
ResultsAll carbapenemase-producing isolates and only one non-producing isolate were resistant to this antibiotic. Both essential agreement and bias were outside the acceptance intervals since MIC values were higher than reference values for all three methods. The Kappa index indicated poor or weak agreement. Changes in clinical categories were observed in 3 isolates.
ConclusionsThe three methods yielded poor agreement with the reference. Despite the differences in MIC values, fewer than 3% involved category changes.
La combinación ceftolozano/tazobactam ha mostrado una actividad excelente frente a Pseudomonas aeruginosa, pero este fármaco no siempre se incluye en los paneles comerciales. El objetivo de este estudio es evaluar el rendimiento de 2 tiras de gradiente (BioMérieux® y Liofilchem®) y un panel de microdilución comercial (Sensititre®, panel EURGNCOL) utilizando esta combinación frente a aislados de P. aeruginosa resistente a los carbapenémicos.
MétodosSe probaron 3 métodos comerciales con 41 aislados productores de metalobetalactamasas y 59 aislados no productores de carbapenemasas de P. aeruginosa. La microdilución de caldo se utilizó como referencia.
ResultadosTodos los aislados productores de carbapenemasas y solo un aislado no productor fueron resistentes a este antibiótico. Tanto la concordancia esencial como el sesgo se encontraron fuera de los intervalos de aceptación, dado que los valores CMI eran superiores que los valores de referencia para los 3 métodos. El índice de Kappa indicó una concordancia pobre o débil. Se observaron cambios en las categorías clínicas en 3 aislados.
ConclusionesLos 3 métodos presentaron una baja concordancia con la microdilución de referencia. A pesar de las diferencias en los valores MIC, menos del 3% implicaron cambios de categoría.
Ceftolozane/tazobactam was recently introduced as a new drug with excellent activity against Pseudomonasaeruginosa1 because it is stable against overproduction of the intrinsic cephalosporinase AmpC.2 This combination was recently included in some commercial panels, such as the Vitek 2 XN12 card (BioMérieux)3 and the Microscan NMDR1 panel (Beckman Coulter)4 for routine antimicrobial susceptibility testing. Validation of other panel types is unknown. Gradient strips for in vitro diagnostic use are also available. When Etest strips were first evaluated with P. aeruginosa isolates, a significant rate of discrepancies between Etest and the reference method was observed.5 The same was true for LiofilmChem gradient strips, where good agreement between microdilution and strips was observed at low concentrations, but with differing results at high concentrations.6 Subsequent analyses have found better correlations for the Etest, but not for LiofilmChem strips, although the number of resistant isolates was not very high7 and below the 50% recommended by the FDA for assessment of commercial devices.8 Carbapenemase-producing P. aeruginosa isolates are resistant to ceftolozane/tazobactam9 and the inclusion of resistance to ceftolozane/tazobactam has been recommended, along with ceftazidime and cefepime resistance, to increase specificity of detection for carbapenemase producers among carbapenem-resistant P. aeruginosa isolates.10 The aim of the study was to evaluate two gradient strips and a Sensititre panel used to assess the activity of this drug against carbapenem-resistant P. aeruginosa isolates from our region, including a higher number of ceftolozane/tazobactam-resistant isolates than in previous comparisons.
Material and methodsA total of 100 carbapenem-resistant P. aeruginosa isolates from 11 hospitals in Andalusia were included in the study, which formed part of the SEIMC Carba project11: 41 were metallo-beta-lactamase-producing (21 IMP-producing; 20 VIM-producing isolates) and 59 non-carbapenemase-producing isolates. The isolates were selected to cover a wide range of ceftolozane/tazobactam MIC values. Eighteen (30%) carbapenem-resistant and non-carbapenemase-producing isolates were considered multidrug-resistant strains (MDR) based on additional resistance to two other conventional antipseudomonal beta-lactam groups (antipseudomonal cephalosporins, piperacillin/tazobactam or aztreonam). Susceptibility to ceftolozane/tazobactam was tested by agar diffusion with two types of commercial gradient strips (BioMérieux and LiofilChem) on Mueller Hinton agar, and by commercial microdilution (Sensititre, EURGNCOL panel; 0.25/4–8/4mg/L ceftolozane/tazobactam; Thermo Fisher Scientific); these results were then compared to broth microdilution, following EUCAST recommendations, as the reference method.12 The MIC results by gradient strip were rounded to the next double dilution step for comparison with microdilution; in the case of the EURGNCOL panel, isolates with MIC>8 and ≤0.5mg/L were grouped together for the comparison, due to the range of concentrations. For reference microdilution tests, fresh panels were prepared with a range of ceftolozane concentrations from 0.12 to 128mg/L (provided by MERCK SHARP and DOHME) and a fixed concentration of 4mg/L tazobactam. Each isolate was tested in parallel by the four methods described above, on the same day, from a single fresh overnight culture on sheep blood agar. The same bacterial suspension adjusted to 0.5 McFarland in 0.9% NaCl solution was used for microdilution, gradient strip diffusion and the Sensititre panel. Quality controls using P. aeruginosa strain ATCC 27853 were included in each series of experiments.
Essential agreement (EA) was considered when the same MIC values (±1 dilution) were obtained for both methods, using EUCAST as the reference. Bias was calculated for each commercial test as the difference between the percentage of results above and below the reference value, according to the recommendations of ISO 20776-2.13 The kappa index was used to evaluate concordance using Landis and Koch guidelines.14 McNemar's test with the Yates correction was used to compare percentages. ISO 20776-2 criteria, acceptable EA rate of ≥90% and interval bias from −30% to 30%, were used to evaluate the performance of the three commercial methods.13
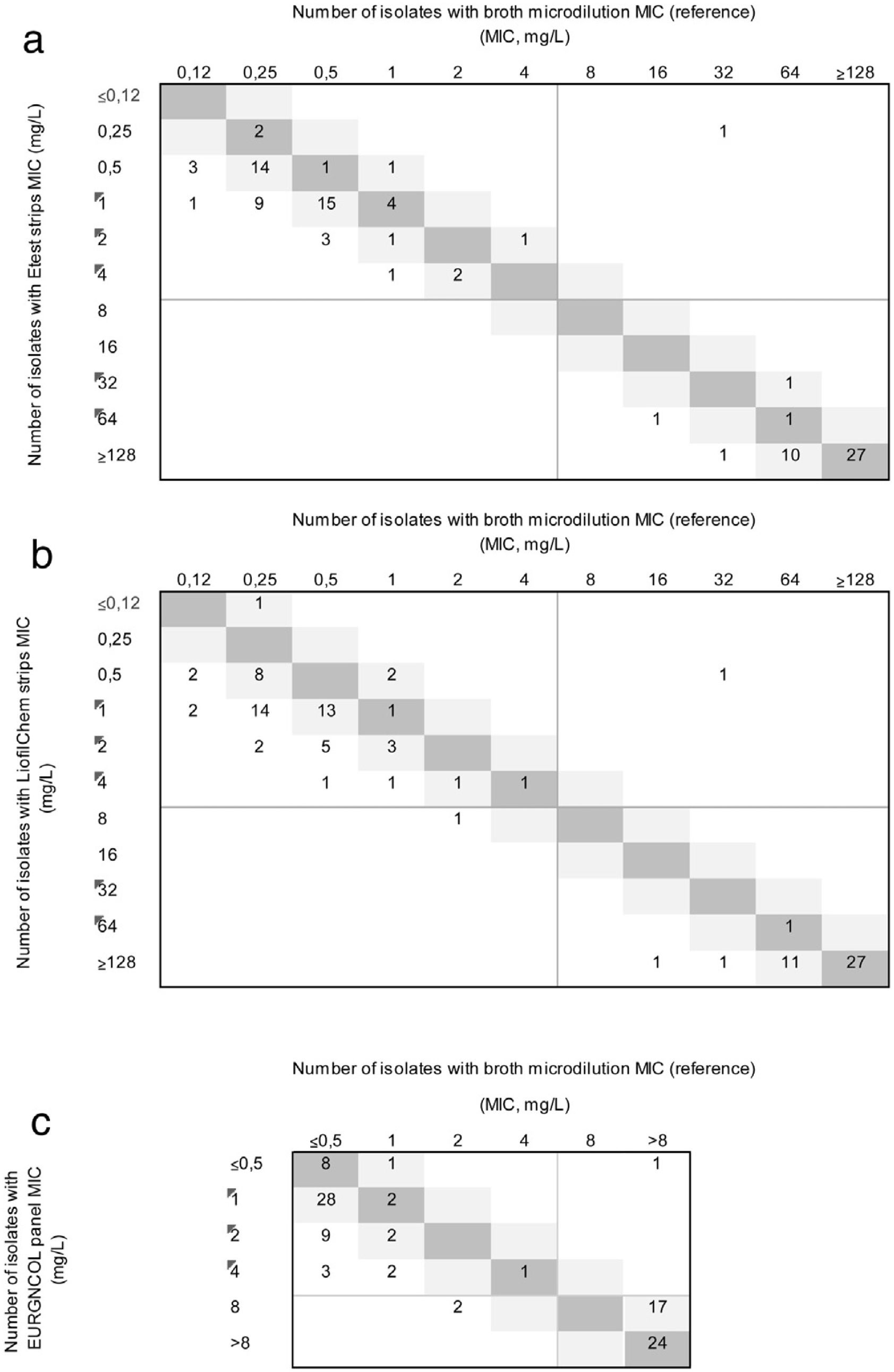
ResultsFifty-eight (58%) of the isolates were susceptible to ceftolozane/tazobactam using the reference method. All carbapenemase-producing isolates (66% of isolates had MIC≥128mg/L) and only one non-carbapenemase-producing isolate (MIC values of 32/4) were resistant to this antibiotic. The MIC50, MIC90, and MIC range of ceftolozane/tazobactam, as determined by the reference method, were 0.25, 1, and 0.12–6mg/L, respectively, for non-carbapenemase-producing isolates, and 64, 128, and 32–256mg/L respectively, for the carbapenemase producers. The EA rate was below the threshold of acceptance in all cases, the highest being with the EURGNCOL panel and the lowest with Liofilmchem (p=0.005). Bias was outside the range of acceptance in all three methods due to MIC values being higher than the reference MIC (Fig. 1), especially in susceptible non-carbapenemase-producing isolates (Table 1). The kappa index was below 0.2 with the LiofilChem strips, indicating poor agreement, and in the 0.21–0.40 range for the BioMérieux strips and panels, indicating fair agreement, but this index was good for panels among non-carbapenemase-producing isolates because of aggregated data (Table 1). Changes in clinical categories were observed in 3 isolates: 1 carbapenemase-producing isolate was resistant by the reference method and susceptible by the three commercial methods, and 2 non-carbapenemase-producing isolates were both susceptible by the reference method, but 1 was resistant using the Liofilchem strip and EURGNCOL panel, and 1 was resistant only with the EURGNCOL panel.
Correlation between ceftolozane/tazobactam MICs as determined by Etest (a); Liofilchem® MIC Test Strips (b); and the Sensititre™ EUROGNCOL panel (c) using microdilution as reference. The number of strains with MICs corresponding to broth microdilution and 1-log2 dilution are indicated in the dark and light grey squares, respectively. EUCAST breakpoints are presented as solid grey lines.
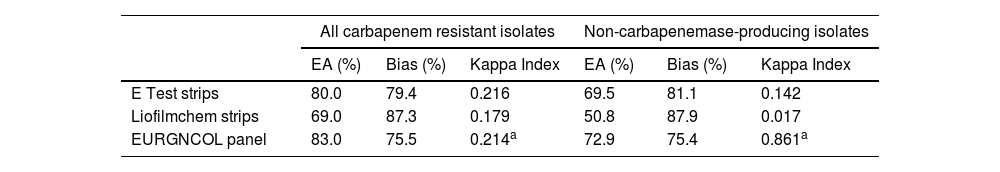
Comparison of in vitro performance of gradient tests and the Sensititre EURGNCOL panel versus the broth microdilution method for the determination of susceptibility of P. aeruginosa isolates to ceftolozane/tazobactam.
| All carbapenem resistant isolates | Non-carbapenemase-producing isolates | |||||
|---|---|---|---|---|---|---|
| EA (%) | Bias (%) | Kappa Index | EA (%) | Bias (%) | Kappa Index | |
| E Test strips | 80.0 | 79.4 | 0.216 | 69.5 | 81.1 | 0.142 |
| Liofilmchem strips | 69.0 | 87.3 | 0.179 | 50.8 | 87.9 | 0.017 |
| EURGNCOL panel | 83.0 | 75.5 | 0.214a | 72.9 | 75.4 | 0.861a |
EA: essential agreement.
In the present study, all three products yielded a higher EA for ceftolozane/tazobactam MICs in P. aeruginosa isolates. Ever since commercial methods for ceftolozane/tazobactam susceptibility testing in P. aeruginosa have been evaluated, the performance of gradient strips has been subject to debate because of the limited number of resistant isolates studied. Our evaluation is the first to include a sufficiently large number of carbapenemase-producing and resistant isolates (42%). In the study involving the largest number of isolates (n=308) to date, carried out by Humphries et al.,15 only 28% of isolates were resistant to ceftolozane/tazobactam. The EA found in our study is lower than that found in other studies using both LiofilmChem strips6,15 and BioMérieux strips15 for P. aeruginosa. Our study obtained higher MIC values for the strips and the Sensititre panel than for broth microdilution. Humphries et al. observed lower MIC values for the LiofilmChem strip, but not for the Etest.15 Changes in clinical categories for the two gradient strips were lower than in previous evaluations, which reported very major errors between 2.5%3 and 25%.16
A limitation of our study is that it included only one isolate with MIC values close to the susceptibility cut-off point. Isolates with intermediate ceftolozane/tazobactam MIC values are rare, since more than 40 mutations are required in non-carbapenemase-producing isolates to raise the MIC value for this antibiotic, which normally only occurs in hypermutator strains.2 The strengths of this study are firstly, that it includes broth microdilution as a reference method, secondly, a large number of isolates were evaluated, including a good proportion of resistant ones, and finally, the multicentre origin of the isolates.
In summary, the three commercial systems correctly categorised ceftolozane/tazobactam susceptibility among carbapenem-resistant P. aeruginosa isolates. Both ceftolozane/tazobactam susceptibility testing options for MDR P. aeruginosa, either gradient diffusion or commercial panel, are more accessible than broth microdilution, but our study showed that additional testing with the reference method is recommended when accurate MIC values are needed.
FundingThis study has been funded by Instituto de Salud Carlos III, Spanish Network for Research in Infectious Diseases (RD16/0016/0001)-co-financed by European Development Regional Fund “A way to achieve Europe”, Operative program Intelligent Growth 2014-2020.
Conflicts of interestNone.