An increased incidence of stroke in HIV-infected patients has already been reported, suggesting that HIV infection may be a cerebrovascular risk factor. The objective of this study was to assess temporal trends in the proportion of HIV infection among patients with stroke in Spain.
MethodsData were obtained from the minimum basic dataset (MBDS) of all patients hospitalized in Spain between 1997 and 2012 with a primary or secondary diagnosis of stroke. The annual proportion of HIV infection and time trends (stratifying by type of stroke and HIV stage) were calculated, and predictors of HIV infection and the social and economic impact of HIV-infected (HIV+) and non-infected (HIV−) patients were analyzed.
ResultsOf 857,371 patients hospitalized with an incident stroke, 2134 (0.25%) had HIV infection. A 2.5% year-on-year increase (OR 1.025, 95% CI 1.015–1.036, p<0.0001) of the proportion of HIV-infected patients was observed due to an increase in the asymptomatic stage of the infection (per year OR 1.077, 95% CI 1.057–1.097, p<0.0001), as the proportion of patients with AIDS remained stable. Factors independently associated with HIV infection and stroke were active smoking, stimulating drugs and hepatitis C virus (HCV) infection. A higher mortality rate, longer hospital stay and a higher cost per hospitalized patient was observed among HIV+ patients.
ConclusionsFrom 1997 to 2012, there was an increase in the proportion of HIV infection among patients hospitalized with stroke irrespective of the classical vascular risk factors, reinforcing the role of HIV infection as a cerebrovascular risk factor.
Se ha observado previamente un aumento de la incidencia de ictus en pacientes con VIH (VIH+), lo que sugiere que esta infección es un factor de riesgo cerebrovascular (FRCV). El objetivo fue analizar las tendencias temporales del porcentaje de VIH+ en pacientes con ictus en España.
MétodosLos datos se obtuvieron del Conjunto Mínimo Básico de Datos (CMBD), incluyendo a todos los pacientes hospitalizados en España entre 1997 y 2012 con un diagnóstico primario o secundario de ictus. Se calcularon el porcentaje anual de infección por VIH y las tendencias temporales (estratificados por el tipo de ictus y el estadio del VIH), así como los factores predictores independientes de infección por VIH en pacientes con ictus. La mortalidad, las estancias hospitalarias y el coste por paciente fueron similares entre los pacientes VIH+ y los pacientes no infectados por el VIH (VIH–).
ResultadosDe los 857.371 pacientes hospitalizados con un ictus incidente, 2.134 (0,25%) presentaban infección por VIH. Se observó un aumento de un 2,5% anual (OR: 1,025; IC del 95%: 1,015-1,036; p<0,0001) en el porcentaje de infección por VIH, secundario a un aumento en el estadio asintomático de la infección (OR anual: 1,077; IC del 95%: 1,057-1,097; p<0,0001), puesto que el porcentaje permaneció estable en pacientes con SIDA. La infección por el virus de la hepatitis C (VHC), el consumo de drogas estimulantes y el tabaquismo activo fueron factores independientemente asociados a sufrir un ictus y presentar VIH. Se observó una mayor mortalidad (OR: 1,81; p<0,0001) y una mayor estancia hospitalaria y coste por paciente hospitalizado en los pacientes VIH+.
ConclusionesDe 1997 a 2012, se ha observado un aumento del porcentaje de infección por VIH en pacientes hospitalizados con ictus independientemente de los factores de riesgo clásicos, lo que refuerza el papel de las infecciones por VIH como FRCV.
According to the WHO, HIV infection represents a global public health issue in terms of morbimortality, with more than 36.7 million people affected and 1 million people died from HIV-related causes at the end of 2016.1 Previous studies reported an increased risk of cardio-2,3 and cerebrovascular diseases4–7 in HIV infection, despite less exposure to classical vascular risk factors in the HIV-infected (HIV+) cohort. Besides, highly active antiretroviral therapy (HAART) has been related to an increased risk of cardio- and cerebrovascular disease in several retrospective8,9 and prospective10–12 studies, as well as in a meta-analysis.13 Nevertheless, this association is controversial due to contrasting evidence, with several other studies where no significant difference was observed in the vascular risk,14–16 even in meta-analysis.17–19 Moreover, a protective effect of HAART has been reported in one study.20
The aim of the study was to assess the trends in proportion of HIV infection among all patients hospitalized in both Spanish public and private hospitals with a primary or secondary diagnosis of stroke, over a period of 16 years (between 1997 and 2012). To explore the factors associated with the trends, we also analyzed the proportion stratifying by type of stroke (ischemic or hemorrhagic) and stage of HIV infection (early – that is, an asymptomatic stage – and advanced – that is, an acquired immune deficiency syndrome, AIDS –) as secondary outcomes. Besides, independent factors associated with HIV+ patients and both ischemic and hemorrhagic strokes, and the socio-economic burden of the disease (mortality, mean stay and mean cost per hospitalization) were also considered as secondary aims.
MethodsData collection. Data were obtained from the Spanish minimum basic dataset (MBDS), a national database which includes information from all discharge reports provided by both public and private hospitals and clinics. Each hospitalization report is encoded and anonymized in the hospital itself by a committee following the International Classification of Diseases, Ninth Revision Clinical Modification –ICD9CM – guidelines before the transmission to the national dataset. A unique hospital and patient identifier allows an anonym linkage of the information displayed of the patient. The minimum variables included are date of admission and discharge, primary and secondary diagnoses, sex, age, procedures, cost per patient, mortality and re-admission among others. More detailed information about MBDS is available at the Spanish Ministry of Health website.21
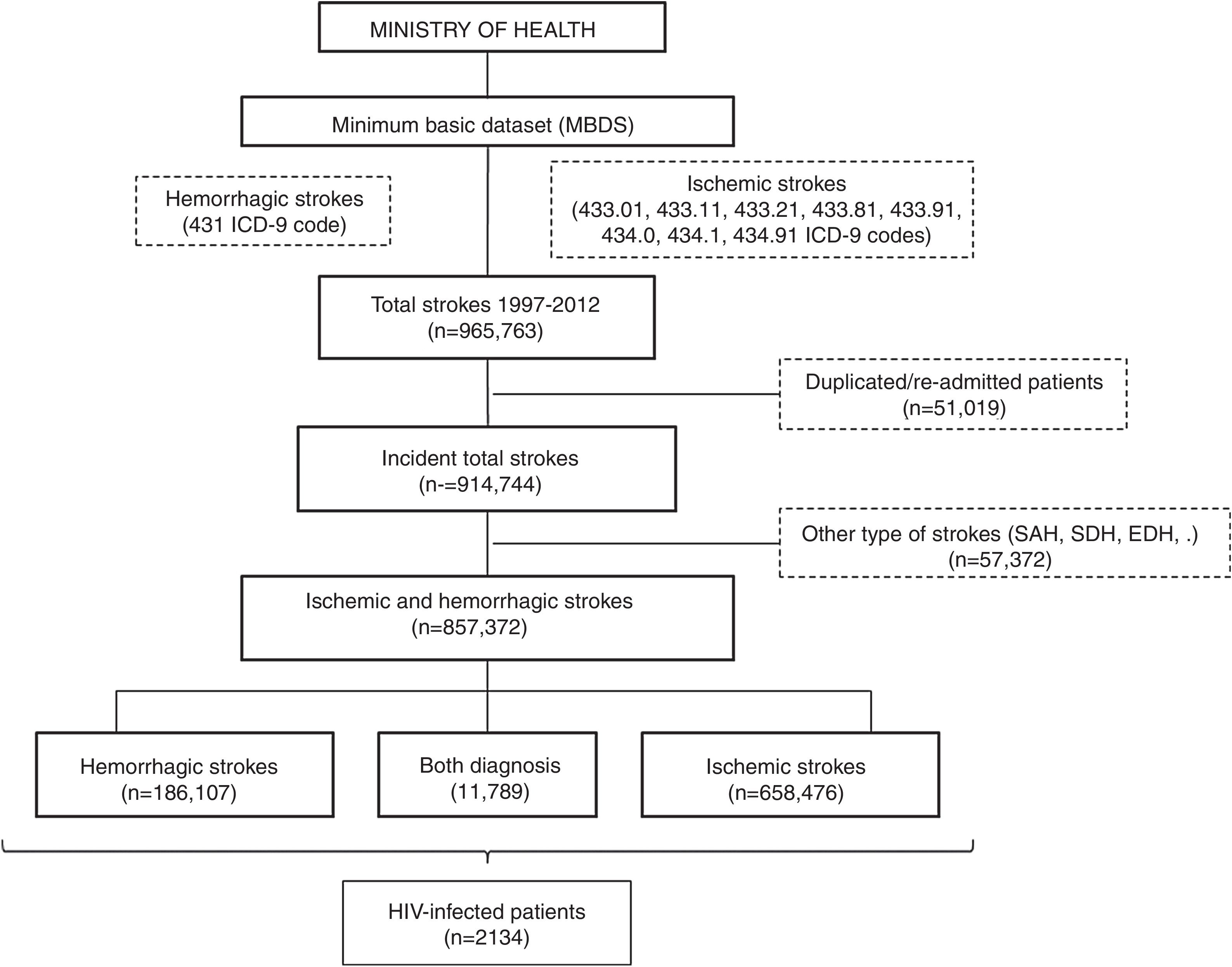
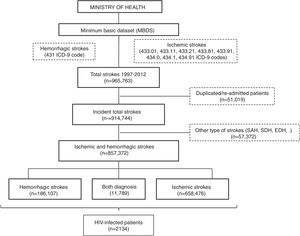
All patients with a primary or secondary diagnosis of stroke at discharge from 1997 to 2012 were included. No further data is available beyond 2012 due to discontinuation of the dataset. Selection was made by requesting to the Ministry of Health for all ischemic (ICD9CM codes 433.01, 433.11, 433.21, 433.81, 433.91, 434.0, 434.1 and 434.91) and hemorrhagic (code 431) subtypes of stroke. We decided not to include transient ischemic attacks due to its probable lack of specificity. Patients with diagnosis different from ischemic and hemorrhagic stroke (e.g. subarachnoid, subdural or epidural hemorrhages or cerebral venous thrombosis), initially provided in the database, were excluded. In order to evaluate only incident cases, all duplicated patients and those with a second admission were also removed (Fig. 1).
With the final cohort, subsequent distinction of patients with asymptomatic HIV infection (code V08) and with AIDS stage (code 042) was performed. Traditional vascular risk factors (hypertension, diabetes mellitus, dyslipidemia, active and former smoking and cardioembolic sources) as well as potential others associated with HIV-infected patients (alcoholism, stimulating drugs consumption and HPV co-infection) were detected. Multiple ICD9CM codes corresponding to a specific risk factor were considered as a sole diagnosis in order to ease statistical analysis (Table I in the Supplemental Content).
Standard protocol approvals, registrations and patient consents. This study was approved by the local ethics committee of University Hospital Ramón y Cajal. All data were public and completely anonymized by the Ministry of Health.
Statistical analysis. Continuous variables were described using means and standard deviations or medians and quartiles depending on whether data were normally distributed or not. The Shapiro–Wilk test was used to test the normality of data distributions. Categorical variables were described using absolute and relative frequencies. Chi-square test or Fisher test was used to analyze categorical variables and T-Student test or Mann–Whitney U test for quantitative variables. A logistic regression model was used to evaluate the temporal evolution on the proportion of HIV+ among all stroke hospitalizations. We performed both unadjusted analysis and adjusted by possible confounding factors in total or ischemic (sex, age, arterial hypertension, diabetes mellitus, dyslipidemia, smoking, cardioembolic sources, alcoholism, stimulating drugs consumption and HPV co-infection) and hemorrhagic strokes (sex, age, hypertension, dyslipidemia, alcoholism, stimulating drugs consumption and HPV co-infection). Subsequent logistic regressions were performed stratifying by asymptomatic HIV infection and AIDS and also by ischemic and hemorrhagic strokes, to isolate a possible factor responsible of the trend. We evaluated the factors independently associated with HIV infection in both type of strokes with a multivariate logistic regression. Discrimination was evaluated by area under roc curve (AUC). All data analyses were conducted using Stata (StataCorp, 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). Statistical hypotheses were tested using p<0.05 as the level of significance.
ResultsA database with 965,763 reports of stroke hospitalizations was received. We removed duplicated/re-admitted patients (51,019) and diagnosis different from the requested (57,372), so the final cohort included 857,372 patients with stroke, of whom a 76.8% (n=658,476) were ischemic, a 21.8% (n=186,107) were hemorrhagic and 1.37% (n=11,789) had both diagnosis (Fig. 1). Among them, we identified 2134 patients with HIV infection (0.25% of all strokes).
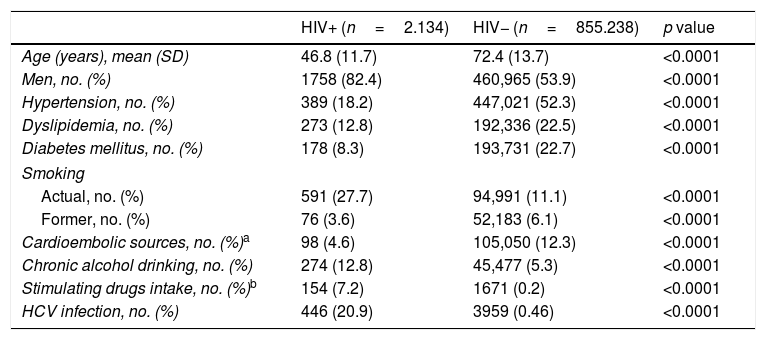
Table 1 outlines the demographic and clinical characteristics in HIV+ and non-infected (HIV−) subjects. Several statistically significant differences can be observed between the two cohorts, with HIV+ patients being younger (mean age of 46.8 years in HIV+ patients vs. 72.4 years in HIV- patients) and having lesser prevalence of hypertension, dyslipidemia, diabetes mellitus and cardioembolic sources. A higher proportion of active smoking, alcohol drinking, stimulating drugs intake and HCV infection was observed. Each natural year increased the mean age of HIV+ patients by 0.87 years (Coef=0.87, CI 95% 0.77–0.98, p<0.0001) and by 0.20 years (Coef=0.20, CI 95% 0.20–0.21, p<0.0001) of the HIV− patients.
Demographic and clinical characteristics of the study cohorts.
| HIV+ (n=2.134) | HIV− (n=855.238) | p value | |
|---|---|---|---|
| Age (years), mean (SD) | 46.8 (11.7) | 72.4 (13.7) | <0.0001 |
| Men, no. (%) | 1758 (82.4) | 460,965 (53.9) | <0.0001 |
| Hypertension, no. (%) | 389 (18.2) | 447,021 (52.3) | <0.0001 |
| Dyslipidemia, no. (%) | 273 (12.8) | 192,336 (22.5) | <0.0001 |
| Diabetes mellitus, no. (%) | 178 (8.3) | 193,731 (22.7) | <0.0001 |
| Smoking | |||
| Actual, no. (%) | 591 (27.7) | 94,991 (11.1) | <0.0001 |
| Former, no. (%) | 76 (3.6) | 52,183 (6.1) | <0.0001 |
| Cardioembolic sources, no. (%)a | 98 (4.6) | 105,050 (12.3) | <0.0001 |
| Chronic alcohol drinking, no. (%) | 274 (12.8) | 45,477 (5.3) | <0.0001 |
| Stimulating drugs intake, no. (%)b | 154 (7.2) | 1671 (0.2) | <0.0001 |
| HCV infection, no. (%) | 446 (20.9) | 3959 (0.46) | <0.0001 |
Abbreviations: HCV: hepatitis C virus; HIV+: HIV-infected patients; HIV−: HIV non-infected patients.
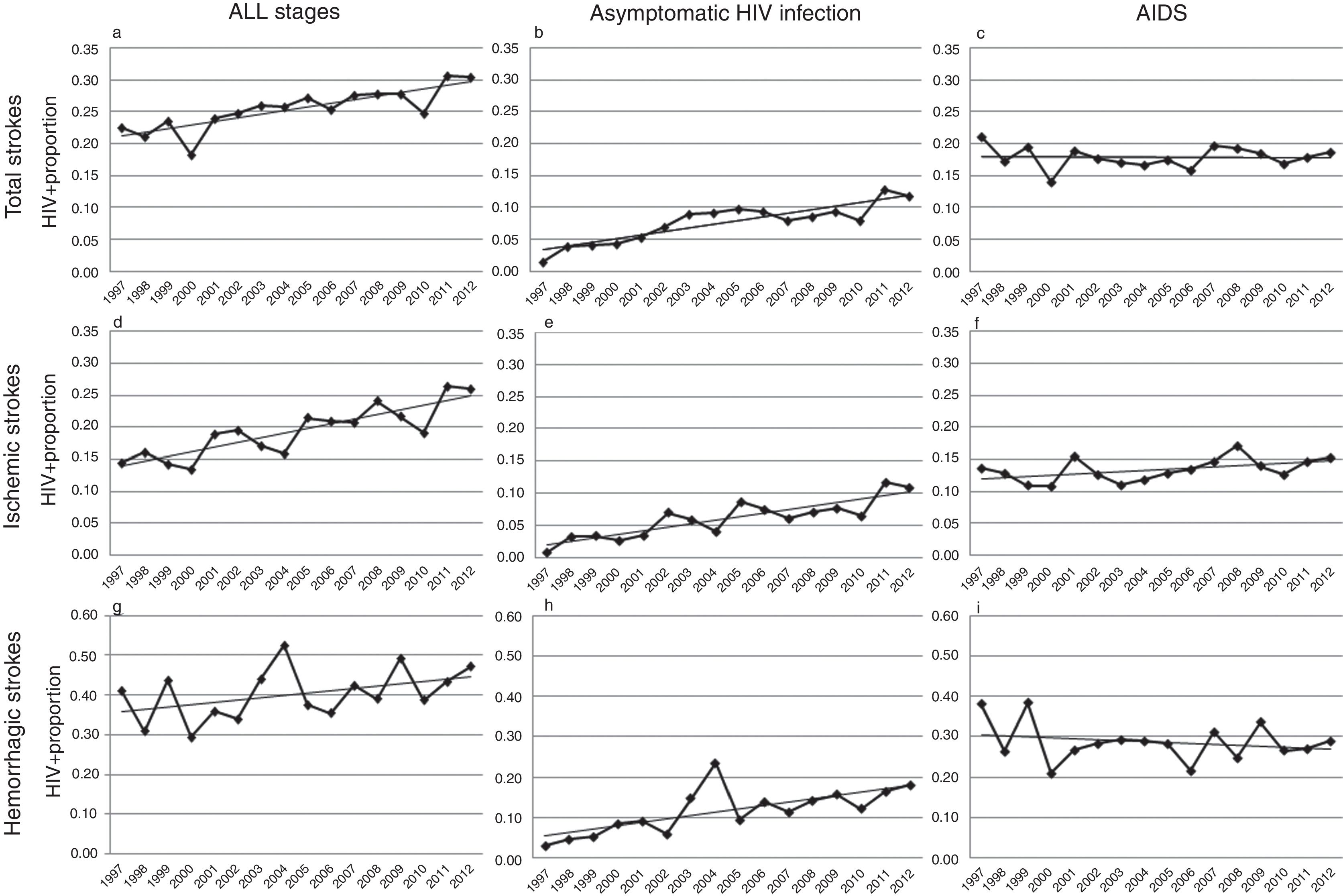
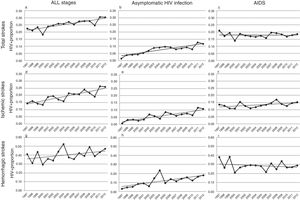
Over the study period, a linear growth of absolute number of patients with stroke was observed between both HIV− and HIV+ subjects (from 33,618 strokes in 1997 to 68,481 in 2012 among HIV− patients and 76 in 1997 and 209 in 2012 among HIV+). In terms of proportion, a significant 2.5% per year increase of HIV infection was noticed in total strokes, without any difference in both unadjusted (per year OR 1.023, CI 95% 1.014–1.033, p<0.0001) and adjusted by vascular risk factors analyses (per year OR 1.025, CI 95% 1.015–1.036, p<0.0001) (Fig. 2a). After stratifying by HIV stage, it was in the asymptomatic stage of HIV infection where a 7.7% per year increment (per year-adjusted OR 1.077, CI 95% 1.057–1.097, p<0.0001) was seen (Fig. 2b), while the proportion of patients with AIDS remained stable (per year-adjusted OR 1.003, CI 95% 0.991–1.015, p=0.62) (Fig. 2c).
Trends in HIV proportion observed among patients with total (a–c), ischemic (d–f) and hemorrhagic (g–i) strokes in the Spain population between 1997 and 2012. For total strokes, a positive trend was detected in all stages (a, trend p value <0.0001) and during the asymptomatic stage of HIV infection (b, trend p value <0.0001), but not for AIDS patients (c, trend p value =0.62). Similar results were observed among ischemic strokes (d–f), with positive trends in all stages (d, trend p value <0.0001) and asymptomatic HIV infection (e, trend p value <0.0001), while AIDS remained stable (f, trend p value=0.13). Finally, HIV infection proportion among hemorrhagic strokes did not significantly increase in all stages (g, trend p value=0.14) or AIDS stage (i, trend p value =0.22), but it did during the asymptomatic HIV infection (h, trend p value <0.0001).
Analyses conducted in ischemic strokes showed also a rise in the proportion of seropositive patients when all HIV stages were considered (per year-adjusted OR 1.035, CI 95% 1.022–1.048, p<0.0001) (Fig. 2d), and again the positive effect was exclusively due to the asymptomatic HIV infection (per year-adjusted OR 1.087, CI 95% 1.062–1.112, p<0.0001) (Fig. 2e), because in AIDS patients it was not statistically significant (per year-adjusted OR 1.012, CI 95% 0.996–1.028, p=0.13) (Fig. 2f).
Among hemorrhagic strokes we observed a positive trend for an increase of HIV infection (per year-adjusted OR 1.012, CI 95% 0.996–1.029) that did not reach the statistical significance in the adjusted analysis (p=0.14) (Fig. 2g). However, a stratified analysis showed that again the asymptomatic stage of HIV infection was related to a significant 7.1% per year increase (per year-adjusted OR 1.071, CI 95% 1.039–1.103, p<0.0001) (Fig. 2h), not observed in the AIDS stage (per year-adjusted OR 0.988, CI 95% 0.969–1.007, p=0.22) (Fig. 2i).
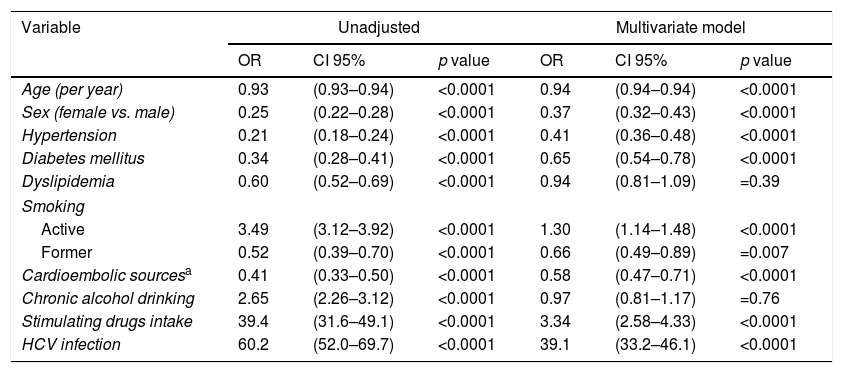
The multivariate logistic regression model was performed in patients with ischemic strokes to isolate independent factors related to the HIV infection (Table 2). Significant lesser odds of hypertension (OR 0.41, CI 95% 0.36–0.48, p<0.0001), diabetes mellitus (OR 0.65, CI 95% 0.54–0.78, p<0.0001), cardioembolic sources (OR 0.58, CI 95% 0.47–0.71, p<0.0001) and former smoking (OR 0.66, CI 95% 0.49–0.89, p=0.007) were observed for HIV infection. No differences were detected in relation to dyslipidemia (OR 0.92, CI 95% 0.81–1.09, p=0.30) and alcoholism (OR 0.96, CI 95% 0.81–1.17, p=0.66) between HIV+ and HIV− patients. Factors independently associated with a higher odds of comorbid HIV diagnosis were active smoking (OR 1.34, CI 95% 1.14–1.48, p<0.0001), stimulating drugs intake (OR 3.34, CI 95% 2.58–4.33, p<0.0001) and a HCV co-infection (OR 39.3, CI 95% 33.2–46.1, p<0.0001). Fewer females were affected by HIV infection (OR 0.38, CI 95% 0.32–0.43, p<0.0001). Whether the same model is repeated in asymptomatic HIV-infected patients, similar results are observed, excepting for an attenuation of the proportion of HCV infection (OR 16.3, CI 95% 11.8–22.4, p<0.0001).
Factors associated with a comorbid HIV diagnosis in patients hospitalized with an ischemic stroke in the Spain population between 1997 and 2012.
| Variable | Unadjusted | Multivariate model | ||||
|---|---|---|---|---|---|---|
| OR | CI 95% | p value | OR | CI 95% | p value | |
| Age (per year) | 0.93 | (0.93–0.94) | <0.0001 | 0.94 | (0.94–0.94) | <0.0001 |
| Sex (female vs. male) | 0.25 | (0.22–0.28) | <0.0001 | 0.37 | (0.32–0.43) | <0.0001 |
| Hypertension | 0.21 | (0.18–0.24) | <0.0001 | 0.41 | (0.36–0.48) | <0.0001 |
| Diabetes mellitus | 0.34 | (0.28–0.41) | <0.0001 | 0.65 | (0.54–0.78) | <0.0001 |
| Dyslipidemia | 0.60 | (0.52–0.69) | <0.0001 | 0.94 | (0.81–1.09) | =0.39 |
| Smoking | ||||||
| Active | 3.49 | (3.12–3.92) | <0.0001 | 1.30 | (1.14–1.48) | <0.0001 |
| Former | 0.52 | (0.39–0.70) | <0.0001 | 0.66 | (0.49–0.89) | =0.007 |
| Cardioembolic sourcesa | 0.41 | (0.33–0.50) | <0.0001 | 0.58 | (0.47–0.71) | <0.0001 |
| Chronic alcohol drinking | 2.65 | (2.26–3.12) | <0.0001 | 0.97 | (0.81–1.17) | =0.76 |
| Stimulating drugs intake | 39.4 | (31.6–49.1) | <0.0001 | 3.34 | (2.58–4.33) | <0.0001 |
| HCV infection | 60.2 | (52.0–69.7) | <0.0001 | 39.1 | (33.2–46.1) | <0.0001 |
Abbreviations: CI: confidence interval; HCV: hepatitis C virus; OR: odds ratio.
In a second multivariate logistic regression model in HIV patients with hemorrhagic stroke (Table II in the Supplemental Content), similar results were observed but they were significantly less exposed to dyslipidemia (OR 0.71, CI 95% 0.51–0.99, p=0.048) and not significantly related to cardioembolic sources (OR 1.08, CI 95% 0.51–2.32, p=0.836). Excellent discrimination was observed, with an AUC of 0.92.
HIV+ patients had higher in-hospital mortality (28.6%), especially those with AIDS (30.8%), in comparison with HIV− patients (18.1%) (OR 1.81, p<0.0001). Asymptomatic HIV infection was associated with a mortality of 24%. However, when mortality is analyzed longitudinally, HIV+ patients had a 6% per year decrease in global deceases during hospitalization (per year OR 0.94, CI 95% 0.92–0.96, p<0.0001), much higher than HIV− patients, who experimented only a 1% per year reduction (per year OR 0.99, CI 95% 0.99–0.99, p<0.0001).
The median stay and cost per patient hospitalized were significantly higher in the HIV+ subjects when compared with the HIV− patients (median [interquartile range, IQR] 11 [6–22] vs. 9 [5–15] days and 6.010 [4.765–8.718] vs. 3.781 [3.384–6.336] €, p<0.0001) respectively.
DiscussionIn the present national population-based study, that analyzed stroke diagnoses of all hospitalizations in Spain between 1997 and 2012, a 2.5% per year increase in the proportion of HIV infection was observed. After adjusting by HIV stage and several vascular risk factors the increment was exclusively seen in the asymptomatic stage of the HIV infection (7.7% per year), being higher in ischemic (8.7% per year) than in hemorrhagic strokes (7.1% per year). Among the AIDS patients, the proportion remained stable after adjusting by known vascular risk factors and demographic variables. These results are consistent with previous multi-institutional population-based studies in other countries.4–7 However, this study has some advantages. We included strokes reported as both primary and secondary diagnosis in order to reduce possible selection bias, as patients with HIV infection may have multiple diagnosis during hospitalization and stroke may not be the primary. This study considered a more extensive period of time (16 years). Besides, we stratified the analyses by the HIV infection stage, as AIDS is associated with an increased mortality and several comorbid complications (such as opportunistic infections and malignancies) related to strokes. We also considered vascular risk factors (alcoholism, stimulating drugs intake and HCV infection) that might be specifically related to HIV infection. Finally, we evaluated the economic burden of strokes associated to HIV infection for our National Health System in terms of median stay and median cost per patient hospitalized.
The increase in the proportion of asymptomatic HIV infection among patients with stroke over the study period may be explained by several factors. An earlier and higher detection of the HIV infection, together with the universal access to antiretroviral treatment in Spain and the decrease in mortality associated with HIV infection may have progressively increased the prevalence of HIV infection in the population. In recent years, the number of new diagnoses of HIV infection in Spain has remained constant.22 However, no data of prevalence of HIV infection without AIDS are available in Spain.23 Therefore, the incidence of stroke among this population cannot be calculated. Thus, we decided to evaluate proportion of HIV among all stroke hospitalizations.
The decrease in the mortality among HIV-infected patients has increased their life expectancy over the years. This might contribute to the rise in the proportion of HIV infection among stroke patients. However, mean age in HIV+ patients with stroke has only increased from 36.9 years in 1997 to 51.3 years in 2012. Thus, this mean age cannot be considered a vascular risk factor responsible for the increase of strokes.
Concerning AIDS patients, there is not an increase in the proportion of strokes associated with them. This may simply reflect the lower number of cases diagnosed since introduction of HAART.22
MBDS do not provide enough data to classify the etiology of stroke. Cardioembolic sources were reported in a 4.6% of patients with HIV infection, much lower than HIV− patients (12.3%). The frequency of lacunar and atherothrombotic stroke cannot be accurately estimated. However, it is presumably lower in HIV+ patients as they have a lower exposure to conventional vascular risk factors (such as male sex, hypertension, diabetes mellitus), even though the proportion of these factors increased from 1997 to 2012 (data not shown). Besides, despite an improvement of the life expectancy over the years in HIV+ patients, the mean age, a crucial vascular risk factor, remained substantially lower in comparison with HIV− patients (46.8 vs. 72.4 years, respectively). A significant proportion of strokes in HIV+ patients may be associated with stimulating drugs intake and HCV infection, as they are independently associated to HIV infection. Relative to HAART, the high prescription of protease inhibitors, which have been associated with dyslipidemia, insulin resistance24,25 and a higher vascular risk,13 has decreased in the recent years in favor of integrase inhibitors (II). II have not been related to an increased vascular risk or metabolic disorders. But despite this hypothetical lower vascular risk associated to II, we still observed an increase of the proportion of HIV infection among stroke patients during the latest years of the period studied, although the effect of the evolution of HAART cannot be analyzed in this study due to the lack of report of the number of patients treated and the type of treatment provided.
The increased risk of strokes among HIV+ patients might be due to the HIV infection per se. The improvement of life expectancy exposes longer the patients to the HIV, a previously reported factor for cerebrovascular events.8–12 Different mechanisms of vascular damage have been hypothesized to account for stroke in association with HIV infection. For ischemic strokes, direct causes include an endothelial dysfunction, a systemic inflammatory cytokine or chemokine dysregulation,26 lipid disorders associated with the HIV infection27 and an enhanced atheroma formation by activated macrophages.28 A HIV-associated vasculopathy has been described in these patients, which may represent a 20% of the causes of stroke.29
The mortality of stroke was higher in HIV+ patients. It might be mostly attributed to AIDS-associated diseases because it is much higher in patients with AIDS and stroke. The decrease of a 6% per year over the study period may reflect the extension of antiretroviral treatment. Similarly, the increased median cost and hospital stay in HIV+ patients may be related to HIV comorbid complications.
Limitations of the present study are those inherent to national population-based studies. The cornerstone of our study is the minimum basic dataset so we are exposed to the errors and bias related to the ICD9CM codification and accuracy of hospitalization reports. Nevertheless, a study validated recently the Spanish MBDS as an accurate and useful tool to deliver valid information for cerebrovascular diseases.30 Moreover, stroke coding by the ICD9CM shows high percentages for sensitivity, specificity and positive predictive value for both ischemic and hemorraghic strokes and even better values for AIDS/HIV infection (Table III in the Supplemental Content). However, despite these limitations, our database has a sample size enough to minimize some bias and strong conclusions can be drawn from the results.
To conclude, our study provides evidence about a raising association between HIV infection and stroke independently of classical vascular risk factors. This was probably due to an increase of the survival related to an earlier detection and a prompt initiation of antiretroviral treatment. A direct consequence in terms of efficiency is a major socioeconomic burden for the National Health System as the cost and stay per hospitalization is higher. Based on previous studies inquiring into the mechanisms of strokes among HIV-infected patients, our results plausibly reinforce the role of the HIV infection as a cerebrovascular risk factor. This evidence encourages the research for HIV infection in young patients with stroke.
Authors’ contributionE Monreal, P Gullón, A Escobar-Villalba, P Pérez-Torre, F Acebrón, C Quereda Rodríguez-Navarro, MJ Pérez-Elías, J Masjuan and I Corral revised the manuscript for content and participated in the study concept or design, analysis or interpretation of data. L Sánchez-Ruano, BM Fernández Félix, A Muriel contributed to clear the original database provided by the Ministry of Health and acted as consultants to the statistical analysis. All authors critically revised the manuscript.
Conflict of interestsThe authors declare that they have no conflicts of interest.
The authors want to thank María Ángeles Gogorcena Aoiz from the Sanitary Activity Information and Statistics area, part of the Innovation and Sanitary Information Vice Presidency of the Equality, Social Services and Health Ministry for the MBDS database contribution.