Disk diffusion is a well standardized method that provides reliable categorical results to guide antimicrobial therapy in numerous types of infections. Based on the guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST), which are widely implemented in Spain, the Spanish Antibiogram Committee (COESANT) has drawn up recommendations for antimicrobial selection by the disk diffusion technique, including selective reporting and its use for the detection of resistance mechanisms. Factors affecting disk diffusion results, along with advantages and shortcomings of the method, are also discussed.
La difusión con discos es un método estandarizado que proporciona resultados fiables para guiar la terapia antimicrobiana en numerosos tipos de infecciones. En base a las directrices del European Committee on Antimicrobial Susceptibility Testing (EUCAST), ampliamente implantadas en España, el Comité Español del Antibiograma (COESANT) ha elaborado recomendaciones para la selección de antimicrobianos para ser estudiados mediante la técnica de difusión con discos, su notificación selectiva en el informe de sensibilidad y su uso para la detección de mecanismos de resistencia. También se discuten los factores que afectan los resultados obtenidos mediante la técnica de difusión con discos junto con las ventajas y desventajas del método.
Disk diffusion is a conventional phenotypic method for antimicrobial susceptibility testing that combines simplicity and cost-effectiveness. Even though automated antimicrobial systems are now widely employed in many clinical microbiology laboratories, the disk diffusion method is still in use, as it provides reliable qualitative results to guide antimicrobial therapy in numerous types of infections. Rigorous compliance with the methodology, the use of high-quality reagents (culture media and antibiotic-containing disks) together with an adequate quality control allow accurate and reproducible results to be obtained.1–3
The method is based on the diffusion of a predefined amount of a given antimicrobial agent contained in a paper disk or tablet, which is placed on the surface of an agar plate previously inoculated with a standardized inoculum of the microorganism. As the drug diffuses, it creates a gradient of concentration, leading to the formation of inhibition zones where the concentration is sufficient to inhibit the growth of the inoculated microorganism. The inhibition-zone diameters are classified into susceptibility categories according to regularly updated clinical breakpoints.4,5 Several factors influence the zone diameters, which can be related to the drug (disk content, diffusion rate, activity against the tested isolate), agar (depth, composition), incubation conditions (temperature, duration, atmosphere) or microorganism (growth rate, inoculum density). Disk diffusion methodology guidelines are provided by the CLSI (Clinical and Laboratory Standards Institute) and EUCAST (European Committee on Antimicrobial Susceptibility Testing), with some differences regarding the testing media for fastidious organisms and the disk concentration for certain antimicrobial agents.6,7 Fortunately, CLSI and EUCAST have created a joint disk-diffusion working group to develop standardized recommendations for disk content selection, which will henceforth be applied to new antimicrobial agents.8,9
For each bacterium–drug combination, clinical breakpoints for interpreting zone diameter are commonly set by selecting an optimal disk content and evaluating the correlation between the minimum inhibitory concentration (MIC) values obtained by reference methods and the inhibition zone diameters.10,11 In 2019, EUCAST revised interpretative clinical categories, redefining category I (susceptible, increased exposure) and establishing arbitrary “off-scale” breakpoints for some species/agents corresponding to a zone diameter of S≥50mm, which indicates that the microorganism may require a high exposure to the drug.
A relevant advantage of the disk diffusion technique is its flexibility in the choice of antimicrobials as opposed to commercial systems in which both the antimicrobials and their concentrations are chosen by the manufacturer. Thus, disk panels can be readily modified according to the specific needs of each center. Moreover, the incorporation of new antimicrobials into disk diffusion antibiograms is easier and less time-consuming compared with commercial panels, which also tend to be costly.12 In contrast, disk diffusion is considered the least expensive of all susceptibility methods. Another benefit is that growth visualization allows the detection of inoculum adequacy, mixed cultures, heteroresistance and interactions between antibiotics as synergy or antagonism effects, which is highly useful for the phenotypic detection of resistance mechanisms. Moreover, interpretive reading of the antibiogram and education can be an advantage of disk diffusion due to its easy adaptability.
One of the main reasons against the use of the disk diffusion method is that it does not provide MIC values, which are used in pharmacokinetic/pharmacodynamic (PK/PD) models for personalized antibiotic therapy, especially in critically ill patients.13 However, as occurs in disk diffusion, MIC determination is inherently variable, influenced by biological factors (strain-to-strain differences) and the type of assay (accepted variation of one doubling dilution), and therefore MIC values cannot be considered as absolute.14
Although the qualitative results obtained by disk diffusion are considered adequate for most infections, choosing the most suitable antibiotic treatment regimen for some types of infections (e.g., endocarditis) or microorganisms (e.g., carbapenemase-producing Enterobacterales) requires establishing MIC values.15 MIC determination can also be useful for the confirmation of unexpected results, when the disk diffusion result falls within the newly defined EUCAST area of technical uncertainty (ATU) or in cases where disk diffusion is unreliable (e.g. vancomycin and staphylococci).
Another shortcoming is that although the method has been validated against the most common bacteria, as stated by the international committees EUCAST and CLSI, it is not yet well standardized against some fastidious and/or slow-growing microbes. It is worth noting that ongoing EUCAST research on setting new disk breakpoints has led to the development of a new disk diffusion method for rapidly growing anaerobic bacteria, and the breakpoints have been published in the 2022 EUCAST document.5,16
Furthermore, the disk diffusion method is labor-intensive, not fully automated, and the interpretation of the inhibition zone diameters is subject to inter-observer variation. The implementation of equipment that allows automatic reading and interpretation of inhibition zone diameters, such as Sirscan (i2a, Montpellier, France), ADAGIO (Bio-Rad, Marnes-la-Coquette, France), or BIOMIC (Giles Scientific Inc., Santa Barbara, USA), has gone some way to address these problems.17–19 This equipment incorporates an expert system to improve the quality of interpretation and allows the storage and further management of data. Disk diffusion could also benefit from automatization of the entire process, including inoculum preparation, streaking of media plates, incubation, and reading, as demonstrated by Cherkaoui et al. using the WASPLab™ system (Copan, Brescia, Italy).20 A study analyzing the most frequently encountered pathogens in blood cultures has shown that this device could also allow early reading (6–12h).21
A recently introduced improvement of the disk diffusion method has been the standardization of rapid susceptibility testing for bloodstream infections with the aim of shortening the turnaround time. The EUCAST rapid method performed directly from positive blood culture bottles provides reliable results within 4–8h and so far has been validated for seven pathogens.22
In 2020, the Spanish Antibiogram Committee (COESANT) and the Study Group on Mechanisms of Action and Resistance to Antimicrobial Agents (GEMARA) from the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) published recommendations for the inclusion of antimicrobials and the selection of concentration ranges in automated systems according to the clinical breakpoints and the epidemiological breakpoints (ECOFF) defined by EUCAST.23
Similarly, the objective here is to provide recommendations on the antimicrobials to be studied using the diffusion disk technique and suggestions for selective reporting. The usefulness of the method for the detection of resistance mechanisms is also outlined.
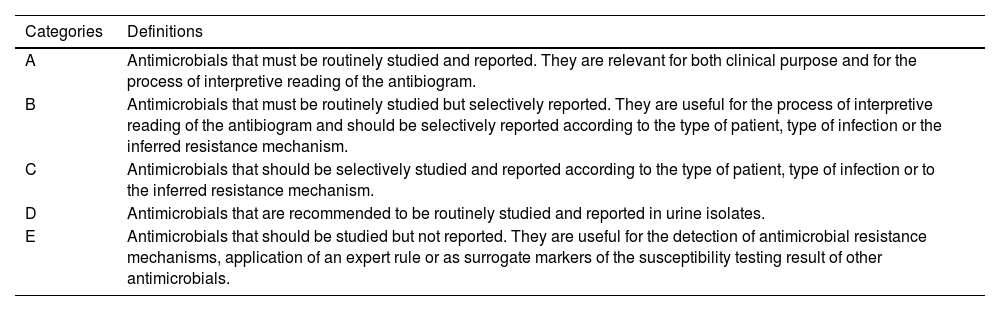
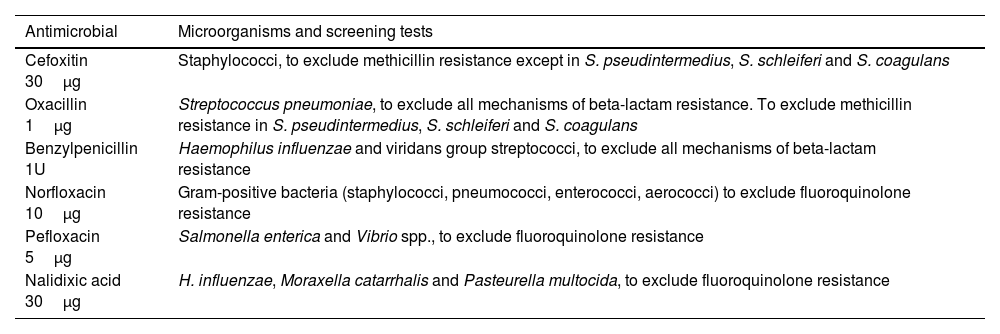
General recommendations for antimicrobial susceptibility testing using the disk diffusion methodThe lists of antimicrobial agents to be tested and reported are shown in supplementary tables S1-S11. Antimicrobials have been divided into five categories (A to E) along with recommendations of testing and selective reporting (Table 1).23 These recommendations should be adapted to each individual center according to the institutional formulary and local antibiotic stewardship programs. Antimicrobial selection criteria are based on microbiological, clinical and PK/PD data, as stated in the previous COESANT document.23 It should be noted that some antimicrobial agents (category E) are used to detect resistance mechanisms or as surrogate markers to extrapolate results for other agents and should not be reported. According to EUCAST guidelines, the list of disk diffusion screening tests that can be used for these purposes is included in Table 2.24
Categories used for the inclusion and reporting of antimicrobial agents in susceptibility testing by disk diffusion.23
| Categories | Definitions |
|---|---|
| A | Antimicrobials that must be routinely studied and reported. They are relevant for both clinical purpose and for the process of interpretive reading of the antibiogram. |
| B | Antimicrobials that must be routinely studied but selectively reported. They are useful for the process of interpretive reading of the antibiogram and should be selectively reported according to the type of patient, type of infection or the inferred resistance mechanism. |
| C | Antimicrobials that should be selectively studied and reported according to the type of patient, type of infection or to the inferred resistance mechanism. |
| D | Antimicrobials that are recommended to be routinely studied and reported in urine isolates. |
| E | Antimicrobials that should be studied but not reported. They are useful for the detection of antimicrobial resistance mechanisms, application of an expert rule or as surrogate markers of the susceptibility testing result of other antimicrobials. |
Antimicrobial agents (category E) used to detect resistance mechanisms or as surrogates for the results of other agents and screening tests.24
| Antimicrobial | Microorganisms and screening tests |
|---|---|
| Cefoxitin 30μg | Staphylococci, to exclude methicillin resistance except in S. pseudintermedius, S. schleiferi and S. coagulans |
| Oxacillin 1μg | Streptococcus pneumoniae, to exclude all mechanisms of beta-lactam resistance. To exclude methicillin resistance in S. pseudintermedius, S. schleiferi and S. coagulans |
| Benzylpenicillin 1U | Haemophilus influenzae and viridans group streptococci, to exclude all mechanisms of beta-lactam resistance |
| Norfloxacin 10μg | Gram-positive bacteria (staphylococci, pneumococci, enterococci, aerococci) to exclude fluoroquinolone resistance |
| Pefloxacin 5μg | Salmonella enterica and Vibrio spp., to exclude fluoroquinolone resistance |
| Nalidixic acid 30μg | H. influenzae, Moraxella catarrhalis and Pasteurella multocida, to exclude fluoroquinolone resistance |
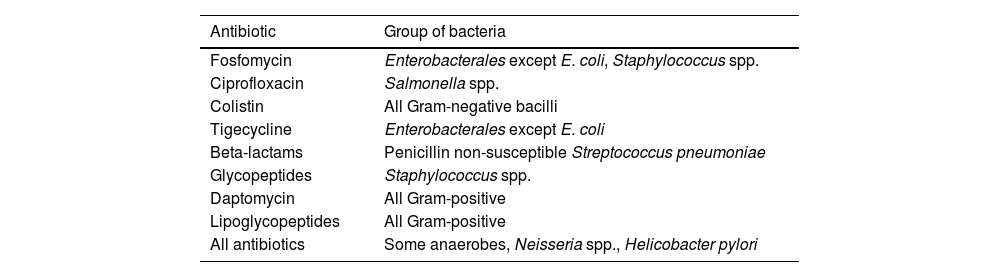
Susceptibility testing by the disk diffusion method is challenging for some drugs, such as colistin and daptomycin, due to their molecular size and physico-chemical traits, and the results for some anaerobes, Neisseria and Helicobacter are unreliable, in which case an MIC method should be used (Table 3).15 There are also some drug-organism combinations for which both disk and MIC EUCAST breakpoints are lacking. In these cases, EUCAST recommends using the PK/PD non-species-related breakpoints only available as MIC values.25 EUCAST also recommends referring to the epidemiologic cutoff (ECOFF) values to determine whether the MIC against the targeted isolate is consistent with the wild type MIC distribution against the species. There may still be cases in which the use of breakpoints defined by other organizations such as the Clinical and Laboratory Standards Institute (CSLI), the Societe Francaise de Microbiologie (SFM) or the U.S. Food and Drug Administration (FDA) could be an alternative when EUCAST breakpoints are unavailable. In these cases, methodological recommendations of each organization regarding disk content and testing media must be taken into account.
Current antibiotic–organism combinations without disk diffusion breakpoints that require MIC determination. (Adapted from ref. 15).
| Antibiotic | Group of bacteria |
|---|---|
| Fosfomycin | Enterobacterales except E. coli, Staphylococcus spp. |
| Ciprofloxacin | Salmonella spp. |
| Colistin | All Gram-negative bacilli |
| Tigecycline | Enterobacterales except E. coli |
| Beta-lactams | Penicillin non-susceptible Streptococcus pneumoniae |
| Glycopeptides | Staphylococcus spp. |
| Daptomycin | All Gram-positive |
| Lipoglycopeptides | All Gram-positive |
| All antibiotics | Some anaerobes, Neisseria spp., Helicobacter pylori |
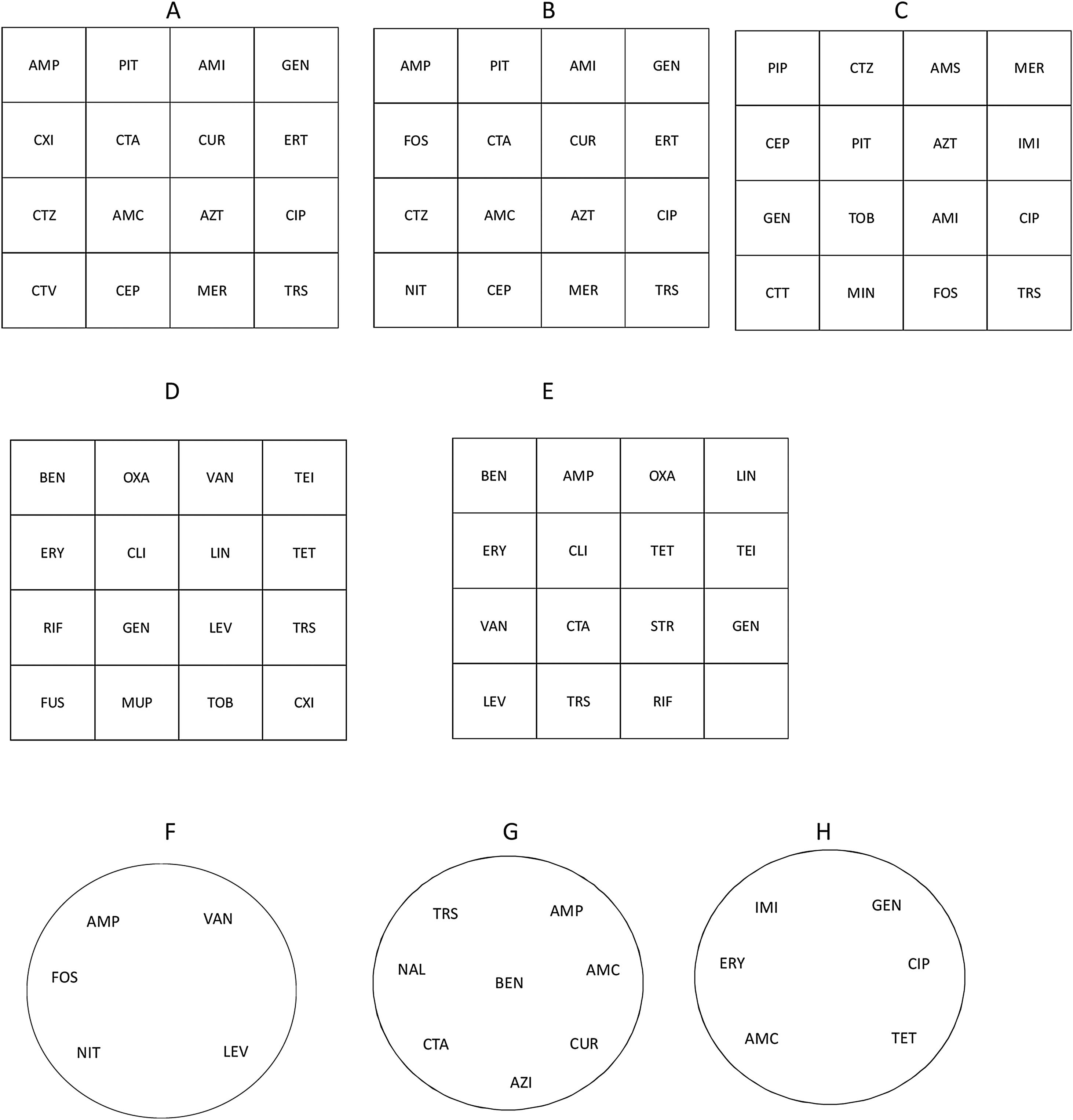
Additional recommendations for the detection of resistance mechanisms in the primary panel by the disk diffusion method and other peculiarities of different bacterial groups are outlined below. Suggested disk diffusion panels for Enterobaterales, Enterobacterales from urinary tract infections (UTI), Pseudomonas spp., Staphylococcus spp., Streptococcus spp./Enterococcus spp., Enterococcus spp. from UTI, Haemophilusinfluenzae/Haemophilus parainfluenzae and Campylobacterjejuni/Campylobactercoli as well as the recommended media are shown in Fig. 1.
Panel distribution of antimicrobial agents to be tested by disk diffusion against Enterobacterales (A), Enterobacterales from urinary tract infections (UTI) (B), Pseudomonas spp. (C), Staphylococcus spp. (D), Streptococcus spp./Enterococcus spp. (E), Enterococcus spp. from UTI (F), Haemophilus influenzae/Haemophilus parainfluenzae (G), and Campylobacter jejuni/Campylobacter coli (H). Figures refer to square (A–E) or round (F–H) plates. Recommended media: Mueller-Hinton agar for Enterobacterales, Pseudomonas spp., Staphylococcus spp., Enterococcus spp.; Mueller-Hinton agar+5% defibrinated horse blood and 20mg/L β-NAD (MH-F) for Streptococcus spp., Haemophilus influenzae/Haemophilus parainfluenzae and Campylobacter jejuni/Campylobacter coli.
AMC: amoxicillin-clavulanate, AMI: amikacin, AMP: ampicillin, AMS: ampicillin-sulbactam, AZI: azithromycin, AZT: aztreonam, BEN: benzylpenicillin, CEP: cefepime, CIP: ciprofloxacin, CLI: clindamycin, CTA: cefotaxime, CTT: ceftolozane-tazobactam, CTV: ceftazidime-avibactam, CTZ: ceftazidime, CUR: cefuroxime, CXI: cefoxitin, ERT: ertapenem, ERY: erythromycin, FOS: fosfomycin, FUS: fusidic acid, GEN: gentamicin, IMI: imipenem, LEV: levofloxacin, LIN: linezolid, MER: meropenem, MIN: minocycline, MUP: mupirocin, NAL: nalidixic acid, NIT: nitrofurantoin, OXA: oxacillin, PIP: piperacillin, PIT: piperacillin-tazobactam, RIF: rifampicin, STR: streptomycin, TEI: teicoplanin, TET: tetracycline, TOB: tobramycin, TRS: trimethoprim-sulfamethoxazole, VAN: vancomycin.
The analysis of antimicrobial resistance patterns and the observation of antibiotic interactions is very useful for the detection of resistance mechanisms. Disk diffusion constitutes a suitable technique for the detection of several beta-lactamases of clinical and epidemiological importance, such as extended spectrum beta-lactamases (ESBLs), AmpC and carbapenemases. Phenotypic confirmation by diffusion techniques is mainly based on the use of beta-lactamase inhibitors and indicator beta-lactam drugs. One of the recommended methods for ESBL detection is the double-disk synergy test, which can be easily incorporated into the primary disk diffusion antibiogram. This method involves placing an amoxicillin-clavulanic acid (10μg) disk at a distance of 30mm (center to center, the distance provided by several types of disk-dispensers) from disks containing third and fourth generation cephalosporins (30μg) used as beta-lactam indicators. The distance may be reduced to 15–20mm according to the inhibition zone diameters. ESBL production is demonstrated if the inhibition zone of any of the indicators is extended by the action of clavulanic acid.26,27
Phenotypic confirmation of ESBL can also be performed by the combination disk test using cephalosporin disks (30μg) with and without clavulanic acid (10μg). An increase in the inhibition zone diameter of ≥5mm for cephalosporin with clavulanic acid compared to cephalosporin alone is considered a positive result. It is worth noting that the cephalosporin disk content initially recommended by EUCAST for the detection of ESBLs (cefotaxime 30μg and ceftazidime 30μg) differed from that used for standard susceptibility testing (cefotaxime 5μg and ceftazidime 10μg). In 2019, the EUCAST technical guidance on the use of the combination disk test to confirm ESBLs in Enterobacterales was modified in favor of the concentration used in standard susceptibility testing.28 Nevertheless, the recommendation for disk content in the double-disk synergy technique currently remains unchanged. Detection of ESBLs in isolates co-producing AmpC beta-lactamases may be challenging due to resistance to clavulanic acid, which can mitigate the synergistic effect. Several approaches could be used to improve ESBL detection in these cases, including the use of AmpC-stable fourth-generation cephalosporins such as cefepime or incorporating cloxacillin (200–250mg/L), an inhibitor of AmpC enzymes, into the medium.
Similarly, the double-disk synergy test or the combination disk test can be performed to detect AmpC beta-lactamases, using disks with AmpC inhibitors such as cloxacillin (500–750μg) or boronic acid (400–600μg) and third generation cephalosporins as indicators. It should be noted that boronic acid inhibitors are not specific for AmpC enzymes and also affect class A beta-lactamases. Inducible AmpC beta-lactamase production can also be detected in a conventional disk diffusion assay by the appearance of a flattening inhibition zone between beta-lactams (such as third generation cephalosporins, aztreonam or piperacillin-tazobactam) and beta-lactam inducers (such as imipenem, cefoxitin or amoxicillin-clavulanate). Additionally, the appearance of scattered colonies near the edge of the inhibition zone of cefoxitin, cefotaxime, ceftazidime and aztreonam has been described as a useful phenotypic indicator to differentiate between plasmidic and chromosomal AmpC.29
Disk diffusion-based tests can also be performed to detect carbapenemase-producing Enterobacterales. The most commonly used method is the combination disk test based on the potentiation of the action of meropenem or imipenem in the presence of specific inhibitors of each class of carbapenemases (boronic acid for class A carbapenemases, and dipicolinic acid or EDTA for class B carbapenemases), in which the inhibition zones of the carbapenem with and without the inhibitor are compared.26,27,30 Commercial kits are available that include cloxacillin to differentiate between AmpC hyperproduction plus porin loss and carbapenemase-production. For OXA-48-type carbapenemases, the only disk-based phenotypic marker is temocillin resistance (zone diameter<11mm), although it lacks specificity. The combination disk method is not included in the primary disk diffusion panel, and is used for positively screened isolates according to the zone diameter cut-off values for carbapenemase-producing Enterobacterales. Therefore, the main drawback of this method is that it requires overnight incubation and has been replaced by other techniques that provide faster results such as biochemical tests, lateral flow immunoassays or molecular assays.27,30
Non-fermentative Gram-negative bacilliFor non-fermenting Gram-negative bacilli other than Pseudomonas spp., Acinetobacter spp., Achromobacter xylosoxidans, Stenotrophomonas maltophilia and Burkholderia pseudomallei, disk diffusion and MIC EUCAST breakpoints are unavailable. In these cases, it is recommended to perform a MIC-based technique and interpret the results according to the EUCAST PK/PD non-species-related breakpoints.25
As for Enterobacterales, disk-based assays using cloxacillin as an inhibitor and ceftazidime as an indicator could be employed to detect AmpC beta-lactamase overproduction in P. aeruginosa. The double-disk synergy test or the combination disk using EDTA or dipicolinic acid and carbapenems can also be useful for the detection of class B carbapenemases in P. aeruginosa and other non-fermentative Gram-negative bacilli.
Staphylococcus spp., Streptococcus spp. and Enterococcus spp.The appearance (sharp or fuzzy) of the inhibition zone edges of certain antimicrobial agents can provide information on resistance mechanisms. For Staphylococcus aureus, isolates with penicillin zone diameters in the susceptible range and sharp zone edges should be considered beta-lactamase producers. Cefoxitin disk diffusion results reliably predicts methicillin resistance in staphylococci, except in Staphylococcus pseudintermedius, Staphylococcus schleiferi and Staphylococcus coagulans. Conventional disk diffusion tests can also detect inducible resistance to clindamycin in Staphylococcus and Streptococcus species, manifested by the appearance of a flattening inhibition zone adjacent to an erythromycin disk (D-zone effect).
Disk diffusion is unreliable for glycopeptide susceptibility testing among staphylococci as it cannot distinguish between wild type isolates and those with non-vanA-mediated glycopeptide resistance. For enterococci, fuzzy zone edges and colonies within the inhibition zone are highly suggestive of glycopeptide resistance and should be investigated further with an MIC method.
Additional uses of diffusion testingThe gradient strip diffusion method combines the principles of dilution and diffusion and allows the MIC to be determined directly. It shares several similarities with the disk diffusion technique regarding inoculum preparation, culture media and incubation conditions, as well as procedural simplicity and versatility in the choice of antimicrobials. The gradient diffusion method generally produces results that match those obtained with standardized dilution methods and has the advantage of providing a more accurate MIC value, due to the use of a higher number of dilutions than the conventional double series. However, it is not considered a reference technique and the degree of concordance with reference dilution methods may vary for some microorganism–drug combinations.
The gradient diffusion method has also been used to detect resistance mechanisms. Double-sided strips containing cephalosporins or carbapenems with and without inhibitors have been developed for the detection of ESBLs, AmpC betalactamases or metallo-beta-lactamases. A method for the screening of heteroresistant vancomycin-intermediate S. aureus has also been described.
Concluding remarksDisk diffusion remains a reliable method for the antimicrobial susceptibility testing of most bacterial pathogens. Unlike commercial automated microdilution systems, the disk diffusion method combines flexibility in the choice of antimicrobials and low cost. It also allows the recognition of phenotypic traits, including inducible and synergistic effects, that are highly useful in detecting certain resistance mechanisms. Although a labor-intensive method, this drawback could be partially resolved by the incorporation of instrumentation for reading zone diameters. Currently, disk diffusion is not well standardized for some bacterial groups; however, the test is being updated according to ongoing research and new disk breakpoints are being set, as reflected in EUCAST and CLSI publications.
Conflict of interestThe authors declare they have no conflict of interest.