Infection of a native joint, commonly referred to as septic arthritis, is a medical emergency because of the risk of joint destruction and subsequent sequelae. Its diagnosis requires a high level of suspicion. These guidelines for the diagnosis and treatment of septic arthritis in children and adults are intended for use by any physician caring for patients with suspected or confirmed septic arthritis. They have been developed by a multidisciplinary panel with representatives from the Bone and Joint Infections Study Group (GEIO) belonging to the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), the Spanish Society of Paediatric Infections (SEIP) and the Spanish Society of Orthopaedic Surgery and Traumatology (SECOT), and two rheumatologists. The recommendations are based on evidence derived from a systematic literature review and, failing that, on the opinion of the experts who prepared these guidelines. A detailed description of the background, methods, summary of evidence, the rationale supporting each recommendation, and gaps in knowledge can be found online in the complete document.
La infección de una articulación nativa, generalmente denominada artritis séptica, constituye una urgencia médica por el riesgo de destrucción articular y las consecuentes secuelas. Su diagnóstico requiere un alto nivel de sospecha. Esta guía de diagnóstico y tratamiento de la artritis séptica en niños y adultos está destinada a cualquier médico que atienda pacientes con sospecha de artritis séptica o artritis séptica confirmada. La guía ha sido elaborada por un panel multidisciplinar en el que están representados el Grupo de Estudio de Infecciones Osteoarticulares (GEIO) de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC), la Sociedad Española de Infectología Pediátrica (SEIP) y la Sociedad Española de Cirugía Ortopédica y Traumatología (SECOT); además han participado dos reumatólogos. Las recomendaciones se basan en la evidencia proporcionada por una revisión sistemática de la literatura y, en su defecto, en la opinión de los expertos que han elaborado la presente guía. En el texto completo online se hace una descripción detallada de los antecedentes, métodos, resumen de la evidencia, fundamentos que apoyan cada recomendación y las lagunas de conocimiento existentes.
- I.
When should the diagnosis of septic arthritis (SA) in children and adults be considered?
- 1.
All acute arthritis should be considered infectious until proven otherwise. A high index of suspicion for infectious arthritis is required because SA is a medical emergency and should be diagnosed as early as possible (A-II).
- 2.
Suspect a diagnosis of SA in any patient with signs/symptoms of arthritis: joint pain, swelling, effusion, warmth, erythema, and/or restriction of movement in one or more joints,
- •
with or without systemic signs/symptoms (fever, chills, shivering), and
- •
with or without risk factors for SA (previous joint disorder, immunosuppressive conditions, recent joint procedures, bacteraemia) (A-II).
- •
- 3.
Increase clinical suspicion of SA in patients with acute monoarticular arthritis especially of large peripheral joints (knee and hip in particular) (A-II).
- 4.
A diagnosis of SA should be considered especially in adults with acute monoarticular or polyarticular arthritis (usually involving two or three joints) with:
- •
inflammatory joint diseases (mainly rheumatoid arthritis),
- •
persistent bacteraemia, and/or
- •
immunosuppression (A-II).
- •
- 5.
Maintain a high index of suspicion for the diagnosis of SA of axial joints (sternoclavicular, acromioclavicular, costochondral, symphysis pubis, sacroiliac and facet joints) because of their lower incidence and often non-specific clinical features (local pain and tenderness) (A-II).
- 6.
In patients with subacute or chronic joint pain and swelling, consider a diagnosis of infectious arthritis caused by other infrequent organisms, such as mycobacteria or fungi, or infrequent bacteria (Borrelia burgdorferi, Brucella spp., Coxiella burnetii, Bartonella spp., Legionella spp., mollicutes [Ureaplasma/Mycoplasma], Nocardia spp., or Tropheryma whipplei) (A-II).
- 1.
- II.
What other possible diseases may be important to consider in patients with suspected SA?
- 1.
In patients with suspected SA, we suggest considering alternative diagnoses, mainly the following:
- •
Non-infectious arthritis, such as crystal-induced arthritis, post-traumatic arthritis, rheumatoid arthritis, and spondyloarthritis (including reactive arthritis, axial spondyloarthritis, psoriatic arthritis, and arthritis associated with inflammatory bowel disease). In children or adolescents, consider juvenile idiopathic arthritis.
- •
Infections of structures adjacent to the joint, such as bursitis, mainly in adults, and osteomyelitis or pyomyositis (typically around the pelvis and hip), mainly in children.
- •
Various viral infections that can present with arthralgias and/or arthritis mimicking septic arthritis.
- •
Transient synovitis and Perthes disease in children with hip involvement (A-II).
- •
- 2.
In adults with suspected SA, it is recommended to rule out crystal arthritis (gout, pseudogout) (A-III). Comment: It is possible to have concomitant infectious and crystal arthritis.
- 1.
- III.
What is the appropriate diagnostic evaluation and initial management of patients with suspected SA?
- 1.
A complete history and physical examination are recommended in all cases of suspected SA (A-III). This can help to differentiate between SA and other disorders and to identify pathogen-specific risk factors.
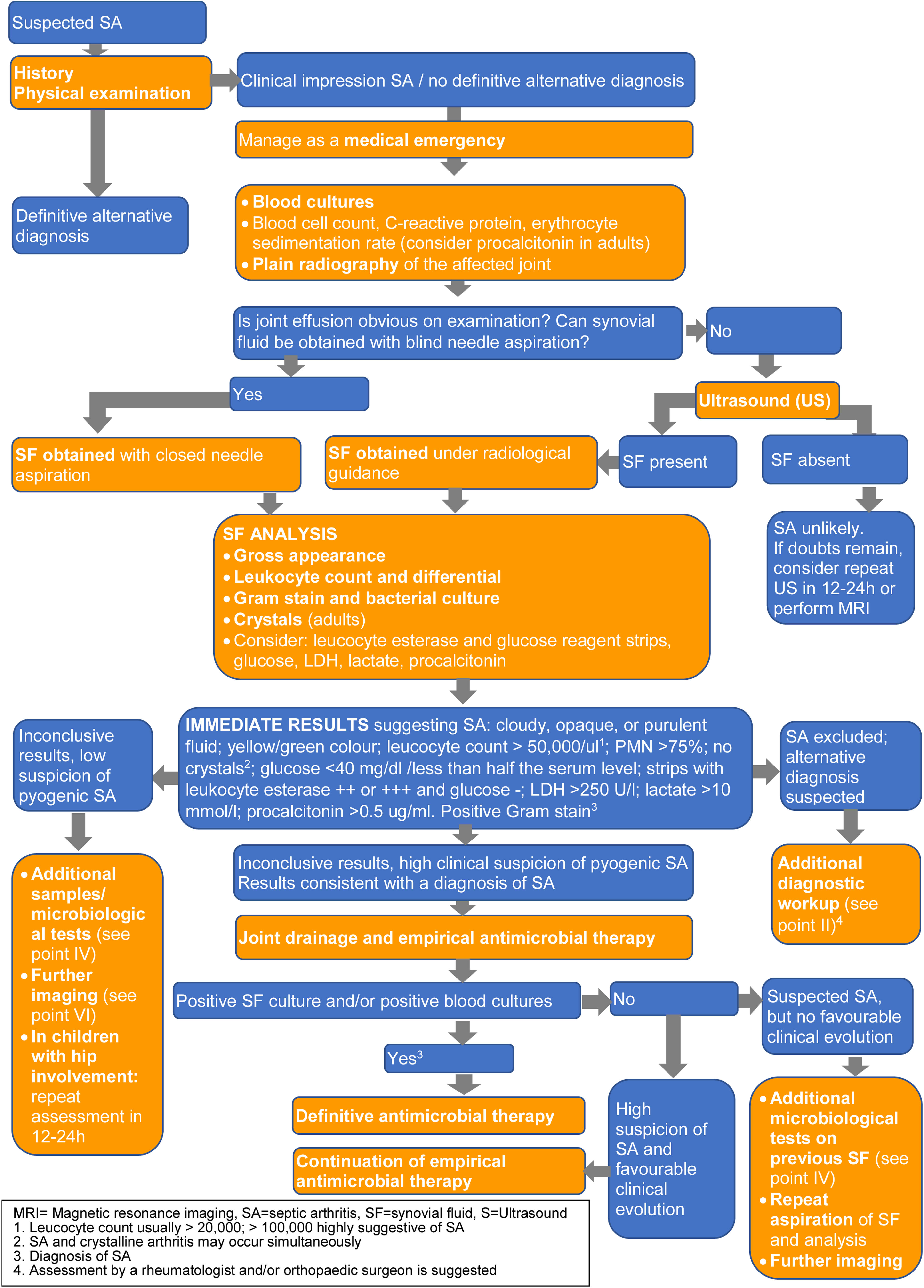
- 2.
A diagnostic algorithm (Fig. 1) showing laboratory and imaging tests (B-III) is provided. These are described in further detail in the following three sections.
- 1.
- IV.
What samples should be collected and what microbiological tests should be performed if SA is suspected?
- 1.
Blood cultures are recommended in all patients with suspected SA and should be obtained prior to antibiotic administration whenever possible (A-II). For blood cultures positive for organisms that commonly cause endocarditis (such as Staphylococcus aureus, viridans group streptococci, or enterococci), we suggest evaluation for endocarditis (B-III).
- 2.
Synovial fluid (SF) samples should be taken as soon as possible in all patients with suspected SA, preferably before initiating antimicrobial therapy (A-II).
- 3.
It is recommended to send the SF in a sterile container for Gram staining, culture and, when indicated, molecular studies (A-II). If there is enough fluid (e.g., more than 2mL) for staining, culture, possible molecular studies and leucocyte count, we suggest bedside inoculation of blood culture bottles with SF (B-II).
- 4.
In patients with suspected SA and negative SF cultures, we suggest obtaining a new sample of SF for microbiological staining and culture (including mycobacteria and fungi), molecular testing (see below) and histopathological analysis, especially if:
- •
they do not respond to empirical therapy against typical SA pathogens and/or
- •
mycobacteria or fungi are suspected (B-II).
- •
- 5.
Molecular methods (broad-range, multiplex or specific polymerase chain reaction [PCR]) for SF analysis or tissue biopsy:
- •
These are not routinely recommended for all SF samples from patients with suspected SA (D-III).
- •
Their use should be previously discussed with a microbiologist (A-III) and considered when SA is suspected in:
- -
All children aged 6 months to 5 years: Kingella kingae-specific PCR (A-II).
- -
Patients with negative SF culture receiving antibiotics before or at arthrocentesis: broad-range or multiplex PCR (A-II).
- -
Patients with negative SF culture who do not improve with empirical antibiotics and/or with clinical and/or epidemiological suspicion of infection with Neisseria gonorrhoeae or fastidious/difficult-to-culture microorganisms, including Brucella spp., B. burgdorferi, Bartonella spp., C. burnetii, Legionella spp., Ureaplasma spp., Mycoplasma spp., and T. whipplei: targeted PCR (B-II).
- -
- •
- 6.
Serological testing for Brucella spp. B. burgdorferi, Bartonella spp., C. burnetii, and/or Mycoplasma spp. is suggested in patients with negative SF culture, especially in the presence of risk factors and/or epidemiological, clinical or radiological evidence (B-III).
- 7.
In patients with suspected mycobacterial or fungal joint infection, as much SF as possible should be sent in a sterile container for culture; synovial biopsy is also recommended because of its higher yield for these organisms (A-III).
- 8.
In patients with suspected gonococcal arthritis, in addition to blood and joint cultures, we suggest N. gonorrhoea culture and nucleic acid amplification testing of genitourinary specimens and/or freshly voided urine, and, if clinically indicated, rectal and oropharyngeal swabs (A-II).
- 1.
- V.
What additional synovial fluid and blood/serum tests should be performed in patients with suspected SA?
- 1.
Recommended tests on SF: gross examination, leucocyte count and polymorphonuclear percentage (A-II). If the amount of SF is low, priority should be given to microbiological tests (A-III). Comment: There is no threshold to accurately diagnose SA or to differentiate SA from other acute arthritis, although the likelihood of SA rises with increasing leucocyte count and PMN percentage. SF leucocyte count >100,000/mm3 or 50,000–100,000/mm3 with >90% PMN are suggestive of infection.
- 2.
Additional markers: determination of SF glucose, lactate dehydrogenase (LDH), serum procalcitonin (PCT) and/or lactate (if available) are suggested, especially if previous initial data (including Gram stain) are inconclusive (C-III). Comment: Low glucose levels and elevated LDH, lactate and PCT levels are common in SA. These SF abnormalities are not reliably diagnostic of SA but may be useful in combination with other data.
- 3.
Use of leucocyte esterase and glucose reagent strip tests in SF may be of value as a rapid screening tool (B-II).
- 4.
SF should be examined for crystals to exclude microcrystalline arthritis in adults (A-II).
- 5.
Recommended blood/serum tests at initial assessment: C-reactive protein (CRP), erythrocyte sedimentation rate, white blood cell (WBC) count and PMN percentage (A-III). Comment: These tests are non-specific and cannot diagnose SA or differentiate it from other forms of arthritis, but their performance can be improved in conjunction with clinical data and other SF analyses. They can also be used as a baseline for serial monitoring of treatment response, particularly CRP.
- 6.
In adults, consider the determination of serum procalcitonin levels, if available. Comment: Although serum procalcitonin levels show low sensitivity, their high specificity may help differentiate between SA and other forms of arthritis (B-II).
- 7.
We suggest a complete blood count and assessment of liver and kidney function as part of the evaluation of patient severity at presentation, as they could influence the choice and dose of antibiotics (B-III).
- 1.
- VI.
What is the role of imaging in patients with suspected SA?
- 1.
Plain radiographs of the affected joint at baseline are suggested in all patients (B-II). Comment: Although not usually helpful for a SA diagnosis, they can show pre-existing joint or bone disease, rule out other diagnoses, and can be used as a reference image to assess future joint damage. Additional imaging is not usually necessary (D-III).
- 2.
Ultrasound is recommended to detect effusions when the physical examination is unclear, and to guide joint aspiration in joints that are difficult to examine, such as the hip or sacroiliac joint (A-II). In children with hip involvement and suspected transient synovitis, ultrasound of both joints is suggested, as bilateral hip effusion is a typical finding of transient synovitis of the hip that may support this diagnosis (B-II).
- 3.
Magnetic resonance imaging (MRI) is recommended for a suspected diagnosis of SA of axial joints (A-III), and when further imaging is needed for suspected spread of infection from the joint to adjacent soft tissues, and/or osteomyelitis (more common in children's joints) (A-II). In children, MRI may be indicated to differentiate transient synovitis of the hip from SA if the diagnosis remains in doubt after the initial evaluation and investigation (A-III).
- 4.
Computed tomography (CT) may be an alternative to MRI when the latter is not readily available (A-II), although CT should generally be avoided in children due to its high radiation index. CT may be an alternative to ultrasound to guide joint aspiration (B-III).
- 5.
Nuclear medicine examinations are not recommended for the diagnosis of SA (D-III).
- 1.
- VII.
General principles of management of SA
- 1.
As a general rule, patients with suspected or documented SA should be admitted to hospital (A-II). Some studies in children treated exclusively with oral outpatient antibiotics showed a favourable outcome when specific criteria were met (BII).
- 2.
Joint drainage is recommended for peripheral bacterial arthritis (except for gonococcal and early mycobacterial infections, which do not usually require joint drainage) and for fungal arthritis (A-II).
- 3.
We recommend joint drainage of large peripheral joints with pyogenic arthritis as soon as possible (A-II).
- 4.
While most patients with early diagnosis of axial joint infection do not require surgery (B-III), drainage of adjacent abscesses and various types of surgery for concomitant osteomyelitis may be necessary, especially if diagnosis is delayed (A-II). MRI is recommended to assess the presence of these complications (A-III).
- 5.
In haemodynamically stable patients without sepsis or septic shock and with clinical and laboratory findings of peripheral pyogenic arthritis, we recommend starting empirical antimicrobial therapy after obtaining blood cultures and SF aspirate, as well as intraoperative specimens if the patient is undergoing urgent surgery (A-II).
- 6.
In patients with haemodynamic instability, sepsis or septic shock, we suggest obtaining blood and SF for culture before starting antimicrobial therapy, if this does not significantly delay initiation of antimicrobial therapy (<45min) (B-III).
- 7.
We recommend that the definitive antibiotic regimen be based on the identified pathogen and its antimicrobial susceptibility or, if no pathogen is identified, on the most likely causative organism(s), to be discussed with an infectious disease specialist or clinical microbiologist whenever possible (A-II).
- 8.
We suggest starting antimicrobial therapy intravenously (B-III).
- 9.
It is recommended to switch to oral antibiotics after a few days (e.g., 2–7 days) of intravenous antibiotics in adults without endocarditis, with negative blood cultures and with clinical and laboratory improvement (provided that appropriate oral antimicrobials can be administered) (A-II). In children with a favourable clinical and analytical evolution after 2–4 days of intravenous antibiotics, switching to the oral route is strongly recommended (A-I).
- 10.
Total duration of antimicrobial treatment in adults without endocarditis:
- •
For large peripheral joints after drainage, we suggest 3–4 weeks for S. aureus (SA) and gram-negative bacilli (GNB), 2–3 weeks for streptococcal arthritis and 1–2 weeks for gonococcal arthritis (B-III).
- •
A longer duration is recommended for SA of axial joints (6 weeks) and SA with adjacent osteomyelitis (A-III) and is also suggested for patients with immunosuppression or a slow/inadequate response to initial treatment (B-III).
- •
Two weeks are recommended for SA of the wrist or hand joints after surgical drainage (this recommendation may not apply to SA caused by methicillin-resistant S. aureus [MRSA]) (A-I).
- •
- 11.
Total duration of antimicrobial treatment in children:
- •
We recommend 2–3 weeks for all uncomplicated SA in children, and 3–4 weeks for SA with osteomyelitis (A-I).
- •
Longer therapy (4–6 weeks) may be required in:
- ∘
Infections caused by MRSA (B-II), Salmonella, Enterobacterales or Pseudomonas aeruginosa (B-III)
- ∘
SA of axial joints (A-III)
- ∘
Newborns and young infants (<3 months) (B-III)
- ∘
Immunocompromised children (B-III)
- ∘
- •
- 1.
- VIII.
What is the recommended initial empirical antimicrobial therapy for SA?
- 1.
Empirical therapy active against S. aureus is always recommended in any patient (adults and children) with suspected SA and negative SF Gram stain (A-II). Additional empirical antimicrobial coverage may be necessary for other pathogens (A-III).
- 2.
In adults with negative SF Gram stain and no specific risk factors for special pathogens or resistant bacteria, we suggest coverage of S. aureus, streptococci and the more common GNB with:
- •
Cloxacillin plus ceftriaxone or monotherapy with amoxicillin–clavulanate (B-III).
- •
A glycopeptide or daptomycin combined with aztreonam or a fluoroquinolone in case of beta-lactam allergy (B-III).
Other options should be considered in the presence of certain risk factors or clinical contexts (B-III).
- •
- 3.
In children without specific risk factors for special pathogens or resistant bacteria and with a negative SF Gram stain, we recommend treatment as follows (A-II):
- •
<3 months: cloxacillin or cefazolin+cefotaxime or gentamicin (avoiding 2 cephalosporins together).
- •
3 months to 2 years: cefuroxime; alternatively, cloxacillin+cefotaxime or amoxicillin–clavulanate.
- •
2–4 years: cefazolin; alternatively, cefuroxime for coverage of Haemophilus influenzae and Streptococcus pneumoniae in under-vaccinated children.
- •
>4 years: cefazolin or cloxacillin.
- •
- 1.
- IX.
What is the definitive antimicrobial therapy for S. aureus SA?
- a)
In adults
- 1.
For methicillin-susceptible S. aureus, intravenous cloxacillin or cefazolin is recommended (A-II). Initial addition of daptomycin may be considered (C-III). Patients allergic to beta-lactams can be treated with vancomycin or daptomycin (A-II).
- 2.
Patients with MRSA SA can be treated with vancomycin or daptomycin (A-II) (initial combination of daptomycin plus a beta-lactam may be considered, C-III).
- 3.
Sequential oral treatment with beta-lactams, levofloxacin, clindamycin or linezolid are possible options, depending on isolate susceptibility and beta-lactam allergy (B-III).
- 4.
The use of rifampin for pure SA is not supported by pathogenesis or evidence. It could be considered in complicated cases with concomitant osteomyelitis (A-III).
- 1.
- b)
In children
- 1.
For methicillin-susceptible S. aureus, initial intravenous cefazolin or cloxacillin is recommended (A-II). Sequential oral treatment with a beta-lactam (i.e., cefadroxil) is recommended (A-II). Clindamycin (A-I), linezolid, levofloxacin (children >6 months), daptomycin (children >1 year) or vancomycin are alternatives for beta-lactam allergy (B-III).
- 2.
For MRSA, initial intravenous clindamycin is recommended if the isolate is susceptible (A-I). Otherwise, the most appropriate antibiotics are linezolid or daptomycin; a glycopeptide would be a valid but less suitable option (B-III). For sequential oral treatment, clindamycin (children >6–8 years) (AI), cotrimoxazole (B-II), levofloxacin (>6 months), or linezolid (B-III) are suggested, depending on isolate susceptibility.
- 1.
- a)
- X.
What is the definitive antimicrobial therapy for streptococcal SA?
- a)
In adults
- 1.
For SA caused by susceptible streptococci, penicillin is the drug of choice. Third-generation cephalosporins (ceftriaxone, cefotaxime) or ampicillin are good alternatives (A-II). In cases of allergy or reduced susceptibility, vancomycin, clindamycin, a fluoroquinolone, or linezolid may be used (B-III).
- 2.
For the oral treatment phase, amoxicillin, cefuroxime, levofloxacin, or moxifloxacin are all good options (A-III).
- 1.
- b)
In children
- 1.
For group A and group B streptococci, and penicillin-susceptible S. pneumoniae, initial intravenous penicillin or ampicillin are the recommended drugs of choice (A-III).
- 2.
Sequential oral treatment with amoxicillin is recommended (A-III).
- 3.
Third-generation cephalosporins (ceftriaxone, cefotaxime), levofloxacin (children >6 months), clindamycin, linezolid or vancomycin are alternatives depending on isolate susceptibility and beta-lactam allergies (C-III).
- 1.
- a)
- XI.
What is the definitive antimicrobial therapy for SA caused by gram-negative bacilli?
- a)
In adults
- 1.
For SA caused by susceptible GNB, initial treatment with an intravenous second- or third-generation cephalosporin is recommended (A-III). For GNB isolates resistant to third-generation cephalosporins, consultation with an infectious disease specialist is recommended (A-III). Initial treatment with aztreonam or a fluoroquinolone is suggested for beta-lactam allergies (B-III).
- 2.
Sequential oral treatment with ciprofloxacin is recommended whenever possible (A-III). Oral beta-lactams or cotrimoxazole are suggested alternative treatments, depending on the susceptibility of the GNB identified (B-III).
- 1.
- b)
In children
- 1.
K. kingae SA can be treated with penicillin or ampicillin. First- and second-generation cephalosporins or amoxicillin–clavulanate are good alternatives (A-II).
- 2.
For SA caused by other GNB, antimicrobial selection should be based on susceptibility (A-III).
- 1.
- a)
- XII.
What is the directed therapy for SA caused by other less common microorganisms?
- •
Candida spp. septic arthritis
- 1.
In surgically treated cases, we suggest 6–8-weeks of therapy with an azole, echinocandin or liposomal amphotericin B (A-III).
- 2.
In neonates with candida SA, an extent-of-disease study is suggested, including lumbar puncture and retinal examination (B-II).
- 1.
- •
Mycobacterium tuberculosis arthritis
- 1.
In patients with early diagnosis tuberculous arthritis (without large abscesses or bone sequestration), tuberculostatic treatment similar to that for tuberculosis at other sites is recommended. Some experts recommend longer treatment (9–12 months) (B-III).
- 2.
It is suggested that treatment be supervised by an expert (B-III).
- 1.
- •
Gonococcal arthritis
- 1.
In adults, we recommend ceftriaxone 1g every 24h (first choice) or cefotaxime 1g intravenously every 8h (alternative) (A-III). After clinical improvement, we suggest switching to an oral agent guided by antimicrobial susceptibility testing: ciprofloxacin 500mg/12h or cefixime 400mg/12h (B-III). Patients with gonococcal arthritis should be screened for other sexually transmitted infections (A-II).
- 2.
In children, we suggest 7 days of cefotaxime (neonates) or ceftriaxone (B-III).
- 1.
- •
- XIII.
What is the treatment for culture-negative septic arthritis?
- 1.
We suggest that culture-negative SA be treated with antimicrobial therapy similar to empirical therapy in patients with Gram stain-negative SF (B-III).
- 2.
In patients who are receiving or have recently received antibiotics, we advise considering antibiotic coverage to tailor antimicrobial therapy (B-III).
- 3.
An accurate epidemiological assessment is required to rule out uncommon or fastidious microorganisms (B-II).
- 1.
- XIV.
Is any adjuvant treatment recommended for SA?
- 1.
In children, nonsteroidal anti-inflammatory drugs may be beneficial during the acute phase while the signs of inflammation are present (A-III).
- 2.
In children with confirmed SA, early administration of a short course of intravenous corticosteroids may accelerate clinical recovery and reduce hospital stay (B-I). Comment: The potential impact of diagnostic delay on non-infectious arthritis and the long-term effects in SA are unclear.
- 3.
In adults, corticosteroid use is not recommended for SA due to the lack of clinical evidence on its effects (D-III).
- 1.
- XV.
What joint drainage procedures are recommended in patients with SA?
- 1.
Joint drainage to treat SA can be performed by closed-needle aspiration (repeated as necessary), arthroscopy or arthrotomy (open surgery) (A-III). We recommend tailoring the optimal drainage procedure to age, affected joint, extent of involvement, time course and other clinical data (A-III).
- 2.
In adults, arthroscopic joint drainage with synovectomy is the suggested first-line procedure for SA of the knee (B-II). Needle aspiration is another treatment option (B-II). For the ankle, elbow or wrist, initial joint drainage may be by needle aspiration or arthroscopy (B-III). For the hip and shoulder, arthroscopy or arthrotomy is the suggested initial procedure (B-II). Open surgery is suggested for cases with unfavourable evolution after repeated aspiration or arthroscopic drainage (B-III).
- 3.
In children, the suggested initial treatment procedure for uncomplicated SA of joints other than the hip is needle aspiration (B-I). For SA of the hip, knee, ankle, shoulder, elbow or wrist, arthroscopy is preferable to open surgery (B-II). We suggest joint drainage by arthrotomy as the first option for hip and shoulder SA in young children, and after more conservative procedures (needle aspiration or arthroscopy) have failed (C-III).
- 1.
- XVI.
What additional measures may be useful to improve the functional outcome of a patient with SA?
Suggestions include:
- 1.
Initiating physiotherapy after surgical joint drainage (B-III).
- 2.
Early mobilisation of the affected joint, initially with passive movement (B-III). In children with hip arthritis, immobilisation in an abduction spica cast is reserved for cases of severe infection at risk of joint dislocation (B-II).
- 3.
Early weight bearing – including partial weight bearing – is discouraged when the hip joint is affected (D-III).
- 4.
Early partial weight bearing is suggested for patients with knee SA, once the pain is controlled (B-III).
- 1.
- XVII.
How should patients be followed up and for how long?
- 1.
Outpatient follow-up with oral antimicrobial therapy (or outpatient parenteral antimicrobial therapy if oral treatment is not possible) is suggested once a favourable clinical and analytical evolution is established (B-III).
- 2.
Clinical (joint pain, inflammation and function) and analytical (blood count, CRP and erythrocyte sedimentation rate) monitoring is suggested (B-III). While patients are receiving antibiotics, we suggest monitoring for possible associated adverse effects (B-III).
- 3.
Outpatient follow-up by orthopaedic and infectious disease specialists is suggested at 1–2 weeks, 4–6 weeks and 3 months after discharge (C-III). We suggest a follow-up period of at least 1 year in adults at risk of long-term adverse outcomes and sequelae (such as those with impaired joint function and/or concomitant osteomyelitis) and in children (preferably by an experienced orthopaedic surgeon) (B-III). In infants with hip/physeal involvement, longer follow-up may be necessary (B-III).
- 1.
The GEIO, a study group belonging to the SEIMC, funded the English revision of the present document (by Janet Dawson, English native official translator). No other funding has been received to develop this manuscript.
Conflict of interestNB has received honoraria for lectures, presentations, or educational activities from MSD, Pfizer and Menarini, and support for attendance at meetings from MSD and Menarini. JLT has received honoraria for lectures, presentations, or educational activities from MSD, Advanz and Menarini, educational grants from MSD, Advanz, Menarini and Pfizer, a research grant from Advanz, and consulting fees from Angellini, TenNor Therapeutics and Debipharm. JPH has received honoraria for lectures, presentations, or educational events from MSD and Astra Zeneca, and for participation on advisory boards from MSD, Pfizer, Menarini, Tillots and Gilead. ENC has received honoraria for lectures, presentations, or educational events from Biomerieux. JC, MDT, LM, OM, DRP and BSD have received honoraria for lectures, presentations or educational events from MSD. The remaining authors report no conflicts of interest related to this document.
Coordinators: Natividad Benito (GEIO-SEIMC), Juan Carlos Martínez-Pastor (SECOT), Jesús Saavedra-Lozano (SEIP).
The complete consensus document is available in Supplementary Material.