The sensitivities of conventional mycobacterial culture in solid or liquid media and acid-fast bacilli (AFB) smear microscopy for Mycobacterium tuberculosis complex (MTBC) detection in extrapulmonary specimens are suboptimal. We evaluated the field performance of the Abbott RealTime MTB assay for the diagnosis of extrapulmonary tuberculosis in a low-prevalence setting.
MethodsThe total number of extrapulmonary specimens with mycobacterial culture and PCR results was 566: sterile fluids (n=278), non-sterile fluids (n=147), lymph node material (n=69) tissue biopsies (n=63), and abscess aspirates (n=9). A composite standard consisting of mycobacterial culture results, clinical treatment response to anti-TB drugs, when administered, and histopathology, radiological and laboratory findings were used as a reference for sensitivity and specificity calculations.
ResultsMycobacterial cultures and PCR were positive in 17 and 28 specimens, respectively. The overall agreement between culture and PCR was moderate (Cohen's kappa coefficient: 0.549; P=0.0001). Taking as a reference our composite standard, the sensitivity of the Abbott PCR assay was 77.7%, the specificity 99.5%, the PPV 95.4%, and the NPV 98.8%. In turn, the sensitivity of the mycobacterial culture was 62.9%, the specificity and PPV 100%, and the NPV 97.9%.
ConclusionThe good field performance of the Abbott RealTime MTB assay makes it valuable for the diagnosis of extrapulmonary tuberculosis in a low-prevalence setting. The use of molecular methods along with culture improves the diagnosis of extrapulmonary tuberculosis.
La sensibilidad del cultivo convencional de micobacterias en medios sólidos o líquidos y la de la microscopía de bacilos ácido-alcohol resistentes para detectar el complejo Mycobacterium tuberculosis en muestras extrapulmonares es subóptima. Evaluamos el rendimiento del ensayo Abbott RealTime MTB para el diagnóstico de la tuberculosis extrapulmonar en un entorno de baja prevalencia.
MétodosEl número total de muestras extrapulmonares con cultivo de micobacterias y resultados de la reacción en cadena de la polimerasa fue de 566: líquidos estériles (n=278), líquidos no estériles (n=147), material de los ganglios linfáticos (n=69), biopsias de tejido (n=63) y aspiraciones de abscesos (n=9). Para calcular la sensibilidad y la especificidad del ensayo se utilizó como referencia un parámetro que incluyó: resultados del cultivo, respuesta clínica al tratamiento con antituberculosos y hallazgos de laboratorio, radiológicos e histopatológicos.
ResultadosLos cultivos de micobacterias y la PCR fueron positivos en 17 y 28 muestras, respectivamente. La concordancia de los resultados obtenidos por ambos métodos fue moderada (coeficiente kappa de Cohen: 0,549; p=0,0001). La sensibilidad de la PCR de Abbott fue del 77,7%, especificidad del 99,5 %, valor predictivo positivo del 95,4% y valor predictivo negativo del 98,8%. La sensibilidad del cultivo fue del 62,9%, la especificidad y el valor predictivo positivo del 100% y el valor predictivo negativo del 97,9%.
ConclusiónEl buen rendimiento del ensayo Abbott RealTime MTB lo hace valioso para el diagnóstico de la tuberculosis extrapulmonar en un entorno de baja prevalencia. El uso de métodos moleculares junto al cultivo mejora el diagnóstico de la tuberculosis extrapulmonar.
Tuberculosis (TB) is a major global health problem, particularly in low/middle income countries.1 Although TB most commonly involves the lungs, it may affect other anatomic sites (extrapulmonary tuberculosis-EPTB-). The incidence of EPTB has increased in recent years, most notably in immunodeficient populations, nowadays representing up to 15% of notified cases worldwide.1 The sensitivities of conventional mycobacterial culture in solid or liquid media and acid-fast bacilli (AFB) smear microscopy for Mycobacterium tuberculosis complex (MTBC) detection in extrapulmonary specimens are suboptimal, likely due to their low bacterial load content, yet culture is considered the gold standard.2 Several nucleic acid amplification tests (NAAT) targeting MTBC have been marketed in the last decade; most of them, however, have only received approval by international agencies for use in respiratory specimens. Notwithstanding, NAAT, especially real-time PCR (polymerase chain reaction) assays, are widely used for EPTB diagnosis with variable success depending upon the platform employed and the specimen type subjected to analysis.3 The Abbott RealTime MTB Assay (Abbott Molecular Inc., Des Plaines, IL, USA), displays high analytical sensitivity with respiratory specimens (limit of detection of 2.45CFU/ml using the H37Rv strain spiked into AFB-negative sputum samples), which appears to exceed that of mycobacterial cultures (nearly 10CFU/ml).4,5 There is scant information on the performance characteristics of this PCR assay with extrapulmonary samples.6,7 In these cited studies archived specimens were used for PCR analyses. Here, we evaluated the clinical yield performance of the Abbott PCR assay for the diagnosis of EPTB in our low prevalence setting.
Patients and methodsStudy settingPatients in this series received medical attention at the Clínico-Malvarrosa (Valencia), Gandía (Valencia), and Sagunto (Valencia) Health Departments in the Valencian Community, a region located in Eastern Spain. According to the Surveillance and Epidemiological Control Service of the Valencian Community the overall rate of TB notification in 2016 at these health departments was 11.9, 11.5 and 13.3 per 100,000 inhabitants, respectively.
Study populationWe retrospectively reviewed AFB-smear microscopy, culture and MTBC PCR results of consecutive extrapulmonary specimens submitted to our laboratory upon clinical suspicion of tuberculosis by the attending physician between February 2016 to February 2019 for routine diagnosis of mycobacterial disease, as well as patients’ clinical charts. The current study was approved by the Ethics Committee at the Hospital Clínico Universitario Fundación INCLIVA.
Conventional mycobacterial proceduresAfter collection, all samples were immediately transported to our laboratory and processed within 24h. AFB-smear microscopic examination by auramine-thiazine red staining was performed using non-concentrated specimens. Positive slides were confirmed by Ziehl-Neelsen staining. All specimens, irrespective of whether they were deemed sterile or non-sterile, were digested and decontaminated with 2% N-acetyl-l-cysteine-sodium hydroxide, neutralized with phosphate buffer (67mM; pH 6.8), followed by centrifugation at 3000×g for 20min.2,8 Biopsy and tissue specimens were thoroughly minced using a pair of sterile scissors and disaggregated in a mortar prior to decontamination. Sediments were resuspended in approximately 3ml of phosphate buffer irrespective of the quantity of original specimens available for the analyses and used for inoculation onto Löwenstein-Jensen (LJ) slant tubes (0.5ml) and Bactec MGIT 960 vials (0.2ml) (Becton, Dickinson and Sparks, MD, USA), which were then incubated at 37°C for 3 months and 8 weeks with continuous monitoring, respectively.9,10 Identification of MTBC isolates was performed by the SD TB Ag MPT 64 Rapid Immunochromatographic test (Standard Diagnostics, Seoul, South Korea). Differentiation of MTBC complex members was achieved using the GenoType MTBC VER 1.X (Hain Lifescience, Hardwiesenstraße, Germany). Species identification of nontuberculous Mycobacteria (NTM) was performed using the GenoType Mycobacterium CM or AS (Hain Lifescience, Hardwiesenstraße, Germany) or 16S rRNA gene sequence analysis.9,10 AFB smears were performed at the respective local laboratories (Hospital Clínico Universitario of Valencia-HCUV-, Hospital Francisco de Borja, Gandía, and Hospital de Sagunto), while culture, identification and PCR assays were performed at HCUV.
Real-time MTB PCR assayThe Abbott Real Time MTB assay detects 8 species belonging to MTBC (M. tuberculosis, M. africanum, M. bovis, M. bovis BCG, M. caprae, M. canetti, M. microti and M. pinnipedii) through the amplification of both the insertion sequence IS6110 and the protein antigen B (PAB) gene. The assay was performed as previously reported.9,10 Briefly, 1.2ml of each decontaminated and concentrated sediment was loaded into the Abbott m2000sp instrument for nucleic acid extraction. Amplification, MTBC detection and data interpretation were performed on the Abbott m2000rt real-time PCR instrument. According to the manufacturer, specimens displaying PCR cycle thresholds ≤40.0 were considered positive.
Statistical methodsA composite standard consisting of mycobacterial culture results, clinical treatment response to anti-MTBC drugs, when administered, and histopathology, radiological and laboratory findings, when available, were used as a reference for sensitivity and specificity calculations (10). A panel of three institutional Infectious Diseases specialists and pneumologist reviewed clinical charts to categorize patients with discordant PCR/culture results as either likely having EPTB or not. In this study, according to recently revised World Health Organization (WHO) criteria11 a bacteriologically confirmed TB case was one from which a biological specimen was positive by smear microscopy, culture or molecular assay.11
The number of true positives (tp), true negatives (tn), false positives (fp), and false negatives (fn) were calculated on a per sample basis and entered into 2×2 tables. Sensitivity and specificity were calculated based on these tables as tp/(tp+fn) and tn/(tn+fp), respectively. Positive predictive value (PPV) was equal to (tp) reported on total positive, and negative predictive value (NPV) was equal to (tn) reported on the total negative. The prevalence of ETPB was not considered for PPV and NPV calculations. Cohens Kappa coefficients were calculated using the statistical software R (http://www.r-project.org).
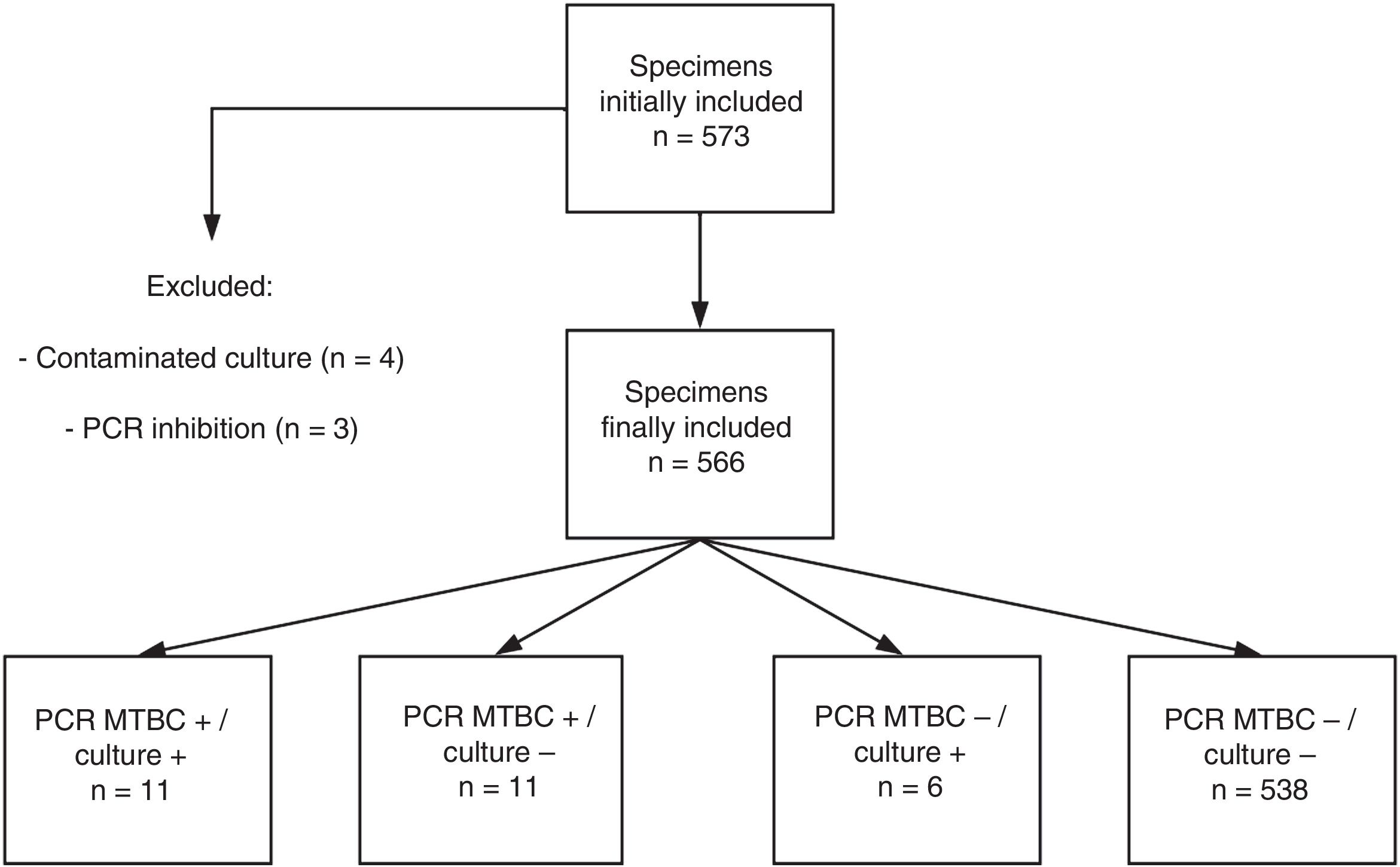
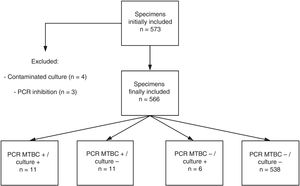
ResultsPatients and specimens included in the studyA total of 573 specimens from 555 patients were analyzed. Redundant specimens (n=15) from a few patients (n=13) were included in the study. No samples from patients under treatment with anti-TB drugs were present in our series. Four specimens yielded contaminated cultures (0.7%) and were excluded from further analyses. PCR inhibition was observed in 3 specimens (0.5%) (Fig. 1). Thus, a total of 566 specimens were finally eligible for comparison purposes. These specimens were sterile fluids (n=278), non-sterile fluids (n=147), lymph node material obtained following surgery (n=44) or fine needle aspiration (n=25), non-lymph node tissue biopsies (n=63), and abscess aspirates (n=9) (Table 1).
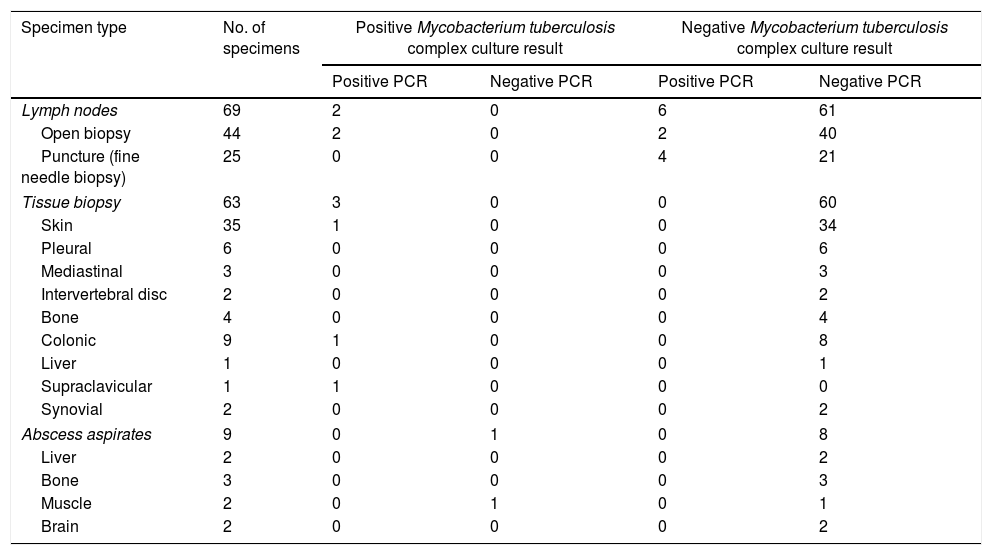
Performance of the Abbott Real Time MTB assay in comparison with and culture for Mycobacterium tuberculosis complex in organ or tissue-derived specimens.
| Specimen type | No. of specimens | Positive Mycobacterium tuberculosis complex culture result | Negative Mycobacterium tuberculosis complex culture result | ||
|---|---|---|---|---|---|
| Positive PCR | Negative PCR | Positive PCR | Negative PCR | ||
| Lymph nodes | 69 | 2 | 0 | 6 | 61 |
| Open biopsy | 44 | 2 | 0 | 2 | 40 |
| Puncture (fine needle biopsy) | 25 | 0 | 0 | 4 | 21 |
| Tissue biopsy | 63 | 3 | 0 | 0 | 60 |
| Skin | 35 | 1 | 0 | 0 | 34 |
| Pleural | 6 | 0 | 0 | 0 | 6 |
| Mediastinal | 3 | 0 | 0 | 0 | 3 |
| Intervertebral disc | 2 | 0 | 0 | 0 | 2 |
| Bone | 4 | 0 | 0 | 0 | 4 |
| Colonic | 9 | 1 | 0 | 0 | 8 |
| Liver | 1 | 0 | 0 | 0 | 1 |
| Supraclavicular | 1 | 1 | 0 | 0 | 0 |
| Synovial | 2 | 0 | 0 | 0 | 2 |
| Abscess aspirates | 9 | 0 | 1 | 0 | 8 |
| Liver | 2 | 0 | 0 | 0 | 2 |
| Bone | 3 | 0 | 0 | 0 | 3 |
| Muscle | 2 | 0 | 1 | 0 | 1 |
| Brain | 2 | 0 | 0 | 0 | 2 |
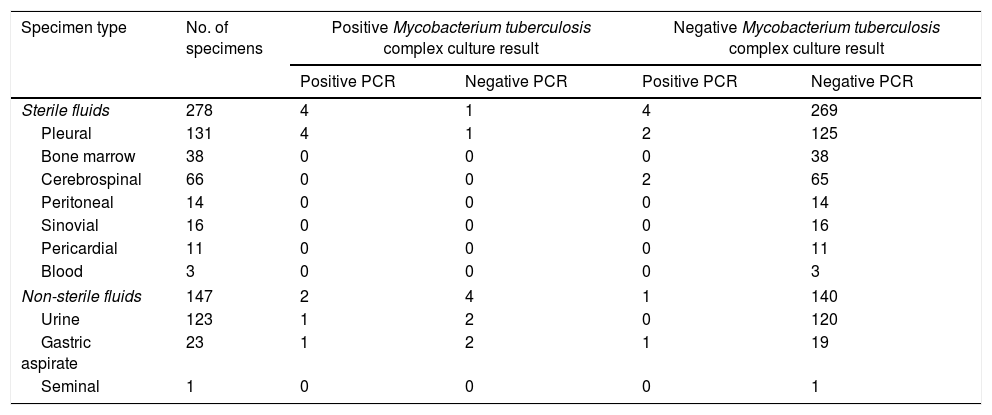
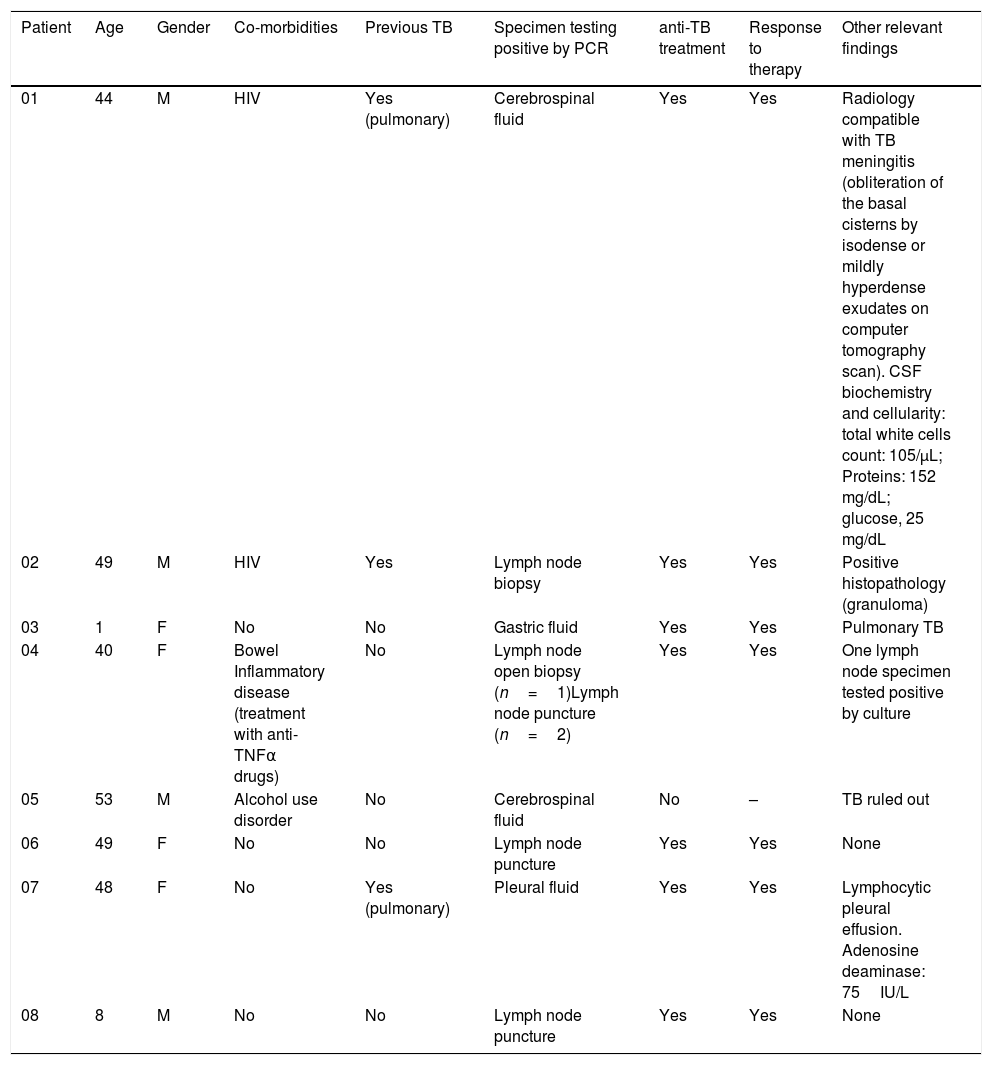
The current series included a total of 25 bacteriologically confirmed cases of EPTB. As shown in Fig. 1, and Tables 1 and 2, 11 specimens (4 pleural fluids, one urine, one gastric aspirate, 2 lymph node material obtained after surgery and 3 non lymph-node tissue biopsies) from unique patients yielded positive MTBC results by both culture and PCR: 10 were Mycobacterium tuberculosis and one Mycobacterium bovis, the latter detected/recovered from an urine specimen, There were 6 specimens (2 urines, 2 gastric fluids, one muscle abscess, and one pleural fluid) from unique patients that tested positive by culture but negative by PCR (5 were Mycobacterium tuberculosis and one Mycobacteriumbovis). Eleven specimens from 8 patients were PCR-positive and culture negative (lymph node material obtained after fine needle aspiration-n=4- or following surgery-n=2-; pleural fluid, n=2; cerebrospinal fluid, n=2; gastric aspirate, n=1). A total of 534 specimens retrieved negative results by AFB microscopy, PCR and culture. Finally, NTMs were isolated from 4 specimens (Mycobacteriumchimera, n=2; Mycobacterium gordonae, n=1; Mycobacterium celatum, n=1), and these yielded negative PCR results. Two specimens from unique patients tested positive by AFB-smear microscopy and these were PCR and culture positive. The overall agreement between culture and PCR was moderate (96% of agreement; Cohen's k: 0.549; P=0.0001). Among the 11 PCR-positive culture-negative specimens, only one (cerebrospinal fluid) was deemed to be a false positive PCR result on the basis of clinical and laboratory grounds (patient 5 in Table 3). This latter specimen yielded a high PCR cycle threshold value (39.7). The specimens yielding true positive PCR results exhibited PCR cycle threshold values ranging from 28.2 to 34.7. Thus, taking as a reference our composite standard, the sensitivity of the Abbott PCR assay was 77.7, the specificity 99.5%, the PPV was 95.4%, and the NPV 98.8%. In turn, the sensitivity of mycobacterial culture was 62.9%, the specificity and PPV 100%, and the NPV 97.9%. The sensitivity of the Abbott PCR and culture differed according to the type of specimen. Specifically, the sensitivity of the PCR assay was 88% with sterile fluids (mainly pleural exudates), 42% with non-sterile fluids and 100% with lymph nodes material, whereas that of culture was 44%, 85% and 25%, respectively.
Performance of the Abbott Real Time MTB assay in comparison with and culture for Mycobacterium tuberculosis complex in sterile and non-sterile fluids.
| Specimen type | No. of specimens | Positive Mycobacterium tuberculosis complex culture result | Negative Mycobacterium tuberculosis complex culture result | ||
|---|---|---|---|---|---|
| Positive PCR | Negative PCR | Positive PCR | Negative PCR | ||
| Sterile fluids | 278 | 4 | 1 | 4 | 269 |
| Pleural | 131 | 4 | 1 | 2 | 125 |
| Bone marrow | 38 | 0 | 0 | 0 | 38 |
| Cerebrospinal | 66 | 0 | 0 | 2 | 65 |
| Peritoneal | 14 | 0 | 0 | 0 | 14 |
| Sinovial | 16 | 0 | 0 | 0 | 16 |
| Pericardial | 11 | 0 | 0 | 0 | 11 |
| Blood | 3 | 0 | 0 | 0 | 3 |
| Non-sterile fluids | 147 | 2 | 4 | 1 | 140 |
| Urine | 123 | 1 | 2 | 0 | 120 |
| Gastric aspirate | 23 | 1 | 2 | 1 | 19 |
| Seminal | 1 | 0 | 0 | 0 | 1 |
Demographic, clinical, and microbiological data of patients who tested positive with the Abbott Real Time MTB PCR and negative by culture for Mycobacterium tuberculosis complex.
| Patient | Age | Gender | Co-morbidities | Previous TB | Specimen testing positive by PCR | anti-TB treatment | Response to therapy | Other relevant findings |
|---|---|---|---|---|---|---|---|---|
| 01 | 44 | M | HIV | Yes (pulmonary) | Cerebrospinal fluid | Yes | Yes | Radiology compatible with TB meningitis (obliteration of the basal cisterns by isodense or mildly hyperdense exudates on computer tomography scan). CSF biochemistry and cellularity: total white cells count: 105/μL; Proteins: 152 mg/dL; glucose, 25 mg/dL |
| 02 | 49 | M | HIV | Yes | Lymph node biopsy | Yes | Yes | Positive histopathology (granuloma) |
| 03 | 1 | F | No | No | Gastric fluid | Yes | Yes | Pulmonary TB |
| 04 | 40 | F | Bowel Inflammatory disease (treatment with anti-TNFα drugs) | No | Lymph node open biopsy (n=1)Lymph node puncture (n=2) | Yes | Yes | One lymph node specimen tested positive by culture |
| 05 | 53 | M | Alcohol use disorder | No | Cerebrospinal fluid | No | – | TB ruled out |
| 06 | 49 | F | No | No | Lymph node puncture | Yes | Yes | None |
| 07 | 48 | F | No | Yes (pulmonary) | Pleural fluid | Yes | Yes | Lymphocytic pleural effusion. Adenosine deaminase: 75IU/L |
| 08 | 8 | M | No | No | Lymph node puncture | Yes | Yes | None |
The use of real-time PCR assays has proven to be helpful in the diagnosis of various forms of EPTB, in particular those in which smear and culture tests are rarely positive, such as pleural, meningitis, abdominal or osteoarticular TB.2,12,13 In a recent meta-analysis3 including data from 25 studies, aggregated estimates of sensitivity and specificity of real-time PCRs for the diagnosis of EPTB were 70% and 99%, respectively. Many of the cited studies used the COBAS TaqMan MTB (Roche Diagnostics), the Xpert MTB/RIF (Cepheid) or laboratory-developed Taqman assays targeting sequences within either the IS6110 or the 16S rRNA genes. In a previous review, Maynard-Smith and colleagues14 evaluated the diagnostic accuracy of the Xpert MTB/RIF for diagnosing different forms of extra-pulmonary tuberculosis and found that the pooled summary estimates of sensitivity when testing smear-positive and smear-negative samples were quite different, as would be presumed (95% vs. 69%, respectively), and that these varied substantially across specimen types, being highest for lymph node tissue (96%) and lowest for pleural fluids (34%).
Here we evaluated the performance of the Abbott RealTime MTB Assay. Of relevance, unlike previous studies evaluating the above6,7 or other real-time PCR assays (i.e. 15.1), a composite standard was used as the reference for the sensitivity and specificity analyses, and importantly the specimens were tested by PCR upon reception in the laboratory. As for the latter, cryopreservation has been shown to decrease the detection yield of MTBC by real-time PCR assays.15
The Abbott RealTime MTB assay was found to perform well, both in terms of sensitivity, which was overall higher than that of conventional mycobacterial culture, and specificity, just yielding one false positive, according to our composite standard, and 6 false-negative results, these mostly being non-sterile fluid specimens (urine and gastric fluid). Notably, the Abbott PCR assay performed particularly well with lymph node material, and pleural fluids. In contrast it performed worse than culture for non-sterile fluids (urine and gastric aspirates). When interpreting our results, it has to be considered that only 2 specimens were positive by AFB-smear microscopic examination by auramine-thiazine red staining, this suggesting that most specimens in our series were paucibacillary. Notably, very few PCR inhibitions were recorded (n=3).
Two recently published studies have evaluated the performance of the Abbott assay with extrapulmonary specimens.6,7 Hinic and colleagues6 examined a limited set of extrapulmonary specimens (n=18) and reported an overall sensitivity and specificity of 100%. In turn, Hofmann-Thiel et al.7 evaluated 107 extrapulmonary samples and found an overall sensitivity of 84.2% and a specificity of 100%. It is important to note that these6,7 were not clinical yield studies, as cryopreserved specimens were used for PCR testing, which may account for the lower sensitivity of the assay reported in the current study.
The current study has several limitations that deserve comment; first, the relatively limited number of specimens either testing positive by culture, PCR or both; second, the sensitivity of culture in particular, and perhaps also that of the PCR assay, could have been underestimated due to our protocol for sample processing by which specimens with low-content material were not prioritized for culture; third, the three specialists assessing clinical charts were not blinded to the test results; this may have introduced a bias.
In summary, despite the above limitations, our data support the feasibility of the Abbott PCR assay for the diagnosis of EPTB in a low prevalence setting. It should be nevertheless taken into account the fact that the Abbott PCR MTB assay do not provide data on the genotypic resistance of MTBC to anti-TB drugs, and also, as other commercially-available molecular assays, neither do inform about the viability of mycobacteria nor about the presence of NTM.
Further studies are warranted to assess the performance of the Abbott assay in particular in sample series enriched in specimen types with very low bacterial DNA content, such as pleural fluids or cerebrospinal fluids.
Financial disclosuresThis research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical statementThe current study was approved by the Ethics Committee at the Hospital Clínico Universitario Fundación INCLIVA.
Informed consentThe need to obtain informed consent was waived because the PCR assays were included in our laboratory test menu.
Conflict of interestsThe authors declare no conflict of interest.
We would like to thank the personnel of the HCU mycobacterial and molecular laboratories for their technical assistance. Estela Giménez holds a Río Hortega research contract from the Carlos III Health Institute (ISCIII) (Ref. CM16/00200).