This update to the document on antiretroviral therapy (ART) in adults, which has been prepared jointly by GeSIDA and the Spanish National AIDS Plan for the last two decades, supersedes the document published in 2017.1 The update provides physicians treating HIV-1-infected adults with evidence-based recommendations to guide their therapeutic decisions. The main difference with respect to the previous document concerns recommended initial ART regimens, only three of which are maintained as preferential. All three include dolutegravir or raltegravir, together with emtricitabine/tenofovir alafenamide or abacavir/lamivudine. Other differences concern the section on switching ART in patients with suppressed viral replication, which now includes new two- and three-drug regimens, and the antiretroviral drugs recommended for pregnant women and patients with tuberculosis. A recommendation has also been added for patients who present with acute HIV infection after pre-exposure prophylaxis.
Esta actualización del documento sobre el tratamiento antirretroviral (TAR) en adultos que GeSIDA y el PNS elaboran desde hace 2 décadas, reemplaza a la de 20171. Su objetivo es proporcionar a los clínicos que tratan a adultos con infección por el VIH-1 recomendaciones basadas en evidencias científicas para guiar sus decisiones terapéuticas. El principal cambio respecto al documento previo incumbe a los regímenes recomendados para el TAR de inicio, solo 3 de los cuales se mantienen como preferentes, incluyendo todos ellos dolutegravir o raltegravir junto con emtricitabina/tenofovir alafenamida o abacavir/lamivudina. Otros cambios conciernen al apartado de cambio del TAR en pacientes con replicación viral suprimida, en el que se han incluido nuevos regímenes de 2 y 3 fármacos, y a los antirretrovirales recomendados en embarazadas o en pacientes con tuberculosis. Se ha añadido también una recomendación para personas que habiendo realizado profilaxis pre-exposición al VIH presentan una infección aguda por dicho virus.
The present consensus document updates previous recommendations of GeSIDA and the National AIDS Plan on ART in adults with HIV infection.1 Summarized below are the recommendations.
Clinical and laboratory evaluation as a guide for ARTGeSIDA has prepared the 2018 consensus document on control and monitoring of HIV infection. All those interested in this topic are recommended to consult the document.
Initial antiretroviral therapyWhen should ART be initiated?
- •
ART should be initiated in all HIV-infected patients (A-I).
Which combination of antiretroviral drugs should be used?
- •
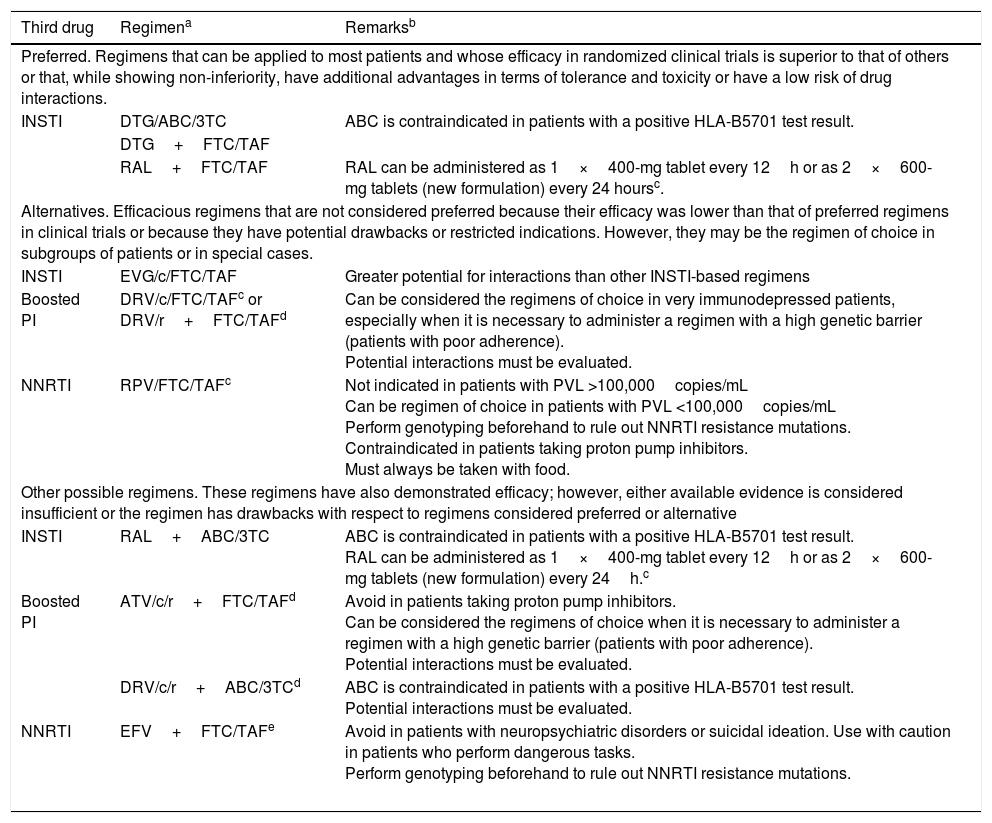
Initial ART can be a combination of 2 nucleoside reverse transcriptase inhibitors (NRTI) and 1 INSTI, 2 NRTI and 1 NNRTI, or 2 NRTI and 1 boosted protease inhibitor (PI) (A-I). Preferred antiretroviral drugs are set out in Table 1.
Table 1.Recommended combinations of initial ARTa
Third drug Regimena Remarksb Preferred. Regimens that can be applied to most patients and whose efficacy in randomized clinical trials is superior to that of others or that, while showing non-inferiority, have additional advantages in terms of tolerance and toxicity or have a low risk of drug interactions. INSTI DTG/ABC/3TC ABC is contraindicated in patients with a positive HLA-B5701 test result. DTG+FTC/TAF RAL+FTC/TAF RAL can be administered as 1×400-mg tablet every 12h or as 2×600-mg tablets (new formulation) every 24 hoursc. Alternatives. Efficacious regimens that are not considered preferred because their efficacy was lower than that of preferred regimens in clinical trials or because they have potential drawbacks or restricted indications. However, they may be the regimen of choice in subgroups of patients or in special cases. INSTI EVG/c/FTC/TAF Greater potential for interactions than other INSTI-based regimens Boosted PI DRV/c/FTC/TAFc or DRV/r+FTC/TAFd Can be considered the regimens of choice in very immunodepressed patients, especially when it is necessary to administer a regimen with a high genetic barrier (patients with poor adherence).
Potential interactions must be evaluated.NNRTI
RPV/FTC/TAFc Not indicated in patients with PVL >100,000copies/mL
Can be regimen of choice in patients with PVL <100,000copies/mL
Perform genotyping beforehand to rule out NNRTI resistance mutations.
Contraindicated in patients taking proton pump inhibitors.
Must always be taken with food.Other possible regimens. These regimens have also demonstrated efficacy; however, either available evidence is considered insufficient or the regimen has drawbacks with respect to regimens considered preferred or alternative INSTI RAL+ABC/3TC ABC is contraindicated in patients with a positive HLA-B5701 test result.
RAL can be administered as 1×400-mg tablet every 12h or as 2×600-mg tablets (new formulation) every 24h.cBoosted PI ATV/c/r+FTC/TAFd Avoid in patients taking proton pump inhibitors.
Can be considered the regimens of choice when it is necessary to administer a regimen with a high genetic barrier (patients with poor adherence).
Potential interactions must be evaluated.DRV/c/r+ABC/3TCd ABC is contraindicated in patients with a positive HLA-B5701 test result.
Potential interactions must be evaluated.NNRTI EFV+FTC/TAFe Avoid in patients with neuropsychiatric disorders or suicidal ideation. Use with caution in patients who perform dangerous tasks.
Perform genotyping beforehand to rule out NNRTI resistance mutations.
ABC, abacavir; ATV/r/c, atazanavir boosted with ritonavir or cobicistat; c, cobicistat; DTG, dolutegravir; DRV/c: darunavir boosted with cobicistat; DRV/r: darunavir boosted with ritonavir; DRV/r/c, darunavir boosted with ritonavir or cobicistat; EVG/c, elvitegravir boosted with cobicistat; EFV, efavirenz; FTC, emtricitabine; INSTI, integrase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitors; RAL, raltegravir; RPV, rilpivirine; TAF, tenofovir-alafenamide; 3TC, lamivudine.
aWhen available, fixed-dose combinations should be used. The use of tenofovir in the form of tenofovir disoproxil with any of its salts (TDx) can be considered an alternative to TAF provided that renal abnormalities and osteopenia are ruled out and there is no risk of developing them.
In drugs from the same family and with the same level of recommendation, the order reflects the preference of the expert panel.
bThe remarks reflect aspects that should be taken into consideration when choosing the regimen; they do not aim to be an exhaustive guide to the precautions to be taken when receiving these drugs. Please see the main text and the appropriate Summary of Product Characteristics for more information.
Cost and pricing of the therapeutic regimens are addressed elsewhere in these guidelines. The cost-effectiveness of the regimens is analyzed formally in an article published simultaneously with the guidelines.
cThe coformulated combinations RPV/TAF/FTC and DRV/c/TAF/FTC, as well as the new formulation of RAL in 600-mg tablets for once-daily administration have been approved by the EMA, although at the time of writing these guidelines, they are not available in Spain.
- •
The NRTI combinations of choice for initial regimens are TAF/FTC and ABC/3TC (AI). Co-formulated preparations are recommended (A-II).
- •
The combination of FTC with tenofovir disoproxil in the form of any of its salt (FTC/TDx) can be used as an alternative to the combination FTC/TAF, provided that renal abnormality and osteopenia are ruled out and there is no risk of developing them (C-III).
- •
The combination ABC/3TC should be avoided in patients with a high PVL (>100,000copies/mL) when combined with an NNRTI or a boosted PI (A-I).
- •
The combination rilpivirine (RPV)/TAF/FTC is considered preferential in patients with a PVL <100,000copies/mL (B-III).
- •
RPV should not be administered to patients with a PVL >100,000copies/mL (A-I).
- •
Efavirenz (EFV) should be avoided in patients with nonstabilized neuropsychiatric disorders or a history of suicidal ideation and in patients who perform dangerous tasks (A-III).
- •
When it is deemed appropriate to initiate a PI-based regimen, the recommendation is for DRV/c/FTC/TAF (A-I) or DRV/r+FTC/TAF (QD) (A-III). Alternatively, ATV/r (or ATV/c)+FTC/TAF (QD) could be prescribed (B-III).
- •
ATV and DRV can be boosted interchangeably with ritonavir 100mg or cobicistat 150mg (B-II).
- •
The combination DRV/r (or DRV/c)+ABC/3TC can also be used, although it has not been formally assessed in a clinical trial (C-III).
- •
Dolutegravir (DTG) coformulated with ABC/3TC, or combined with TAF/FTC (A-I) or raltegravir (RAL, 400mg BID o 1200mg QD) combined with FTC/TAF (A-III) are considered preferred regimens for initial treatment (A-I).
- •
The combination EVG/c/TAF/FTC is preferred over EVG/c/TDF/FTC owing to its greater efficacy, better tolerability profile and the possibility of administering it with an estimated glomerular filtration rate (eGFR) >30mL/min (A-I).
- a)
NRTI
Switching from ABC/3TC to TDF/FTC or TAF/FTC
- •
At present, this committee cannot recommend switching ABC/3TC to TDF/FTC with the aim of reducing cardiovascular risk (C-I) nor switching ABC/3TC to TAF/FTC with the aim of improving lipid profile, kidney function, or bone mineral density (C-I).
Switching from TDF to ABC
- •
The switch from TDF to ABC is a valid option in patients with osteopenia or osteoporosis associated with TDF (A-II).
Switching from TDF/FTC to TAF/FTC
- •
This switch is virologically safe and is associated with improved bone mineral density and kidney function (A-I).
- •
- b)
NNRTI
Switching from EFV/TDF/FTC to RPV/TDF/FTC
- •
In patients with adverse central nervous system (CNS) effects caused by EFV, this switch can improve the symptoms (A-II).
Switching from RPV/TDF/FTC or EFV/TDF/FTC to RPV/TAF/FTC
- •
Those switching are virologically safe and are associated with improved bone mineral density and kidney function (A-I).
- •
- c)
Protease inhibitors
Switching from ATV/r or DRV/r to ATV/c or DRV/c
- •
In patients receiving treatment with ATV/r or DRV/r, switching to ATV/c (A-I) or DRV/c (A-II) is an option that reduces the pill burden. The results of bioequivalence studies lead this Committee to recommend ATV/c or DRV/c interchangeably in contexts that affect ATV/r or DRV/r as a component of triple regimens. Data on dual regimens or monotherapy are not sufficient to recommend using the drugs interchangeably.
Switching from a boosted PI+TDF/FTC to DRV/c/TAF/FTC
- •
This switch is virologicaly safe and is associated with improved bone mineral density and tubular function (AI).
- •
- a)
Switching from NNRTI to INSTI
Switching from EFV to RAL
- •
This switch is an option in patients with CNS adverse events (A-II) or dyslipidemia (A-I) caused by EFV.
- •
- b)
Switching from boosted PI to an NNRTI
Switching from boosted PI to RPV/FTC/TDF
- •
This switch is a valid option in patients with gastrointestinal disorders or dyslipidemia (A-I).
- •
- c)
Switching from boosted PI to INSTI
Switching from boosted PI to RAL
- •
Switching to RAL+2 active NRTI is a valid option for patients with dyslipidemia taking ART with NRTI+1 boosted PI (A-I).
Switching from boosted PI+2 NRTI to DTG+2 NRTI
- •
This switch is virologically safe and is associated with a significant improvement in lipid profile (A-I).
- •
- d)
Switching to EVG/c/FTC/TAF from TDF-containing regimens
- •
Switching from EVG/c/FTC/TDF, EFV/FTC/TDF, or ATV/r+FTC/TDF to EVG/c/FTC/TAF is virologically safe and is also associated with improved bone mineral density and kidney function (A-I).
- •
- e)
Switching to DTG/ABC/3TC from regimens containing 2 NRTI and PI, NNRTI, or INSTI
- •
This switch is virologically safe (B-I).
- •
- a)
Dual therapy with 3TC and ATV/r or DRV/r
Switching from 2 NRTI plus ATV/r, DRV/r, or LPV/r to 3TC plus ATV/r or DRV/r
- •
This switch is an option if the clinician wishes to avoid or prevent the adverse effects caused by NRTI. This option requires the patient to fulfill the following criteria: 1) no chronic hepatitis B; 2) PVL <50copies/mL for at least 6 months; and 3) no mutations in the protease gene or previous virological failure to boosted PI or 3TC (A-I).
- b)
Monotherapy with PI/r
- •
Given the greater risk of a virological rebound, this Committee considers that monotherapy with DRV/r is less virologically safe than dual therapy with DRV/r+3TC. Thus, it can be considered an exceptional option that requires the patient to fulfill the same criteria as for dual therapy with DRV/r+3TC (C-I).
- c)
Dual therapy with dolutegravir and rilpivirine
Switching to DTG+RPV from regimens that contain 2 NRTI and PI, NNRTI, or INI
- •
This switch is virologically safe (A-I).
- d)
Monotherapy with dolutegravir
- •
Switching to monotherapy with DTG is not virologically safe and cannot be recommended (C-I).
- •
The causes of virological failure (VF) should be analyzed (A-III).
- •
Resistance and viral tropisms (except when the co-receptor is not CCR5 or when MVC is not expected to be included in the rescue regimen) should be assessed in order to design the best alternative regimen. The test should be performed while the patient is receiving the failed treatment or as soon as possible after suspension of the failed treatment. If the results of previous genotyping tests are available, all the resistance mutations detected should be evaluated (A-I).
- •
Switching ART because of VF should be performed early to avoid accumulation of mutations and to facilitate the response to the new treatment (A-III).
- •
The objective of rescue ART is to achieve a PVL <50copies/mL (A-II).
- •
The new ART regimen should contain 3 totally active antiretroviral drugs. If this is not possible, 2 fully active drugs should be combined with other drugs that maintain partial virological activity (A-I). Regimens with only 2 active antiretroviral drugs based on a boosted PI may be a reasonable option when it is not possible to use NRTI or construct a simple regimen with 3 active drugs (A-I).
- •
In patients who have experienced VF, DRV/r is the PI/r that has proven most efficacious in all the rescue lines. When major resistance mutations are present, the recommended dose is 600/100mg BID (A-I).
- •
DTG is the INSTI of choice in patients who experience VF and who are INSTI-naïve or ITINN-naïve (A-I), or in cases where other INSTI fail. In the case of previous failure to RAL or EVG, the recommended dose of DTG is 50mg BID, accompanied by optimized background therapy (A-II).
- •
The use of tipranavir/ritonavir (TPV/r), ENF, or thymidine analogs is restricted to patients with no other therapeutic options (A-III).
- •
In patients with low-grade VF (PVL detectable but ≤200copies/mL), genotyping can be performed with a 2–3-mL plasma sample (A-II). If genotyping does not reveal resistance mutations, an ART regimen with a high barrier to resistance should be maintained. Genotyping and a new ART regimen are recommended in patients with a higher PVL (>200copies/mL) based on both resistance mutations and previous ART. In any case, ART should not be intensified with a single drug (A-III).
- •
ART should not be suspended in patients with advanced VF and no therapeutic options (A-II). In this situation, the approach should involve antiretroviral drugs that reduce viral replicative capacity and do not lead to resistance mutations that might compromise future treatments (A-III).
- •
In patients who experience VF and for whom a suppressive ART regimen cannot be designed, the recommendation is to consult with clinicians and virologists specialized in resistance and rescue therapy who are involved in restricted access programs in order to design a nonsuppressive “bridging” regimen while waiting for active drugs to become available (B-III).
- •
Before initiating ART, the patient should be prepared and factors likely to limit adherence should be identified and corrected (A-III).
- •
Once ART has been initiated, a first check-up should be made after 2–4 weeks to verify adherence and correct adherence problems if necessary (A-III).
- •
Adherence should be monitored and reinforced at visits to the doctor (A-III).
- •
Adherence should be monitored by a multidisciplinary team including a doctor, nursing staff, specialists in psychological support, and a hospital pharmacist (A-III).
- •
In the case of patients whose adherence is irregular, it is recommended to use regimens based on boosted PI, preferably DRV because of its high genetic barrier to resistance, in order to prevent the development of resistance (A-II).
- •
Using fixed dose combinations of antiretroviral drugs simplifies ART and thus facilitates continued adherence. The use of whole regimens in a single tablet is the most efficient strategy for preventing selective poor adherence (A-II).
- a)
Immediate adverse effectsAvoid the use of antiretroviral drugs whose immediate adverse effects are similar to clinical manifestations or laboratory abnormalities that are already present in a specific patient (A-II).HLA-B*5701 testing is mandatory before prescribing ABC, since it has a negative predictive value of almost 100% for the risk of hypersensitivity reaction to this drug (A-I).If the adverse effect is very intense or long-lasting or cannot be tolerated by the patient, the potential culprit antiretroviral drug(s) should be switched (A-I).
- b)
Late adverse effectsART should be tailored by evaluating the risk or presence of chronic diseases in such a way that the regimen selected does not contain antiretroviral drugs that can favor the onset or progression of these diseases (A-II).Withdrawal of some of the antiretroviral drugs involved in late adverse effects can improve the underlying clinical abnormality, although other factors are generally considered to be more important. Priority should be given to interventions to address these factors (A-II).
- •
All medications, natural products, and alternative medicines taken by the patient should be recorded in the clinical history in order to evaluate potential interactions (A-III).
- •
Contraindications should be taken into account and the corresponding dose adjustments made where necessary (A-I).
- •
ART should be started as soon as possible to obtain the maximum benefit (A-II).
- •
ART should be done with the same preferential regimens used to treat chronic HIV infection (A-I) (Table 1). A regimen comprising 2 NRTI and an INSTI could reduce PVL more rapidly during the first 4–8 weeks than PI or NNRTI and, thus, make it easier to reduce transmission of HIV (A-I).
- •
If the results of resistance testing are not available, it is preferable to begin with a regimen based on DTG or boosted DRV until the results become available (A-II). If the patient had recently started pre-exposure prophylaxis (PrEP), a fourth drug should be added to the regimen until the results of the resistance test become available (C-III).
- •
The preferred regimen for initial ART is the combination of 2 NRTI and 1 INSTI or a boosted PI (A-III).
- •
The use of NNRTI, MVC, or ENF is not indicated for the treatment of HIV-2 infection (A-I).
- •
Administration of ART during pregnancy is discussed in a consensus document prepared by the PNS in collaboration with GeSIDA and other scientific societies.
- •
If HIV infection is diagnosed during pregnancy, ART should be initiated as early as possible because of the possibility of intrauterine transmission (A-I).
- •
The choice of specific antiretroviral drugs should be based on resistance studies, and drug safety. If there are no resistance mutations, the regimen of choice is TDF or ABC+3TC or FTC+RAL (A-I) or ATV/r (A-I) or DRV/r (A-II).
- •
The combination TDF/FTC+LPV/r is not recommended.
- •
Intrapartum treatment with intravenous ZDV is indicated if the PVL is >1000copies/mL or unknown at delivery (A-I) or if it is between 50 and 999copies/mL (B-III).
- •
Elective cesarean delivery is indicated at week 38 in women with a pre-labor PVL of >1000copies/mL (A-II).
In most opportunistic infections, ART should be started as soon as possible (preferably within the first 15 days after starting treatment for the infection) (A-II).
Patients with Pneumocystis jiroveci pneumonia (PJP) should start ART during the 2 weeks following the diagnosis of PJP (A-I).
In patients with cryptococcal meningitis, initiation of ART should be deferred for 5 weeks because of the greater risk of death associated with early initiation (A-I).
b) ART and tuberculosisTreatment of tuberculosis in HIV-infected adults was the subject of a consensus document from GeSIDA/PNS, which is available for consultation.
The optimal time for initiating ART depends on the CD4+ T-lymphocyte count. If the CD4+ T-lymphocyte count is <50cells/μL, ART should be started as soon as possible, but not later than the first 2 weeks (A-I). If the CD4+ T-lymphocyte count is >50cells/μL, initiation of ART can be delayed until the intense phase of anti-tuberculosis treatment has been completed (8 weeks) (A-I).
Choice of NRTI: ABC, TDF, 3TC, and FTC can be used with no added risks (A-I). However, a relevant interaction could occur between TAF and the rifamycins, with a decrease in absorption and in the plasma concentration of TAF, since TAF is transported by glycoprotein P (P-gp) and the rifamycins induce the activity of this protein.
Choice of the third drug. EFV at standard dose is the antiretroviral drug of choice (A-I). The alternative regimens include RAL at 800mg/12h (A-II), although 400mg/12h has proven to be efficacious. There are arguments against administering rifampicin with RAL in its new once-daily formulation, namely, it reaches lower trough levels than with the standard twice-daily regimen. Pharmacokinetic studies support the administration of rifampicin with MVC at 600mg/12h or with DTG at 50mg/12h (A-III).
Drugs that cannot be used. RPV, ETR, PI, and EVG should not be co-administered with rifampicin. (A-II).
Immune reconstitution inflammatory syndrome (IRIS). If the patient develops IRIS, neither ART nor anti-tuberculosis medication should be interrupted (A-III). The symptoms of IRIS can by managed by adding non-steroidal anti-inflammatory drugs in mild to moderate cases (A-III) or corticosteroids in severe forms (A-II). Administration of prednisone (40mg/d for 2 weeks, followed by 20mg/d for a further 2 weeks) prevents the development of IRIS in patients with at least 100 CD4/μL (A-I).
c) Renal insufficiencyFor a complete overview of renal disorders in HIV-infected patients, please consult the consensus document drafted by GeSIDA, the SEN, and the SEQC3.
- •
It is necessary to adjust the dose of NRTI, except for ABC (A-II).
- •
No dose adjustment is required for NNRTI, PI, ENF, RAL, or DTG (A-II).
- •
The dose of MVC should be adjusted if it is used in combination with potent CYP3A4 inhibitors such as PI (A-II).
- •
Co-formulations of antiretroviral drugs are not advised in patients with significant renal insufficiency. EVG/c/FTC/TDF should not be used in patients with an eGFR <70mL/min. EFV/FTC/TDF, RPV/EFV/TDF, and DTG/ABC/3TC should not be used in patients with eGFR <50mL/min. EVG/c/FTC/TAF should not be used in patients with eGFR <30mL/min. In these cases, antiretroviral drugs should be administered separately and the appropriate adjustments made (B-III).
- •
In patients with renal insufficiency (any stage), kidney function, including tubular function in the case of patients taking TDF, should be closely monitored and nephrotoxic drugs avoided (A-III).
- •
In patients with advanced chronic renal insufficiency, the dose should be adjusted according to the recommendations of the summary of product characteristics (A-II). In the absence of contraindications, the combination of ABC+3TC (adjusted for eGFR) with an NNRTI or a non-boosted INSTI (DTG or RAL) or DRV/r can be used (A-III).
In conjunction with the AEEH, SEIMC recently drafted guidelines for the management of hepatitis C. Please consult the guidelines for more detailed information.
- •
In patients who require treatment for hepatitis C, it is generally preferable to initiate ART before starting treatment for HCV infection A-III.
Any antiretroviral drug can be used in patients with chronic liver disease and normal liver function, including patients with cirrhosis (Child–Pugh, class A) (A-I). In patients with (Child–Pugh stage A or B) cirrhosis, INSTI, RPV and DRV do not require dose adjustments and are the drugs of choice (A-I). In patients with Child–Pugh stage C disease, RAL and DTG does not require dose adjustment and ATV/r and FPV/r is safe (A-III).
- •
With the exception of sofosbuvir, currently used DAA present significant pharmacokinetic interactions with antiretroviral drugs that may require doses to be adjusted or coadministration to be contraindicated (A-I). An updated pharmacologic interaction software package should be used before prescribing a DAA-containing regimen in a patient on ART (A-III).
- •
ART should be initiated with a regimen including TDF or TAF and FTC or 3TC in patients coinfected with HIV and HBV (A-I).
Please refer to the relevant GeSIDA documents for complete information on cancer in HIV-infected patients.
- •
Patients with any type of cancer who are not receiving ART should initiate therapy as soon as possible (A-II).
- •
RAL should be the antiretroviral drug of choice in patients receiving chemotherapy (A-III). DTG can be considered an alternative in cases of resistance to RAL (C-III).
Please consult the pharmaco-economic study yearly published by GeSIDA.
- •
Cost-effectiveness criteria should be taken into account when deciding on initial ART (A-III).
This consensus document was drafted without grant aid or other funding, whether collective or individual, from any private institutions. The conflicts of interest not associated with this document declared by the Members of the Editorial Board are set out below.
Koldo Aguirrebengoa has acted as a consultant for AbbVie, Gilead Sciences, Janssen Cilag, and ViiV Healthcare. He has participated in clinical trials and studies for AbbVie, Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme and has received payment for lectures from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
José R. Arribas has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. He has received grant support for clinical research from Janssen, Merck Sharp & Dohme, and Gilead Sciences and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Vicente Boix has acted as a consultant for AbbVie, Janssen Cilag, Gilead Sciences, Merck Sharp & Dome, and ViiV Healthcare. He has received clinical research grants from Gilead Sciences, Janssen Cilag, and ViiV Healthcare and payment for talks by Bristol-Myers Squibb, Janssen Cilag, Merck Sharp & Dome, and ViiV Healthcare. He has also received payment for developing training materials for Bristol-Myers Squibb, Janssen Cilag, Merck Sharp & Dome, and ViiV Healthcare.
Juan Berenguer has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Janssen Therapeutics, Merck Sharp & Dohme, and ViiV Healthcare. He has received clinical research grant support from Bristol-Myers Squibb, Merck Sharp & Dohme, and ViiV Health care and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Health care.
José R. Blanco has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Janssen Therapeutics, Merck Sharp & Dohme, and ViiV Healthcare. He has received clinical research grant support from Bristol-Myers Squibb y Gilead Sciences, and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Pere Domingo has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and ViiV Healthcare. He has received clinical research grant support from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and ViiV Healthcare and payment for talks from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, and ViiV Healthcare.
Vicente Estrada has acted as a consultant for Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare. He has received payment for clinical research from Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme and for talks from Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme.
María José Galindo has acted as a consultant for Abbott Laboratories, AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme. She has received clinical research grants from Abbott Laboratories, Boehringer Ingelheim, GlaxoSmithKlein, and Janssen Cilag and payment for talks from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and Roche. She has participated in the preparation of training materials for AbbVie, GlaxoSmithKlein, Janssen Cilag, Pfizer, and ViiV Healthcare.
Federico García has acted as an adviser/consultant and has given talks for AbbVie, Gilead Sciences, Hologic, Merck Sharp & Dohme, Roche Diagnostics, Werfen, and ViiV. He has also received research grants from Gilead Sciences, Merck Sharp & Dohme, Roche Diagnostics, and ViiV Healthcare.
José M. Gatell has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, and ViiV Healthcare. He has received clinical research grant support from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, and ViiV Healthcare and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, and ViiV Healthcare.
Juan González García has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare. Has received clinical research grant support and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. He has also received financial support to attend scientific meetings from Bristol-Myers Squibb, Gilead Sciences and Janssen Cilag.
Félix Gutiérrez has acted as a consultant for and received payment for lectures and talks from Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
José Antonio Iribarren declares that he has no conflicts of interest.
Hernando Knobel has acted as a consultant for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks and designing training materials for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Josep M. Llibre has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for training activities from Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
José López Aldeguer has received payment for talks from Gilead Sciences.
Juan C. López Bernaldo de Quirós has acted as a consultant for Janssen and ViiV Healthcare and has received lecture fees from AbbVie, Bristol-Myers Squibb, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Luis F. López Cortés has received unrestricted research funding, consultancy fees, and lecture fees from and have served on the advisory boards of Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Juan E. Losa has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Fernando Lozano has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck-Sharp & Dohme, and ViiV Healthcare and has received fees for educational talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck-Sharp & Dohme, and ViiV Healthcare.
Ana Mariño has received grant aid for attendance at conferences and meetings from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and ViiV Healthcare.
Esteban Martínez has received fees for medical training activities or participation in advisory boards from Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare. His institution has received grants for clinical research studies in which he acted as the principal investigator.
José M. Miró has acted as a consultant for the laboratories Angelini, AbbVie, Bristol-Myers Squibb, Genentech, Gilead Sciences, Medtronic, Merck Sharp & Dohme, Novartis, and Sanofi and has received clinical research grant support from Cubist, Gilead Sciences, Merck Sharp & Dohme, Novartis, Fondo de Investigaciones Sanitarias (FIS) del Instituto de Salud Carlos III (Madrid), Fundación para la Investigación y Prevención del Sida en España (FIPSE, Madrid), Ministerio de Sanidad, Servicios Sociales e Igualdad (MSSSI, Madrid), National Institutes of Health (NIH, Bethesda, MA, USA), and NEAT. He has also received payment for talks from Merck Sharp & Dohme and Novartis.
María Luisa Montes has acted as a consultant for Janssen Cilag and Gilead Sciences. She has received clinical research grants from AbbVie, Intercept, Merck-Sharp & Dome, ViiV Healthcare, Fondo de Investigaciones Sanitarias (FIS) del Instituto de Salud Carlos III (Madrid), Fundación para la Investigación y Prevención del Sida en España (FIPSE), and Ministerio de Sanidad, Servicios Sociales e Igualdad (MSSSI, Madrid). She has also received payment for talks from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck-Sharp & Dome, and Roche Pharma.
Santiago Moreno has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and Roche Pharma and has received clinical research grant support from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and Roche Pharma. He has also received payment for talks from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and Roche Pharma.
Eugenia Negredo declares that she has no conflicts of interest.
María J. Pérez Elías has acted as a consultant for AbbVie, Boehringer Ingelheim, Gilead Sciences, Janssen Cilag, and ViiV Healthcare. She has received clinical research grants from Gilead Sciences, Janssen Cilag, Merck-Sharp & Dome, and ViiV Healthcare and has payment for talks at events funded by Gilead Sciences, Janssen Cilag, Merck-Sharp & Dome, and ViiV Healthcare.
José A. Pérez Molina has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from Bristol-Myers Squibb, Merck Sharp & Dohme, and ViiV Healthcare.
Daniel Podzamczer has received research grants and/or consultancy fees and/or lecture fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Rosa Polo declares that she has not received any grant aid or subsidies associated with this document.
Joaquín Portilla has acted as a consultant for AbbVie, Gilead Sciences, and Janssen; he has received payment for talks from AbbVie, Bristol Myers-Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
Eva Poveda has received grants for attending conferences and scientific meetings from Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare. She has received payment for talks from Janssen Cilag and Merck Sharp & Dohme and grants for developing research projects and biomedical training activities from Gilead Sciences and Janssen Cilag.
Federico Pulido has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Esteban Ribera has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Antonio Rivero has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare. He has received clinical research grant support from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare and payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare.
Rafael Rubio has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, and Janssen Cilag and has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, Roche Pharma, and ViiV Healthcare.
Jesús Santos has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and ViiV-Healthcare and has received fees for educational talks from Bristol-Myers Squibb, Gilead sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV-Healthcare.
José Sanz Moreno has participated in clinical trials sponsored by Bristol-Myers Squibb, and ViiV Healthcare. He has received payment for consultancy work and preparing training presentations for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Jesús Sanz Sanz has acted as a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. He has received payment for talks from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare, and for preparing training presentations from ViiV Healthcare.
Sergio Serrano has received clinical research grants from Merck Sharp & Dohme. He has received payment for giving training presentations for Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare and for developing training presentations for Gilead Sciences.
Javier de la Torre has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Montserrat Tuset has received clinical research grant support from Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme and payment for talks from Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Miguel Angel von Wichmann has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare. He has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme and for developing training presentations from AbbVie.
The Board of GeSIDA and the National AIDS Plan acknowledge the contributions and opinions of Pablo Bachiller, Almudena Blanco, Inmaculada Clotet, Manuel Cotarelo, Pedro Ferrer, Julián Olalla, Óscar Rincón, Felipe Rodríguez-Alcántara, Francisco J. Rodríguez-Gómez y Nuria Sánchez.