The aim was to investigate the in vitro activity of ceftobiprole and dalbavancin against a collection of coagulase-negative staphylococci (CoNS) isolates with reduced susceptibility to daptomycin or resistant to linezolid and/or glycopeptides.
MethodsA total of 228 CoNS were tested using the Vitek-2 AST-626 cards (bioMérieux) and MIC of daptomycin, linezolid, vancomycin and teicoplanin were confirmed by Etest Strips (bioMérieux). Susceptibility testing for ceftobiprole and dalbavancin were performed by CLSI broth microdilution methodology. Results were interpreted according to 2021 EUCAST clinical breakpoints.
ResultsCeftobiprole and dalbavancin were active against 96.0% and 93.0% of CoNS, respectively, MIC90 were 2 and 0.125mg/L. MICs of ceptobiprole were higher against S. hominis and S. haemolyticus (MIC90 4mg/L). Dalbavancin exhibited higher MICs against S. haemolyticus and CoNS with reduced susceptibility to daptomycin and resistant to teicoplanin.
ConclusionCeftobiprole and dalbavancin demonstrated a high in vitro activity against our collection of CoNS isolates.
El objetivo fue evaluar la actividad in vitro de dalbavancina y ceftobiprol frente a estafilococos coagulasa negativos (ECN) con sensibilidad disminuida a daptomicina y/o resistentes a linezolid o glucopéptidos.
MétodosSe testó la sensibilidad de 228 ECN con tarjetas VITEK®2 AST-626 (bioMérieux) y las CMI de daptomicina, linezolid, vancomicina y teicoplanina fueron confirmadas con tiras Etest® (bioMérieux). El ensayo de sensibilidad frente a ceftobiprol y dalbavancina se realizó mediante microdilución en caldo (metodología CLSI). Los resultados se interpretaron siguiendo los puntos de corte de EUCAST 2021.
ResultadosCeftobiprol y dalbavancina fueron activos en el 96,0 y 93% de ECN, las CMI90 fueron 2 y 0,125mg/L, respectivamente. Las CMI de ceftobiprol fueron superiores en Staphylococcus hominis y Staphylococcus haemolyticus (CMI90 4mg/L). Dalbavancina exhibió mayores CMI en S. haemolyticus y en ECN con sensibilidad disminuida a daptomicina o resistentes a teicoplanina.
ConclusiónCeftobiprol y dalbavancina han demostrado una potente actividad in vitro frente a esta colección de ECN.
The increasing prevalence of drug-resistant Gram-positive cocci requires new agents to treat these infections. Ceftobiprole is a fifth-generation cephalosporin with a broad spectrum of antimicrobial activity, including Gram-positive and Gram-negative pathogens. Just like other β-lactams, it exhibits an inhibitory action on peptidoglycan transpeptidases by binding to penicillin-binding proteins (PBPs). Ceftobiprole has a high affinity for PBP2a of methicillin-resistant Staphylococcus aureus and coagulase-negative staphylococci (CoNS), which represents an important advantage. Ceftobiprole is approved for the treatment of community and hospital-acquired pneumonia, excluding ventilator-associated pneumonia.1,2
Dalbavancin is a semi-synthetic lipoglycopeptide antibiotic and it has excellent bactericidal activity against Gram-positive pathogens, including methicillin-resistant staphylococci. Dalbavancin binds to the terminal carbon of the d-alanyl-d-alanine peptide and inhibits the last stages of cell wall synthesis. Unlike other glycopeptides, it has a lipophilic chain that binds to the bacterial cellular membrane, thus enhancing its activity. Dalbavancin has been approved for treatment of acute bacterial skin infections.3,4
Methicillin-resistant CoNs are among the main causes of nosocomial infections.5 The large proportion of methicillin-resistant CoNS strains and the emergence of strains with reduced susceptibility to daptomycin or resistant to glycopeptides and/or linezolid are a global concern.5 Furthermore, new antibiotics such as ceftobiprole and dalbavancin have been introduced for the treatment of severe infections caused by these microorganisms.
The aim of this study was to investigate the in vitro activity of ceftobiprole and dalbavancin against a collection of CoNS isolates with reduced susceptibility to daptomycin or resistant to linezolid, vancomycin and/or teicoplanin.
MethodsBacterial isolatesA total of 228 non-duplicate CoNS isolates from clinical samples, collected between January 2012 and March 2016 at Marques de Valdecilla University Hospital (Spain), were studied. All isolates were tested using the Vitek-2 AST-626 cards (bioMérieux, France) and subsequently stored in vials of tryptic soy broth with glycerol at −80°C. At the time of the study, the strains were thawed and the minimal inhibitory concentration (MIC) of daptomycin, linezolid, vancomycin and teicoplanin were confirmed by Etest Strips (bioMérieux, France) according to 2021 EUCAST breakpoints6: 7 strains with reduced daptomycin susceptibility (2mg/L); 111 linezolid resistant (range: 8–256mg/L); 115 teicoplanin resistant (range: 8–64mg/L) and 1 strain vancomycin resistant (8mg/L).
The species included in the study were Staphylococcus epidermidis (n=187), Staphylococcus hominis (22), Staphylococcus haemolyticus (16), Staphylococcus warneri (3) and Staphylococcus capitis (1).
The isolates were recovered from blood (significant bacteremia, 106; 46.5%), skin and soft tissues (43; 18.9%), abdominal specimens (24; 10.5%), osteoarticular specimens (19; 8.3%), cerebrospinal fluid (17; 7.5%), urine (13; 5.7%) and respiratory tract (6; 2.6%). Microorganisms were identified at the species level by MALDI-TOF MS (Vitek MS, bioMerieux).
Ceftobiprole and dalbavancin susceptibility testingSusceptibility testing for ceftobiprole (Basilea Pharma) and dalbavancin (Med Chem Express) was performed following the Clinical and Laboratory Standards Institute (CLSI) broth microdilution methodology. Custom plates were prepared manually in the laboratory and incubated at 35±2°C for 16–20h in ambient atmosphere. MIC of dalbavancin was determined in the presence of polysorbate-80 (0.002%) according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations.6,7
Inocula were prepared from 18hours blood agar plates by direct colony suspension and contained 5×105CFU/mL. Quality control strain S. aureus ATCC 29213 was included in each set of experiments to assure proper test conditions and procedures.
Results were interpreted according to EUCAST breakpoints version 11.0, January 2021.6 Dalbavancin: susceptible ≤0.125mg/L, resistant>0.125mg/L. In the case of ceftobiprole, breakpoints for S. aureus were used (susceptible≤2mg/L, resistant>2mg/L).
Statistical analysisDifferences between MICs were analyzed using Kruskal–Wallis and Bonferroni tests. P-value<0.05 was considered statistically significant. Statistical analysis was performed using SPSS-Statistics version 20.0 (IBM-SPSS, Chicago, IL, USA).
ResultsAntimicrobials were tested against 228 CoNS isolates from clinical samples. MICs distributions for ceftobiprole and dalbavancin are shown in Tables 1 and 2.
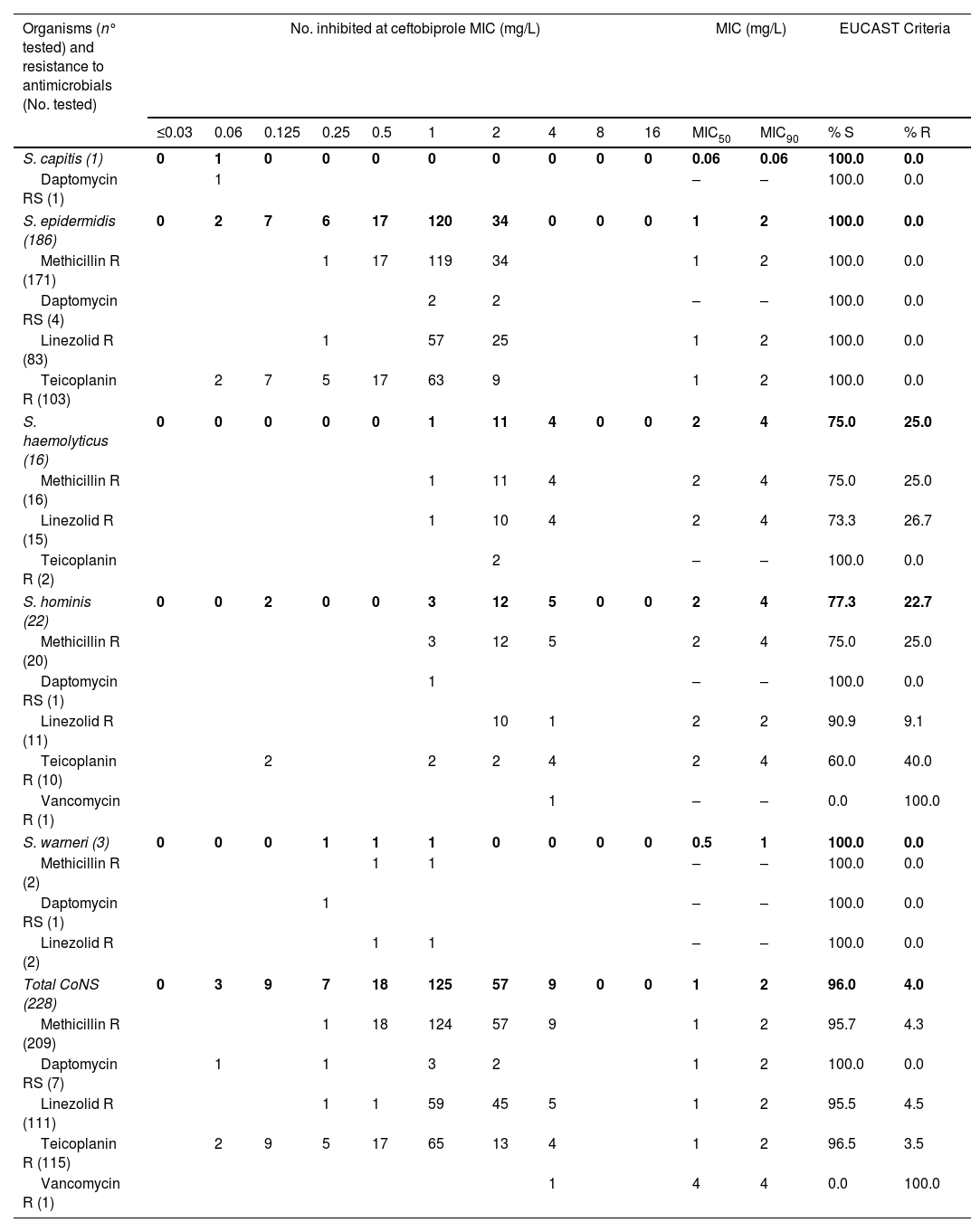
In vitro activity of ceftobiprole against coagulase-negative staphylococci with different resistance phenotypes.
| Organisms (n° tested) and resistance to antimicrobials (No. tested) | No. inhibited at ceftobiprole MIC (mg/L) | MIC (mg/L) | EUCAST Criteria | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | MIC50 | MIC90 | % S | % R | |
| S. capitis (1) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.06 | 0.06 | 100.0 | 0.0 |
| Daptomycin RS (1) | 1 | – | – | 100.0 | 0.0 | |||||||||
| S. epidermidis (186) | 0 | 2 | 7 | 6 | 17 | 120 | 34 | 0 | 0 | 0 | 1 | 2 | 100.0 | 0.0 |
| Methicillin R (171) | 1 | 17 | 119 | 34 | 1 | 2 | 100.0 | 0.0 | ||||||
| Daptomycin RS (4) | 2 | 2 | – | – | 100.0 | 0.0 | ||||||||
| Linezolid R (83) | 1 | 57 | 25 | 1 | 2 | 100.0 | 0.0 | |||||||
| Teicoplanin R (103) | 2 | 7 | 5 | 17 | 63 | 9 | 1 | 2 | 100.0 | 0.0 | ||||
| S. haemolyticus (16) | 0 | 0 | 0 | 0 | 0 | 1 | 11 | 4 | 0 | 0 | 2 | 4 | 75.0 | 25.0 |
| Methicillin R (16) | 1 | 11 | 4 | 2 | 4 | 75.0 | 25.0 | |||||||
| Linezolid R (15) | 1 | 10 | 4 | 2 | 4 | 73.3 | 26.7 | |||||||
| Teicoplanin R (2) | 2 | – | – | 100.0 | 0.0 | |||||||||
| S. hominis (22) | 0 | 0 | 2 | 0 | 0 | 3 | 12 | 5 | 0 | 0 | 2 | 4 | 77.3 | 22.7 |
| Methicillin R (20) | 3 | 12 | 5 | 2 | 4 | 75.0 | 25.0 | |||||||
| Daptomycin RS (1) | 1 | – | – | 100.0 | 0.0 | |||||||||
| Linezolid R (11) | 10 | 1 | 2 | 2 | 90.9 | 9.1 | ||||||||
| Teicoplanin R (10) | 2 | 2 | 2 | 4 | 2 | 4 | 60.0 | 40.0 | ||||||
| Vancomycin R (1) | 1 | – | – | 0.0 | 100.0 | |||||||||
| S. warneri (3) | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0.5 | 1 | 100.0 | 0.0 |
| Methicillin R (2) | 1 | 1 | – | – | 100.0 | 0.0 | ||||||||
| Daptomycin RS (1) | 1 | – | – | 100.0 | 0.0 | |||||||||
| Linezolid R (2) | 1 | 1 | – | – | 100.0 | 0.0 | ||||||||
| Total CoNS (228) | 0 | 3 | 9 | 7 | 18 | 125 | 57 | 9 | 0 | 0 | 1 | 2 | 96.0 | 4.0 |
| Methicillin R (209) | 1 | 18 | 124 | 57 | 9 | 1 | 2 | 95.7 | 4.3 | |||||
| Daptomycin RS (7) | 1 | 1 | 3 | 2 | 1 | 2 | 100.0 | 0.0 | ||||||
| Linezolid R (111) | 1 | 1 | 59 | 45 | 5 | 1 | 2 | 95.5 | 4.5 | |||||
| Teicoplanin R (115) | 2 | 9 | 5 | 17 | 65 | 13 | 4 | 1 | 2 | 96.5 | 3.5 | |||
| Vancomycin R (1) | 1 | 4 | 4 | 0.0 | 100.0 | |||||||||
MIC, minimum inhibitory concentration; MIC50/90, MICs required to inhibit 50% and 90% of the isolates, respectively; S, susceptible; R, resistant; RS, reduced susceptibility; EUCAST ceftobiprole clinical breakpoint R>2mg/L. S. aureus breakpoints were assumed for CoNS.
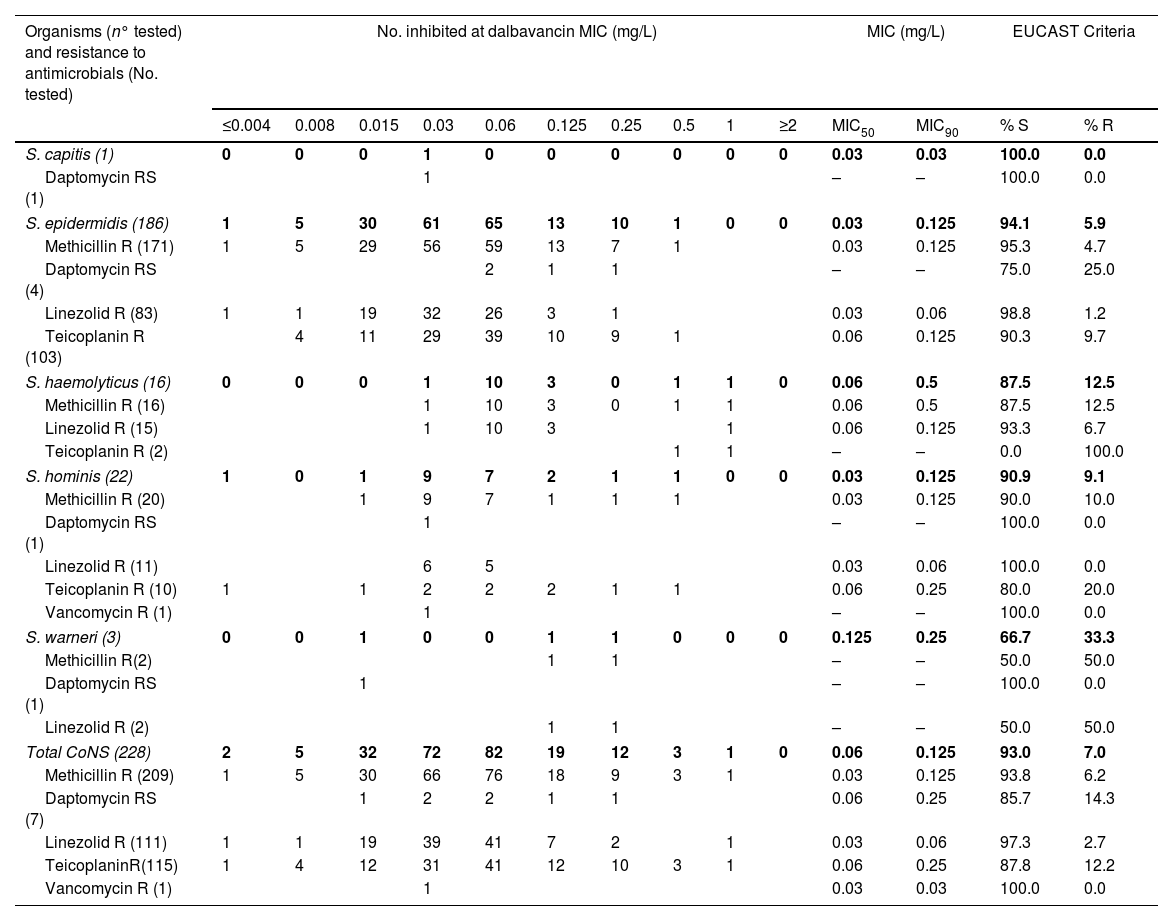
In vitro activity of dalbavancin against coagulase-negative staphylococci with different resistance phenotypes.
| Organisms (n° tested) and resistance to antimicrobials (No. tested) | No. inhibited at dalbavancin MIC (mg/L) | MIC (mg/L) | EUCAST Criteria | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.004 | 0.008 | 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | ≥2 | MIC50 | MIC90 | % S | % R | |
| S. capitis (1) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.03 | 0.03 | 100.0 | 0.0 |
| Daptomycin RS (1) | 1 | – | – | 100.0 | 0.0 | |||||||||
| S. epidermidis (186) | 1 | 5 | 30 | 61 | 65 | 13 | 10 | 1 | 0 | 0 | 0.03 | 0.125 | 94.1 | 5.9 |
| Methicillin R (171) | 1 | 5 | 29 | 56 | 59 | 13 | 7 | 1 | 0.03 | 0.125 | 95.3 | 4.7 | ||
| Daptomycin RS (4) | 2 | 1 | 1 | – | – | 75.0 | 25.0 | |||||||
| Linezolid R (83) | 1 | 1 | 19 | 32 | 26 | 3 | 1 | 0.03 | 0.06 | 98.8 | 1.2 | |||
| Teicoplanin R (103) | 4 | 11 | 29 | 39 | 10 | 9 | 1 | 0.06 | 0.125 | 90.3 | 9.7 | |||
| S. haemolyticus (16) | 0 | 0 | 0 | 1 | 10 | 3 | 0 | 1 | 1 | 0 | 0.06 | 0.5 | 87.5 | 12.5 |
| Methicillin R (16) | 1 | 10 | 3 | 0 | 1 | 1 | 0.06 | 0.5 | 87.5 | 12.5 | ||||
| Linezolid R (15) | 1 | 10 | 3 | 1 | 0.06 | 0.125 | 93.3 | 6.7 | ||||||
| Teicoplanin R (2) | 1 | 1 | – | – | 0.0 | 100.0 | ||||||||
| S. hominis (22) | 1 | 0 | 1 | 9 | 7 | 2 | 1 | 1 | 0 | 0 | 0.03 | 0.125 | 90.9 | 9.1 |
| Methicillin R (20) | 1 | 9 | 7 | 1 | 1 | 1 | 0.03 | 0.125 | 90.0 | 10.0 | ||||
| Daptomycin RS (1) | 1 | – | – | 100.0 | 0.0 | |||||||||
| Linezolid R (11) | 6 | 5 | 0.03 | 0.06 | 100.0 | 0.0 | ||||||||
| Teicoplanin R (10) | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 0.06 | 0.25 | 80.0 | 20.0 | |||
| Vancomycin R (1) | 1 | – | – | 100.0 | 0.0 | |||||||||
| S. warneri (3) | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0.125 | 0.25 | 66.7 | 33.3 |
| Methicillin R(2) | 1 | 1 | – | – | 50.0 | 50.0 | ||||||||
| Daptomycin RS (1) | 1 | – | – | 100.0 | 0.0 | |||||||||
| Linezolid R (2) | 1 | 1 | – | – | 50.0 | 50.0 | ||||||||
| Total CoNS (228) | 2 | 5 | 32 | 72 | 82 | 19 | 12 | 3 | 1 | 0 | 0.06 | 0.125 | 93.0 | 7.0 |
| Methicillin R (209) | 1 | 5 | 30 | 66 | 76 | 18 | 9 | 3 | 1 | 0.03 | 0.125 | 93.8 | 6.2 | |
| Daptomycin RS (7) | 1 | 2 | 2 | 1 | 1 | 0.06 | 0.25 | 85.7 | 14.3 | |||||
| Linezolid R (111) | 1 | 1 | 19 | 39 | 41 | 7 | 2 | 1 | 0.03 | 0.06 | 97.3 | 2.7 | ||
| TeicoplaninR(115) | 1 | 4 | 12 | 31 | 41 | 12 | 10 | 3 | 1 | 0.06 | 0.25 | 87.8 | 12.2 | |
| Vancomycin R (1) | 1 | 0.03 | 0.03 | 100.0 | 0.0 | |||||||||
MIC, minimum inhibitory concentration; MIC50/90, MICs required to inhibit 50% and 90% of the isolates, respectively; S, susceptible; R, resistant; RS, reduced susceptibility; EUCAST dalbavancin clinical breakpoint R>0.125mg/L.
In the case of ceftobiprole, 219 (96.0%) CoNS isolates were susceptible and 9 (4.0%) were resistant (4 S. haemolyticus and 5 S. hominis), with MICs of 4mg/L. For dalbavancin, 16 (7.0%) CoNS were not susceptible, with MICs of 0.25–1mg/L (11 S. epidermidis, 2 S. haemolyticus, 2 S. hominis and 1 S. warneri).
In the collection, 7/228 CoNS isolates showed reduced susceptibility to daptomycin. All of them were susceptible to ceftobiprole, MIC50 and MIC90 values were 1 and 2mg/L respectively. Dalbavancin MIC50 and MIC90 were 0.06 and 0.25mg/L and one strain (S. epidermidis) resulted resistant to dalbavancin (MIC 0.25mg/L).
Of all isolates tested, 111 (48.7%) were resistant to linezolid. Five isolates (4.5%) resistant to linezolid showed resistance to ceftobiprole (4 S. haemolyticus and 1 S. epidermidis), and 3 CoNS were resistant to dalbavancin. The MIC50/90 were 1/2mg/L for ceftobiprole and 0.03/0.06mg/L for dalbavancin.
Ceftobiprole was active against 96.5% of CoNS resistant to teicoplanin and the MIC50/90 were 1/2mg/L. All isolates resistant to ceftobiprole corresponded to S. hominis. In addition, the only strain resistant to vancomycin (S. hominis) was also resistant to ceftobiprole, showing a MIC of 4mg/L, and susceptible to dalbavancin (MIC 0.03mg/L).
The dalbavancin MIC range in glycopeptide-resistant strains was ≤0.004–1mg/L and MIC50/90 values were 0.06mg/L and 0.25mg/L respectively. The percentage of resistance was 12.2% and the species that showed higher MICs were S. haemolyticus and S. hominis.
DiscussionTreatment of infections caused by CoNS may be difficult because there are strains resistant to multiple antibiotics.5 In our work, 96.0% of CoNS showed susceptibility to ceftobiprole and 93.0% to dalbavancin. Ceftobiprole MICs were significantly higher in S. hominis and S. haemolyticus than in S. epidermidis (p<0.05), whilst dalbavancin exhibited higher MICs in S. haemolyticus than S. epidermidis (p<0.05) as well as against CoNS resistant to teicoplanin and with reduced susceptibility to daptomycin. In the latter cases, the observed differences were not significant (p>0.05).
Heriksen et al. studied 650 CoNS and reported a ceftobiprole MIC50/90 of 1/2mg/L and 100% susceptibility.8 In a study of Pfaller et al., ceftobiprole was tested against 439 CoNS and 100.0% of strains were susceptible, with a MIC50/90 of 0.5/1mg/L.9
A ceftobiprole surveillance study in Europe published resistance rates of 9.0% for methicillin-resistant CoNS.10 Another study that included methicillin-resistant CoNS collected in 2015 in Europe reported resistance rates of 14.3%.11 In our study, ceftobiprole resistance rates against methicillin-resistant CoNS strains was lower (4.3%).
In a study by Sader et al. regarding dalbavancin activity against a set of 5008 CoNS strains from USA and Europe (2014–2018), MIC50/90 were 0.03/0.06mg/L and 99.1% of the strains were susceptible. S. haemolyticus and S. saprophyticus were the species with highest MICs (MIC90 0.12mg/L).12 In our study, dalbavancin MICs against S. haemolyticus were also higher than against other CoNS species (MIC90 0.5mg/L). These results show that antimicrobial susceptibility may vary according to the species studied.
In another study, dalbavancin MIC90 was 0.25mg/L against 15 teicoplanin resistant CoNS. It was active but showed higher MICs than against linezolid resistant staphylococci (MICs≤0.06mg/L).13 Comparing with our results, MICs (MIC90 0.25mg/L) against CoNS strains resistant to teicoplanin and these with reduced susceptibility to daptomycin were also higher compared to isolates showing methicillin and linezolid resistance.
A study published in 2018 that included 1992 CoNS showed that dalbavancin was the most active agent against CoNS with MIC90 0.06mg/L and only 0.4% of strains resistant. The most common species of CoNS were S. epidermidis and S. lugdunensis, of which 99.7% and 100.0%, were dalbavancin susceptible, respectively.14 Our results showed a higher MIC90 (0.125mg/L) and a resistance rate of 7.0%. Differences may be explained by the composition of CoNS study collections.
According to the data reported in the literature, CoNS have shown a low potential to develop resistance to ceftobiprole and dalbavancin.
Our results are consistent with data previously published by other authors, but it is nevertheless important to carry out further studies evaluating the susceptibility of ceftobiprole and dalbavancin in different CoNS species and in multi-resistant strains.
In conclusion, ceftobiprole and dalbavancin demonstrated a high in vitro activity against CoNS isolates with reduced susceptibility to daptomycin or resistant to linezolid and/or glycopeptides. Both may be a good therapeutic alternative in infections caused by these microorganisms. Therefore, further studies are required so as to expand the clinical indications for these antimicrobials.
FinancingThis research has not received specific aid from public sector agencies, the commercial sector or non-profit entities.
Conflicts of interestThe authors declare no conflict of interest.