The prescription of antiretroviral treatment (ART) that contains pharmacokinetic enhancers such as ritonavir and cobicistat is frequent. The objective of this study was to analyse the potential interactions of ART that include these molecules in their formulation with the patient's home medication, as well as the clinical management of those potentially serious.
MethodsProspective study conducted in the pharmacy care clinic of a third level hospital between January and December of 2018. Those HIV+patients with an ART containing cobicistat or ritonavir were included in the study. Potential interactions between ART and concomitant medication were analysed in three databases (Micromedex®, Drugs.com and Liverpool), the interventions carried out were detailed, and adverse drug reactions analysed.
Results968 patients were included with a total of 2148 prescriptions (274 different medications). A total of 86 interventions were performed regarding potential interactions in patients. The most frequent were substitutions of corticoid treatments, treatment suspensions and closer monitoring of treatments. A total of possible adverse drug reactions were analysed. The degree of agreement in the severity classification of the interactions for cobicistat and ritonavir was good among the three databases. It was remarkable Micromedex® as the most complete because it has more registered medications.
ConclusionThe interactions between ART with pharmacokinetic enhancers in its composition and concomitant medication is frequent and requires a significant variety of interventions. The check of interactions in different databases is recommended since they can cause adverse drug reactions.
La prescripción de tratamiento antirretroviral (TAR) que contiene potenciadores farmacocinéticos como ritonavir y cobicistat es frecuente. El objetivo de este estudio fue analizar las interacciones potenciales del TAR que incluyen estas moléculas en su formulación con la medicación domiciliaria del paciente, así como el manejo clínico de aquellas potencialmente graves.
MétodosEstudio prospectivo en la consulta de atención farmacéutica de un hospital de tercer nivel entre enero y diciembre de 2018. Se incluyeron en el estudio aquellos pacientes VIH+ con un TAR que contuviera cobicistat o ritonavir. Se analizaron las interacciones potenciales entre el TAR y la medicación concomitante en tres bases de datos (Micromedex®, Drugs.com y Liverpool), se detallaron las intervenciones realizadas, y se analizaron las reacciones adversas encontradas.
ResultadosSe incluyeron 968 pacientes con un total de 2.148 prescripciones (274 principios activos diferentes). Se realizaron un total de 86 intervenciones relativas a interacciones potenciales en los pacientes. Las más frecuentes fueron sustituciones de tratamientos corticoideos, supensiones de tratamiento y monitorizaciones más estrechas. Se analizaron un total de doce sospechas de reacción adversa. El grado de concordancia en la clasificación de la gravedad de las interacciones para cobicistat y ritonavir fue buena entre las tres bases de datos. Resultó destacable Micromedex® como la más completa por tener más principios activos registrados.
ConclusiónLas interacciones entre el TAR con potenciadores farmacocinéticos en su composición y la medicación concomitante es frecuente y requiere de una importante variedad de intervenciones. El chequeo de interacciones en distintas bases de datos es recomendable ya que pueden ocasionar reacciones adversas a medicamentos.
Today, the life expectancy of HIV patients is very close to that of uninfected people.1 This is undoubtedly the result of the high efficacy of currently available antiretroviral therapies (ART).2 With an inordinate number of drug combinations available on the market, most of the guidelines prioritise combinations that are formulated in one single tablet, which is known as a single-tablet regimen (STR). However, although all STRs show very similar efficiencies, their safety profiles are not the same, since some include pharmacokinetic enhancers, also known as boosters, in their formulation, and whose interaction profile is less favourable. This is one of the main reasons that, in the most recent updates to the European and American Guidelines, these regimens have ceased to be front line and have been positioned as alternative treatment lines.2–5
Pharmacokinetic enhancers are drugs that increase the concentration of other active substances in the blood. In HIV in particular, they have long been used as enhancers of protease inhibitors, and more recently of integrase inhibitors. The use thereof makes it possible to decrease the dose and frequency of administration. Ritonavir was the first enhancer marketed, and later cobicistat was introduced, which has two theoretical advantages over the former; a lack of antiretroviral activity (which gives it an absence of risk in the development of resistance), and a slightly narrower interaction spectrum, especially with regard to the induction of certain enzymes.6
The main problem with enhancer-containing regimens is the non-specific inhibition of cytochrome P450 as well as of various transporters involved in the metabolism, distribution and elimination of numerous drugs. This can result in the toxicity at normally safe doses of certain treatments, such as certain statins, whose use in combination with these enhancers may increase the risk of rhabdomyolysis owing to an increase in their plasma concentration,7 or the risk of iatrogenic Cushing's syndrome by altering the metabolism of corticosteroid treatments, consequentially deregulating the endocrine system.8 On the other hand, by inhibiting the metabolism, it can also cause the inhibition of the bioactivation of certain prodrugs, such as clopidogrel, and therefore they do not reach therapeutic concentrations. While it is true that many other antiretrovirals have also been linked to significant potential interactions (e.g. the enzyme induction produced by efavirenz or nevirapine, or the interaction between metformin and dolutegravir), the fact is that regimens containing pharmacokinetic enhancers have been shown to be an independent factor in increasing the risk of drug interactions.9 One tool that can be of considerable use in identifying the causality of an adverse drug reaction (ADR) is the Naranjo algorithm.10 In turn, to identify the probability that two drugs have had an interaction in a patient, the DIPS algorithm can be used in a complimentary manner.11
From this perspective, it is worth noting the importance of the pharmacist's role in the treatment and monitoring of HIV patients, since the intervention of a pharmacist has been shown to reduce potentially inappropriate prescriptions as well as contraindicated medications.12 Clinical pharmacists are beginning to have an increasingly defined role in the care and monitoring of these patients, and their functions are detailed in internationally renowned guidelines.13 In the particular case of drug interactions, pharmacists are experts in consulting databases as well as in solving potentially serious interactions in collaboration with physicians.14
The main objective of this paper is to analyse the potential interactions of ARTs that include pharmacokinetic enhancers in their formulation with the patient's home medication, as well as the clinical management of those that are potentially serious. Secondary objectives include determining the causality of the interaction in the appearance of ADRs detected in the pharmaceutical care consultation and analysing the agreement between the databases most commonly used by clinicians to resolve these interactions.
MethodsA prospective study was carried out in the pharmaceutical care department of a third-level hospital in the Community of Madrid between January and December 2018. During this period, patients attended the department an average of 6 times (visits every 2 months), and on all occasions they were attended by a specialised pharmacist in an HIV monographic consultation. HIV+ patients with an ART containing cobicistat or ritonavir were included in the study. Sex, age and concomitant medication (including nutritional supplements and herbal products) were recorded by conducting an interview with the pharmacist and consulting the Horus® primary care programme.
During the visit with the pharmacist, an anamnesis was performed to detect possible ADRs derived from the interaction between the enhancer and home treatment. In order to determine the causality of the ADR, the Naranjo algorithm was used.10 In order to find out the probability that the interaction had existed in that specific patient, the DIPS algorithm was used.11
Patients were divided into two groups according to their ART enhancer: cobicistat or ritonavir. Interactions with the rest of the treatment were analysed using three databases: HIV Drug Interactions from the University of Liverpool,15 the Drug Information Database (Drugs.com)16 and Micromedex®.17 The interactions were classified into five groups: severe interaction, moderate interaction, mild interaction, with no known interaction, and unregistered active ingredient. Since Micromedex® classifies interactions differently than the other two databases, it was established that contraindicated was equivalent to severe, significant was equivalent to moderate, and those classified as mild or moderate were equated with mild, since the mild classification it is used for less than 1% of all the active ingredients included in this database. In all cases in which the pharmacist believed the interaction could have clinical significance or put the patient at risk, a pharmaceutical intervention was carried out and the prescribing physician was contacted. The pharmaceutical interventions carried out were classified into 5 groups: suspension of the home drug, replacement of the home drug, change of dose of the home drug (increase or decrease), stricter monitoring of the treatment, and change of ART.
Frequencies, medians and ranges were used for the analysis of demographic data and prescriptions. For the analysis of the concordance between the databases, the weighted kappa coefficient was used by means of the use of bi-squared weights for categorical ordinal data, multiple observers and incomplete designs using STATA® version 12 software (Stata Corp). The value of the kappa (k) coefficient was interpreted according to the agreement indices of Landis and Koch, considering 0–0.2 “insignificant”, 0.21–0.4 “discrete”, 0.41–0.6 “moderate”, 0.61–0.8 “substantial” and 0.81–1 “almost perfect”.18
The study did not require the collection of informed consent from the patients, since the activity carried out by the pharmacists for the study is included within their usual healthcare activity.
ResultsThe study included 968 patients (81.4% men, with a median age of 50 years [interquartile range: 34–66]) with a total of 2148 prescriptions (median of 2 medications per patient). The median age for the group of patients receiving cobicistat (897 patients) was 50 years (interquartile range: 40–56), with a median of one drug per patient, while for ritonavir (71 patients) the median was 55 years old (interquartile range: 49–58) with 3 medications per patient. Excluding the ARV treatment, 20.7% of patients (14.1% for the cobicistat group and 33.8% for the ritonavir group) were considered polymedicated (5 drugs or more).
The prescriptions corresponded to 274 different active ingredients. The most commonly prescribed medications in the study population were calcifediol (n=338 prescriptions), atorvastatin (n=153) and omeprazol (n=98). The most prescribed pharmacological group was N (central nervous system) (n=63 active substances), followed by A (digestive system and metabolism) (n=43) and C (cardiovascular system) (n=41).
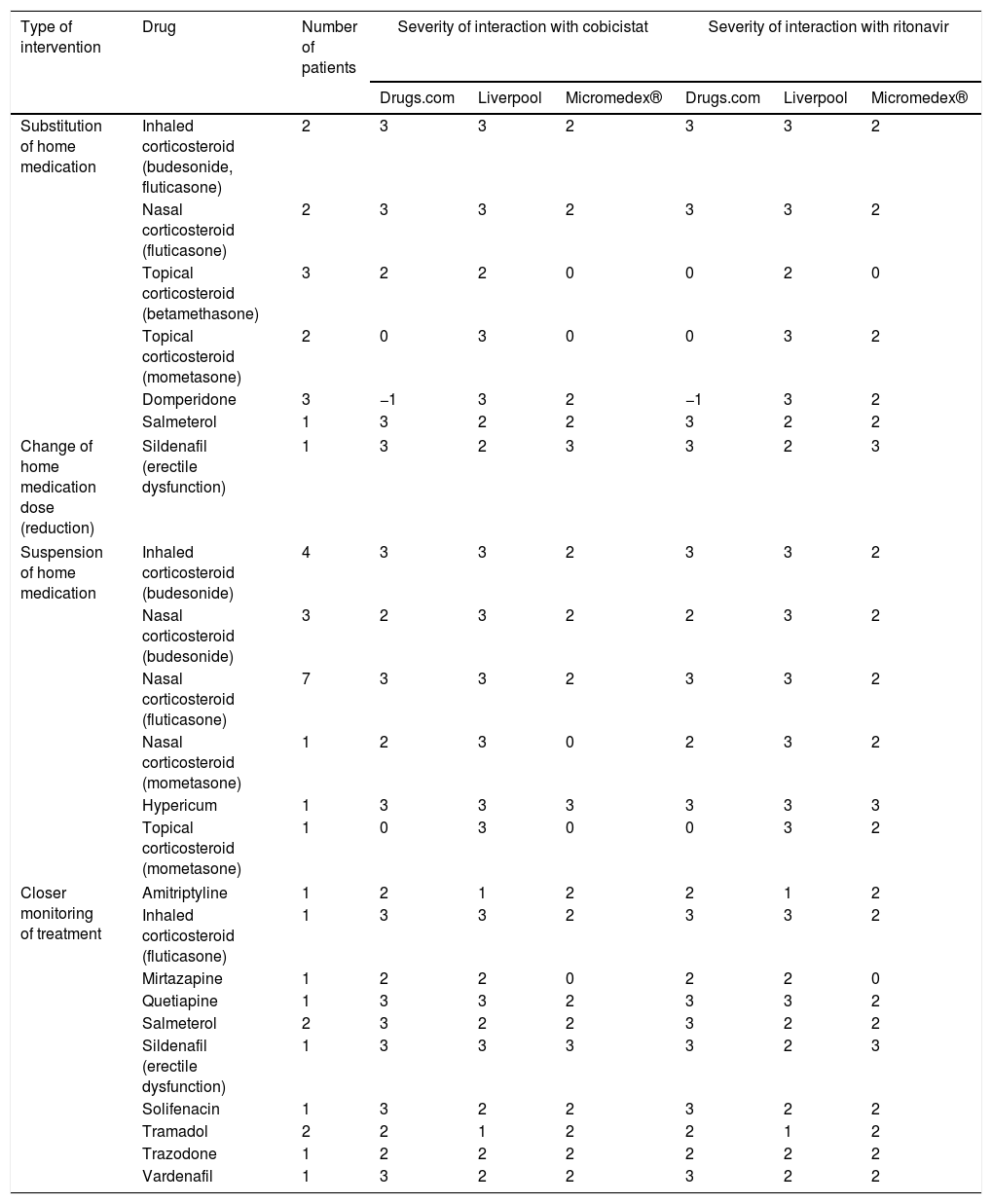
A total of 86 interventions involving potential interactions were performed in 80 patients (6 patients had two interventions). Table 1 summarises the interventions performed by pharmacists without the need for approval by the prescribing physician. Most consisted of drug suspensions for minor pathologies, such as corticosteroid-based nasal sprays. In the case of home drug substitutions, worthy of note were products that included creams with powerful corticosteroids for others of lesser potency, or prokinetics such as domperidone for others with less potential for interaction such as metoclopramide.
Pharmaceutical interventions that did not require the approval of the attending physician.
| Type of intervention | Drug | Number of patients | Severity of interaction with cobicistat | Severity of interaction with ritonavir | ||||
|---|---|---|---|---|---|---|---|---|
| Drugs.com | Liverpool | Micromedex® | Drugs.com | Liverpool | Micromedex® | |||
| Substitution of home medication | Inhaled corticosteroid (budesonide, fluticasone) | 2 | 3 | 3 | 2 | 3 | 3 | 2 |
| Nasal corticosteroid (fluticasone) | 2 | 3 | 3 | 2 | 3 | 3 | 2 | |
| Topical corticosteroid (betamethasone) | 3 | 2 | 2 | 0 | 0 | 2 | 0 | |
| Topical corticosteroid (mometasone) | 2 | 0 | 3 | 0 | 0 | 3 | 2 | |
| Domperidone | 3 | −1 | 3 | 2 | −1 | 3 | 2 | |
| Salmeterol | 1 | 3 | 2 | 2 | 3 | 2 | 2 | |
| Change of home medication dose (reduction) | Sildenafil (erectile dysfunction) | 1 | 3 | 2 | 3 | 3 | 2 | 3 |
| Suspension of home medication | Inhaled corticosteroid (budesonide) | 4 | 3 | 3 | 2 | 3 | 3 | 2 |
| Nasal corticosteroid (budesonide) | 3 | 2 | 3 | 2 | 2 | 3 | 2 | |
| Nasal corticosteroid (fluticasone) | 7 | 3 | 3 | 2 | 3 | 3 | 2 | |
| Nasal corticosteroid (mometasone) | 1 | 2 | 3 | 0 | 2 | 3 | 2 | |
| Hypericum | 1 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Topical corticosteroid (mometasone) | 1 | 0 | 3 | 0 | 0 | 3 | 2 | |
| Closer monitoring of treatment | Amitriptyline | 1 | 2 | 1 | 2 | 2 | 1 | 2 |
| Inhaled corticosteroid (fluticasone) | 1 | 3 | 3 | 2 | 3 | 3 | 2 | |
| Mirtazapine | 1 | 2 | 2 | 0 | 2 | 2 | 0 | |
| Quetiapine | 1 | 3 | 3 | 2 | 3 | 3 | 2 | |
| Salmeterol | 2 | 3 | 2 | 2 | 3 | 2 | 2 | |
| Sildenafil (erectile dysfunction) | 1 | 3 | 3 | 3 | 3 | 2 | 3 | |
| Solifenacin | 1 | 3 | 2 | 2 | 3 | 2 | 2 | |
| Tramadol | 2 | 2 | 1 | 2 | 2 | 1 | 2 | |
| Trazodone | 1 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Vardenafil | 1 | 3 | 2 | 2 | 3 | 2 | 2 | |
3: severe interaction; 2: moderate interaction; 1: mild interaction; 0: with no known interaction; −1 unregistered active ingredient.
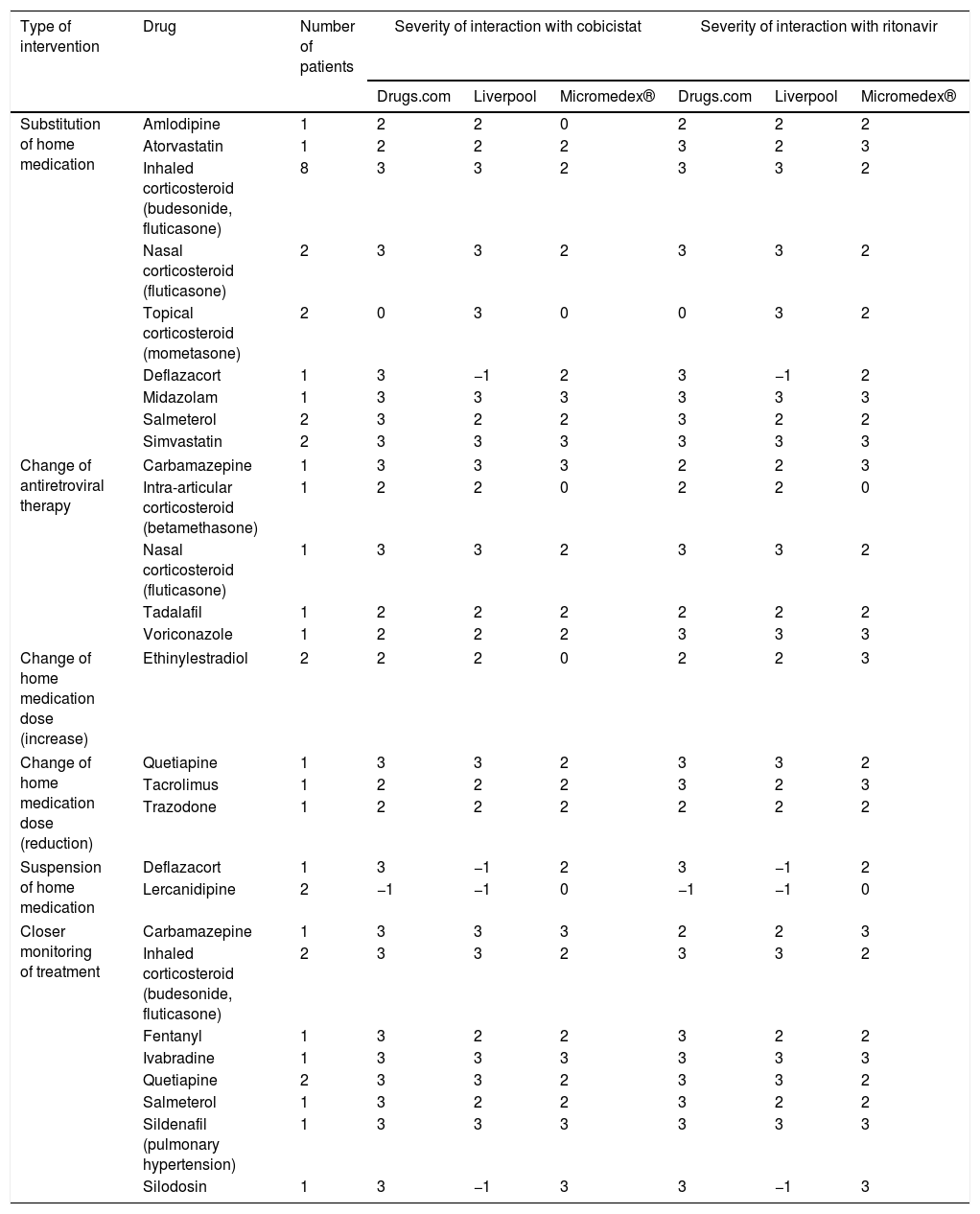
The interventions performed by the doctors are shown in Table 2. Most were substitutions for therapeutic equivalents, mainly of inhaled corticosteroids for lung diseases, with some not dependent on cytochrome P450, such as beclomethasone. In addition, ART changes were made in 5 patients, in whom the enhancers were removed as the rest of the medication could not be changed. In the first patient, the change was due to the recent diagnosis of epilepsy and the need to introduce carbamazepine. The second patient had to undergo corticosteroid-based infiltrations. Another patient had to be treated with tadalafil for pulmonary hypertension. The fourth patient was diagnosed with aspergillosis and had to maintain prolonged voriconazole treatment. The final patient had to maintain corticosteroid treatment to treat chronic allergic rhinitis.
Pharmaceutical interventions that did not require the approval of the attending physician.
| Type of intervention | Drug | Number of patients | Severity of interaction with cobicistat | Severity of interaction with ritonavir | ||||
|---|---|---|---|---|---|---|---|---|
| Drugs.com | Liverpool | Micromedex® | Drugs.com | Liverpool | Micromedex® | |||
| Substitution of home medication | Amlodipine | 1 | 2 | 2 | 0 | 2 | 2 | 2 |
| Atorvastatin | 1 | 2 | 2 | 2 | 3 | 2 | 3 | |
| Inhaled corticosteroid (budesonide, fluticasone) | 8 | 3 | 3 | 2 | 3 | 3 | 2 | |
| Nasal corticosteroid (fluticasone) | 2 | 3 | 3 | 2 | 3 | 3 | 2 | |
| Topical corticosteroid (mometasone) | 2 | 0 | 3 | 0 | 0 | 3 | 2 | |
| Deflazacort | 1 | 3 | −1 | 2 | 3 | −1 | 2 | |
| Midazolam | 1 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Salmeterol | 2 | 3 | 2 | 2 | 3 | 2 | 2 | |
| Simvastatin | 2 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Change of antiretroviral therapy | Carbamazepine | 1 | 3 | 3 | 3 | 2 | 2 | 3 |
| Intra-articular corticosteroid (betamethasone) | 1 | 2 | 2 | 0 | 2 | 2 | 0 | |
| Nasal corticosteroid (fluticasone) | 1 | 3 | 3 | 2 | 3 | 3 | 2 | |
| Tadalafil | 1 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Voriconazole | 1 | 2 | 2 | 2 | 3 | 3 | 3 | |
| Change of home medication dose (increase) | Ethinylestradiol | 2 | 2 | 2 | 0 | 2 | 2 | 3 |
| Change of home medication dose (reduction) | Quetiapine | 1 | 3 | 3 | 2 | 3 | 3 | 2 |
| Tacrolimus | 1 | 2 | 2 | 2 | 3 | 2 | 3 | |
| Trazodone | 1 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Suspension of home medication | Deflazacort | 1 | 3 | −1 | 2 | 3 | −1 | 2 |
| Lercanidipine | 2 | −1 | −1 | 0 | −1 | −1 | 0 | |
| Closer monitoring of treatment | Carbamazepine | 1 | 3 | 3 | 3 | 2 | 2 | 3 |
| Inhaled corticosteroid (budesonide, fluticasone) | 2 | 3 | 3 | 2 | 3 | 3 | 2 | |
| Fentanyl | 1 | 3 | 2 | 2 | 3 | 2 | 2 | |
| Ivabradine | 1 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Quetiapine | 2 | 3 | 3 | 2 | 3 | 3 | 2 | |
| Salmeterol | 1 | 3 | 2 | 2 | 3 | 2 | 2 | |
| Sildenafil (pulmonary hypertension) | 1 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Silodosin | 1 | 3 | −1 | 3 | 3 | −1 | 3 | |
3: severe interaction; 2: moderate interaction; 1: mild interaction; 0: with no known interaction; −1: unregistered active ingredient.
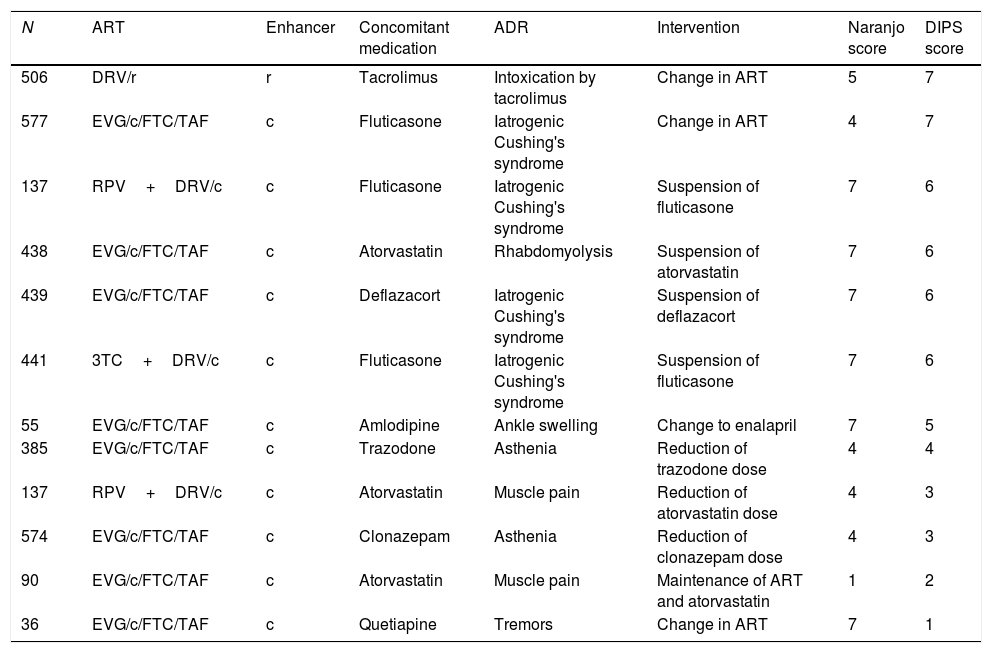
Twelve suspected ADRs caused by interaction with the pharmacokinetic enhancer were detected in 11 patients. The drugs involved in the interaction and their degree of causality are shown in Table 3. More than half of the interactions were classified with “probable” causality by the interaction, and in 3 of them the physician had to make a change in the patient's ART.
Causality of identified adverse reactions.
| N | ART | Enhancer | Concomitant medication | ADR | Intervention | Naranjo score | DIPS score |
|---|---|---|---|---|---|---|---|
| 506 | DRV/r | r | Tacrolimus | Intoxication by tacrolimus | Change in ART | 5 | 7 |
| 577 | EVG/c/FTC/TAF | c | Fluticasone | Iatrogenic Cushing's syndrome | Change in ART | 4 | 7 |
| 137 | RPV+DRV/c | c | Fluticasone | Iatrogenic Cushing's syndrome | Suspension of fluticasone | 7 | 6 |
| 438 | EVG/c/FTC/TAF | c | Atorvastatin | Rhabdomyolysis | Suspension of atorvastatin | 7 | 6 |
| 439 | EVG/c/FTC/TAF | c | Deflazacort | Iatrogenic Cushing's syndrome | Suspension of deflazacort | 7 | 6 |
| 441 | 3TC+DRV/c | c | Fluticasone | Iatrogenic Cushing's syndrome | Suspension of fluticasone | 7 | 6 |
| 55 | EVG/c/FTC/TAF | c | Amlodipine | Ankle swelling | Change to enalapril | 7 | 5 |
| 385 | EVG/c/FTC/TAF | c | Trazodone | Asthenia | Reduction of trazodone dose | 4 | 4 |
| 137 | RPV+DRV/c | c | Atorvastatin | Muscle pain | Reduction of atorvastatin dose | 4 | 3 |
| 574 | EVG/c/FTC/TAF | c | Clonazepam | Asthenia | Reduction of clonazepam dose | 4 | 3 |
| 90 | EVG/c/FTC/TAF | c | Atorvastatin | Muscle pain | Maintenance of ART and atorvastatin | 1 | 2 |
| 36 | EVG/c/FTC/TAF | c | Quetiapine | Tremors | Change in ART | 7 | 1 |
c: cobicistat; DIPS: Drug Interaction Probability Scale (<2 points: doubtful; 2–4 points: possible; 5–8 points: probable; >8 points: very probable); DRV: darunavir; EVG: elvitegravir; FTC: emtricitabine; N: patient number; r: ritonavir; ADR: adverse drug reaction; RPV: rilpivirine; TAF: tenofovir alafenamide; ART: antiretroviral treatment; 3TC: lamivudine.
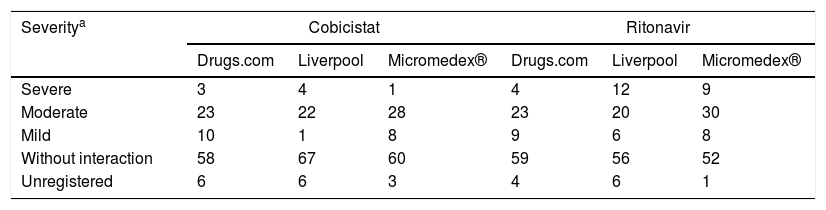
With regard to the classification of interactions according to severity in the different databases, Micromedex® stood out as the most complete, with more than 97% of the registered active principles. Furthermore, this database was the one that assigned lower severity indexes to the active ingredients analysed. The complete analysis is shown in Table 4.
Classification of severity of the interaction of the active substances of home medications with pharmacokinetic enhancers.
| Severitya | Cobicistat | Ritonavir | ||||
|---|---|---|---|---|---|---|
| Drugs.com | Liverpool | Micromedex® | Drugs.com | Liverpool | Micromedex® | |
| Severe | 3 | 4 | 1 | 4 | 12 | 9 |
| Moderate | 23 | 22 | 28 | 23 | 20 | 30 |
| Mild | 10 | 1 | 8 | 9 | 6 | 8 |
| Without interaction | 58 | 67 | 60 | 59 | 56 | 52 |
| Unregistered | 6 | 6 | 3 | 4 | 6 | 1 |
The degree of concordance in the severity classification of the interactions for cobicistat and ritonavir was good (k=0.65 and k=0.62, respectively, between the three databases). Comparing databases in pairs, it was found that the most discordant pair was Micromedex® versus Liverpool, with a concordance found for cobicistat and moderate ritonavir, k=0.53 and k=0.59, respectively, and the highest concordance was found between Liverpool and Drugs.com with k=0.74 for both cobicistat and ritonavir.
DiscussionThe number of drug interactions between home medication and ART with enhancers is high. Despite this, a pharmaceutical intervention was required in only 8 out of 100 patients. In addition, it was noted that in one in 7 of these patients who underwent surgery, a suspicion of an ADR was detected.
Most of the interventions were related to the use of corticosteroids for different indications (44 interventions out of 86). There are relatively numerous clinical cases and published reviews relating to this type of interaction and its serious consequences in patients,8,17–19 and hence the 3 databases consulted classify the majority of corticosteroids with a moderate-severe interaction potential. In the case of minor pathologies (such as rhinitis), the pharmacist recommended either the suspension or substitution of the drug, thus contributing to the safe use of the drugs and alleviating the healthcare burden on the physician. In more complex cases, the pharmacist contacted the physician, who normally changed the corticosteroid to one of lower potency or whose metabolism was not affected by cytochrome P450, such as beclomethasone, as stated in the GESIDA Guide.3 In the cases in which the physician preferred to keep the original corticosteroid, the ART was changed for another one without an enhancer. This occurred in a patient who required the intra-articular administration of corticosteroids, since it has been described that the interaction can also occur via this route.8 In addition, during the pharmaceutical care visit, pharmacists suspected iatrogenic Cushing's syndrome in four cases and brought it to the attention of their prescribing physicians, a fact already well described in the literature, including by inhalation.19–21
The prescription of statins in these patients was also highly relevant. It should be noted that HIV patients have a higher risk of cardiovascular accident owing to multifactorial causes, and it is therefore important to keep their cholesterol and triglyceride levels controlled. The problem with statins is that those most commonly used in clinical practice, such as simvastatin and atorvastatin, have a high profile of interactions with these enhancers. In the case of simvastatin and lovastatin, the guidelines contraindicate their use,4 and recommend they be replaced with others.22,23 Physicians typically opt for atorvastatin and rosuvastatin, high-potency statins that are classified as moderate to severe interaction in the three databases consulted. In this sense, it is noteworthy how in two of the databases (Drugs.com and Micromedex®) the interaction between atorvastatin and ritonavir is at the highest level of severity, while for atorvastatin and cobicistat none of the databases contraindicates their associated use. The difference is that despite the fact that both enhancers inhibit the cytochrome P450 and the OATP1B1 transporter, cobicistat does not inhibit the P-gp transporter and therefore blocks to a lesser extent the metabolism of this statin, which is highly dependent on these three elements for its metabolism and elimination. In conclusion, it is still necessary to monitor the use of these drugs in these patients and to monitor muscle pain, and to monitor CPK,7 and in certain cases, one enhancer change for another may be justified if the statin cannot be changed.
Other notable interventions were those related with psychiatric medication, such as quetiapine (5 patients), which increases its systemic exposure by more than 6 times when administered together with potent cytochrome P450,24 inhibitors, and also increases the plasma half-life, making intentional poisoning with this type of drug even more dangerous.25 For Molas et al. this was also the most prescribed drug, with potentially serious interactions in a study with a population very similar to ours.9 Also worthy of note is the prescription of phosphodiesterase inhibitors, such as sildenafil (3 patients), tadalafil (one patient) or vardenafil (one patient). In cases where the medicine was used recreationally, the pharmacist advised on the necessary dose adjustments, since specific recommendations appear in the technical data sheets in this regard, and in cases of pulmonary hypertension, the doses were reviewed by their pulmonologists and infectious disease physicians. This interaction appears as moderate or severe in the three databases consulted. There is a case in the bibliography in which this interaction probably influenced the death of a patient.26
It is also important to note the differences found in the severity ratings of the interaction between the three databases. Micromedex® was the most complete as it has more active ingredients included in its database, including plants, and it was also the one with the lowest severity of interactions. In this sense, the importance of checking the interactions of plants and herbal products needs to be stressed, since they are falsely believed to be innocuous and are not always so.27 Likewise, Drugs.com is notable for classifying the severity of the interaction of an active ingredient according to the route of administration used, and Liverpool for completing the consultation with drugs of abuse. In our case, we found that several patients took red yeast rice to regulate their cholesterol. Both Liverpool and Drugs.com classify the interaction of this supplement with ritonavir or cobicistat as potentially severe, as it is a product with a high content of lovastatin, directly involved in the metabolism pathway inhibited by enhancers. Although Micromedex® has registered the supplement, it offers no information on the potential interaction. Also worthy of note is the case of domperidone, classified as a severe interaction in Micromedex® and Liverpool, while not registered in Drugs.com, or silodosin, also classified as severe in Micromedex® and Drugs.com, while not registered in Liverpool. In the bibliography we consulted, there are other studies similar to ours in which databases of HIV+ patients are compared. Ramos et al. found a weighted kappa of 0.61 between Micromedex® and Drugs.com.28 Meanwhile, Monteith et al. checked the agreement between 6 databases (including Micromedex® and Drugs.com) for psychiatric medication and found the best concordance for severe interactions (0.695).29 These discrepancies highlight the need to consult various databases.
Careful review of interactions is a fundamental part of the pharmacotherapeutic follow-up carried out by pharmacists in a pharmaceutical care consultation for HIV+ patients, since this is also a population in which the frequency thereof is very high owing to the nature of the drugs used,30 and which is increasingly poly-medicated and ageing.31 It is not just about entering the drugs that the patient takes in one of the multiple software packages or web pages available, but also interpreting these results and applying them to each patient in particular, since on many occasions these alerts may lack clinical significance.32 In addition, excessive alerts and notices to doctors can lead to what is called “fatigue” and the recommendations made not being followed.33
As limitations, it should be noted that interactions were only analysed in patients undergoing treatment with pharmacokinetic enhancers and concomitant medications, but not between concomitant medications with each other, so only a small proportion of all patients receiving ARV treatment were studied, and not all possible interactions in these selected patients. Furthermore, we did not specifically ask about the use of substances of abuse, despite their high potential for interaction and frequent use in the current population.34 Despite having identified patients with ADRs, we have not conducted a study of the costs of avoided ADRs, which could have a considerable impact.30
As strong points, we would like to highlight that, despite this being a single-centre study, the sample was high, with almost a thousand patients included. In addition, we have reviewed interactions with food supplements and herbal products, substances that are often not considered in medication reconciliation processes and which can have potentially fatal interactions.27 In a study very similar to ours, Hollywood et al. found that up to 43% of the medicines taken by their study population are of the OTC (over the counter) type and therefore do not need a prescription and may go unnoticed if a correct consultation is not performed.35
It should also be noted that we consulted more than one database, when the rest of the studies usually choose a single one to conduct their studies. Lastly, it is important to note that not only have we analysed the potential of the interactions, but we have also found out the causality of those ADRs related to the interactions.
We can conclude that interactions between an ART with pharmacokinetic enhancers in its composition and concomitant medication are frequent and require a wide variety of interventions, ranging from monitoring treatments more closely, to substitutions for therapeutic equivalents, to changes in the ATR itself. Furthermore, these interactions are often the cause of ADRs, which is why active surveillance by physicians and pharmacists and their close collaboration become essential for the safe use of these drugs. Checking interactions in different databases is highly recommended, since in many cases the information between them is complementary, the agreement between them is sometimes poor, and some are incomplete.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Vélez-Díaz-Pallarés M, Esteban-Cartelle B, Montero-Llorente B, Gramage-Caro T, Rodríguez-Sagrado MÁ, Bermejo-Vicedo T. Interacciones de cobicistat y ritonavir en pacientes con VIH y sus consecuencias clínicas. Enferm Infecc Microbiol Clin. 2020;38:212–218.