Our aim was to evaluate the variability in the processing of osteoarticular samples for microbiological diagnosis between different national centers.
MethodsAn online survey with 13 questions was designed and it was sent through SEIMC to the Microbiology Departments of the different centers.
ResultsWe obtained 72 answers. In more than half of the tertiary-care centers, at least 5 samples are received. Approximately half of the centers vortex bones/implant samples. Sonication is used mostly in tertiary-care centers. Cultures for aerobic and anaerobic bacteria are performed during 6-10 days of incubation but with differences in liquid culture media. Molecular diagnosis is performed in less than 50% of the centers. Universal PCR (16S rRNA) is the most widely used technique.
ConclusionWe found heterogeneity especially in the use of sonication, liquid culture media or molecular diagnosis, probably in direct relation to the resources and capacities of each center.
Nuestro objetivo fue evaluar la variabilidad en el procesamiento de muestras osteoarticulares para el diagnóstico microbiológico entre distintos centros nacionales.
MétodosSe diseñó una encuesta online con 13 preguntas y se difundió a través de la SEIMC a los servicios de microbiología de los distintos centros.
ResultadosObtuvimos 72 respuestas. En más de la mitad de los centros, de tercer nivel, se reciben al menos 5 muestras. Aproximadamente la mitad de los centros agitan con vórtex las muestras de hueso/implante, utilizándose la sonicación sobre todo en centros de tercer nivel. Se realizan cultivos para bacterias aerobias y anaerobias durante 6-10 días de incubación, pero con diferencias en los medios de cultivo líquidos. El diagnóstico molecular se realiza en menos del 50% de los centros, siendo la PCR universal (ARNr 16S) la técnica más empleada.
ConclusionesExiste heterogeneidad fundamentalmente en el empleo de sonicación, los medios líquidos de cultivo o el diagnóstico molecular, probablemente en relación directa con los recursos y capacidades de cada centro.
Population ageing and an increase in traffic accidents, as well as developments in technology, have led to increased numbers of surgical procedures with orthopaedic implants. This has in turn led to non-negligible rates of osteoarticular infections (1%-2% in prosthetic joints and up to 15% in complex open fractures). Such infections are associated with significant morbidity and mortality, in addition to economic costs for the healthcare system. Hence, multidisciplinary management is of growing importance for optimal diagnosis and treatment.1
Microbiological diagnosis of these infections is complex, since in some cases the micro-organisms responsible grow slowly and are involved in biofilm formation. This renders their isolation in culture and microbiological identification more difficult. Furthermore, chronic infections may have a patchy distribution such that multiple samples from different anatomical sites are needed to boost the odds of recovering the causal micro-organism.1 Culture remains the gold standard for diagnosing osteoarticular infections.2 Our objective was to evaluate variability in the processing of osteoarticular samples for microbiological diagnosis at different hospitals across Spain.
MethodsA survey with 13 single-answer and multiple-answer questions regarding microbiological processing of osteoarticular samples was prepared (Table 1). The survey was sent online through the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [Spanish Society of Infectious Diseases and Clinical Microbiology] (SEIMC) to all its members, who had a 30-day period to complete it. After the 30 days had elapsed, the survey was closed and the results obtained were compiled. The first two questions were about the level of care and management at the hospital; the other 11 questions were about the amount and type of osteoarticular samples received as well as the pre-processing and processing of said samples for purposes of microbiological diagnosis. The questions covered matters such as the use of rapid staining, solid and/or liquid culture media, incubation times and molecular diagnostic techniques.
Single-answer and multiple-answer questions put to the hospitals surveyed.
| Question | Answers |
|---|---|
| 1. The hospital at which you work is:a | - A public hospital. |
| - A privately managed public hospital. | |
| - A private hospital. | |
| 2. At what type of hospital do you work?a | - A primary hospital (a local hospital with few specialisations and up to approximately 200 beds). |
| - A secondary hospital (a provincial or regional hospital with several specialisations and up to approximately 800 beds). | |
| - A tertiary hospital (a highly specialised central, university or reference hospital with up to approximately 1,500 beds). | |
| 3. In prosthetic joint infection, how many osteoarticular samples do you usually receive per patient?a | - ≤3 samples. |
| - 5 samples. | |
| - >5 samples. | |
| - It varies. | |
| 4. In prosthetic joint infection, what type of samples are received?b | - Joint/synovial fluid. |
| - Tissue/biopsy. | |
| - Bone. | |
| - Prosthetic/osteosynthesis materials. | |
| 5. Regarding sample processing:b | - Centrifugation is performed on fluid samples. |
| - Staining is performed on tissue/biopsy. | |
| - Vortex mixing is performed on bone and/or prosthetic/osteosynthesis materials. | |
| - Sonication is performed on prosthetic/osteosynthesis materials. | |
| 6. What types of rapid staining do you use for direct sample examination?b | - Gram staining. |
| - Calcofluor white staining. | |
| - Ziehl–Neelsen and/or auramine staining. | |
| 7. Regarding seeding in solid media:a | - Seeding is performed only in aerobic media. |
| - Seeding is performed only in anaerobic media. | |
| - Seeding is performed in aerobic and anaerobic media. | |
| - Seeding is performed only in liquid media, not in solid media. | |
| 8. Regarding seeding in liquid media:a | - Thioglycolate or BHI broth is used. |
| - Direct blood culture bottle spiking is used. | |
| - Thioglycolate or BHI broth plus direct blood culture bottle spiking is used. | |
| - Seeding is performed only in solid media, not in liquid media. | |
| 9. What incubation conditions do you use for fluid samples (joint/synovial fluid)?a | - Incubation for 2-5 days at 37 °C in CO2/anaerobiosis. |
| - Incubation for 6-10 days at 37 °C in CO2/anaerobiosis. | |
| - Incubation for 11-14 days at 37 °C in CO2/anaerobiosis. | |
| - Incubation for 15-21 days at 37 °C in CO2/anaerobiosis. | |
| 10. What incubation conditions do you use for solid samples (tissue/biopsy, etc.)?a | - Incubation for 2-5 days at 37 °C in CO2/anaerobiosis. |
| - Incubation for 6-10 days at 37 °C in CO2/anaerobiosis. | |
| - Incubation for 11-14 days at 37 °C in CO2/anaerobiosis. | |
| - Incubation for 15-21 days at 37 °C in CO2/anaerobiosis. | |
| 11. With respect to molecular biology diagnostic techniques (PCR):b | - They are performed in parallel to culture. |
| - They are performed in cases of negative culture. | |
| - They are performed in cases of antibiotic therapy prior to sample collection. | |
| - They are not performed. | |
| - Other. | |
| 12. If they are performed, what samples are they performed on?b | - On joint/synovial fluid. |
| - On tissue/biopsy. | |
| - On fluid from bone and/or prosthetic/osteosynthesis materials mixed in a vortex mixer or sonicated. | |
| - On any sample. | |
| 13. If they are performed, what type of technique is used?b | - Universal (16S) PCR. |
| - In-house PCR for the most common pathogens. | |
| - Commercial PCR for the most common pathogens (FilmArray®, Unyvero®, etc.). | |
| - Specific PCR for Staphylococcus aureus. | |
| - Other. |
BHI: brain heart infusion; CO2: carbon dioxide; PCR: polymerase chain reaction.
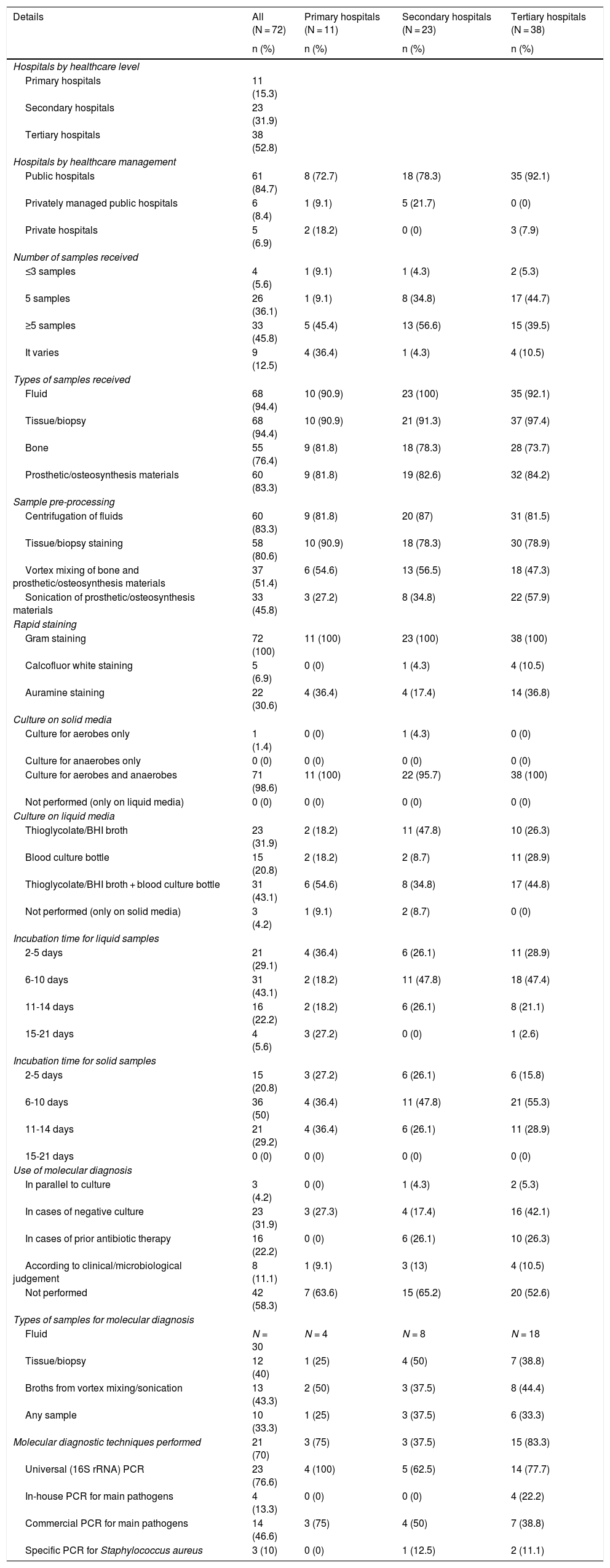
A total of 72 responses from different hospitals across Spain were compiled. By level of care, more than half (52.8%) were tertiary hospitals, followed by secondary hospitals (31.9%) and primary hospitals (15.3%). The vast majority were public hospitals (84.7%). The details drawn from the survey, taken together and broken down by type of hospital, are shown in Table 2. At least five osteoarticular samples per patient were received by the microbiology departments at approximately half the secondary and tertiary hospitals; the primary hospitals showed more variability. At all the hospitals, in more than 90% of cases, fluid or tissue/biopsy samples were received, followed closely by bone samples and samples of prosthetic/osteosynthesis materials (73%-84%). More than 75% of the hospitals pre-processed fluids by centrifugation and tissues/biopsies by staining, while around 50% of them pre-processed bone and/or prosthetic/osteosynthesis materials in a vortex mixer. Sonication of prosthetic/osteosynthesis materials was performed at 45.8% of the hospitals; at tertiary hospitals specifically, this figure rose to 57.9%. Gram staining was performed in all cases (100%) at all hospitals, whereas calcofluor white and auramine staining were done occasionally. In virtually all cases, solid culture media were used for both aerobic and anaerobic micro-organisms. The different hospitals varied with respect to liquid culture media; however, the vast majority (95.8%) used some sort of liquid medium, and 43.1% of them used a combination of thioglycolate broth or brain heart infusion (BHI) broth plus the blood culture bottle. Incubation times for liquid and solid media also varied quite a bit, but tended to be six to 10 days and rarely more than 14 days. Molecular diagnosis on direct samples was performed at up to 41.7% of the hospitals and, when used, was performed on any type of sample with no clear distinction. In most cases, it was done in cases of negative cultures and prior administration of antibiotic therapy to the patient. Among the hospitals that used molecular biology techniques, the most commonly used technique was in-house universal (16S ribosomal RNA [rRNA]) polymerase chain reaction (PCR) testing at 76.6% of hospitals, followed by commercial PCR testing with selective detection of the main pathogens causing osteoarticular infections. At 26.7%, universal 16S PCR testing was combined with other techniques such as in-house and commercial PCR testing for the most common osteoarticular pathogens as well as specific PCR testing for Staphylococcus aureus.
Microbiological processing of osteoarticular samples at the different hospitals surveyed.
| Details | All (N = 72) | Primary hospitals (N = 11) | Secondary hospitals (N = 23) | Tertiary hospitals (N = 38) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Hospitals by healthcare level | ||||
| Primary hospitals | 11 (15.3) | |||
| Secondary hospitals | 23 (31.9) | |||
| Tertiary hospitals | 38 (52.8) | |||
| Hospitals by healthcare management | ||||
| Public hospitals | 61 (84.7) | 8 (72.7) | 18 (78.3) | 35 (92.1) |
| Privately managed public hospitals | 6 (8.4) | 1 (9.1) | 5 (21.7) | 0 (0) |
| Private hospitals | 5 (6.9) | 2 (18.2) | 0 (0) | 3 (7.9) |
| Number of samples received | ||||
| ≤3 samples | 4 (5.6) | 1 (9.1) | 1 (4.3) | 2 (5.3) |
| 5 samples | 26 (36.1) | 1 (9.1) | 8 (34.8) | 17 (44.7) |
| ≥5 samples | 33 (45.8) | 5 (45.4) | 13 (56.6) | 15 (39.5) |
| It varies | 9 (12.5) | 4 (36.4) | 1 (4.3) | 4 (10.5) |
| Types of samples received | ||||
| Fluid | 68 (94.4) | 10 (90.9) | 23 (100) | 35 (92.1) |
| Tissue/biopsy | 68 (94.4) | 10 (90.9) | 21 (91.3) | 37 (97.4) |
| Bone | 55 (76.4) | 9 (81.8) | 18 (78.3) | 28 (73.7) |
| Prosthetic/osteosynthesis materials | 60 (83.3) | 9 (81.8) | 19 (82.6) | 32 (84.2) |
| Sample pre-processing | ||||
| Centrifugation of fluids | 60 (83.3) | 9 (81.8) | 20 (87) | 31 (81.5) |
| Tissue/biopsy staining | 58 (80.6) | 10 (90.9) | 18 (78.3) | 30 (78.9) |
| Vortex mixing of bone and prosthetic/osteosynthesis materials | 37 (51.4) | 6 (54.6) | 13 (56.5) | 18 (47.3) |
| Sonication of prosthetic/osteosynthesis materials | 33 (45.8) | 3 (27.2) | 8 (34.8) | 22 (57.9) |
| Rapid staining | ||||
| Gram staining | 72 (100) | 11 (100) | 23 (100) | 38 (100) |
| Calcofluor white staining | 5 (6.9) | 0 (0) | 1 (4.3) | 4 (10.5) |
| Auramine staining | 22 (30.6) | 4 (36.4) | 4 (17.4) | 14 (36.8) |
| Culture on solid media | ||||
| Culture for aerobes only | 1 (1.4) | 0 (0) | 1 (4.3) | 0 (0) |
| Culture for anaerobes only | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Culture for aerobes and anaerobes | 71 (98.6) | 11 (100) | 22 (95.7) | 38 (100) |
| Not performed (only on liquid media) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Culture on liquid media | ||||
| Thioglycolate/BHI broth | 23 (31.9) | 2 (18.2) | 11 (47.8) | 10 (26.3) |
| Blood culture bottle | 15 (20.8) | 2 (18.2) | 2 (8.7) | 11 (28.9) |
| Thioglycolate/BHI broth + blood culture bottle | 31 (43.1) | 6 (54.6) | 8 (34.8) | 17 (44.8) |
| Not performed (only on solid media) | 3 (4.2) | 1 (9.1) | 2 (8.7) | 0 (0) |
| Incubation time for liquid samples | ||||
| 2-5 days | 21 (29.1) | 4 (36.4) | 6 (26.1) | 11 (28.9) |
| 6-10 days | 31 (43.1) | 2 (18.2) | 11 (47.8) | 18 (47.4) |
| 11-14 days | 16 (22.2) | 2 (18.2) | 6 (26.1) | 8 (21.1) |
| 15-21 days | 4 (5.6) | 3 (27.2) | 0 (0) | 1 (2.6) |
| Incubation time for solid samples | ||||
| 2-5 days | 15 (20.8) | 3 (27.2) | 6 (26.1) | 6 (15.8) |
| 6-10 days | 36 (50) | 4 (36.4) | 11 (47.8) | 21 (55.3) |
| 11-14 days | 21 (29.2) | 4 (36.4) | 6 (26.1) | 11 (28.9) |
| 15-21 days | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Use of molecular diagnosis | ||||
| In parallel to culture | 3 (4.2) | 0 (0) | 1 (4.3) | 2 (5.3) |
| In cases of negative culture | 23 (31.9) | 3 (27.3) | 4 (17.4) | 16 (42.1) |
| In cases of prior antibiotic therapy | 16 (22.2) | 0 (0) | 6 (26.1) | 10 (26.3) |
| According to clinical/microbiological judgement | 8 (11.1) | 1 (9.1) | 3 (13) | 4 (10.5) |
| Not performed | 42 (58.3) | 7 (63.6) | 15 (65.2) | 20 (52.6) |
| Types of samples for molecular diagnosis | ||||
| Fluid | N = 30 | N = 4 | N = 8 | N = 18 |
| Tissue/biopsy | 12 (40) | 1 (25) | 4 (50) | 7 (38.8) |
| Broths from vortex mixing/sonication | 13 (43.3) | 2 (50) | 3 (37.5) | 8 (44.4) |
| Any sample | 10 (33.3) | 1 (25) | 3 (37.5) | 6 (33.3) |
| Molecular diagnostic techniques performed | 21 (70) | 3 (75) | 3 (37.5) | 15 (83.3) |
| Universal (16S rRNA) PCR | 23 (76.6) | 4 (100) | 5 (62.5) | 14 (77.7) |
| In-house PCR for main pathogens | 4 (13.3) | 0 (0) | 0 (0) | 4 (22.2) |
| Commercial PCR for main pathogens | 14 (46.6) | 3 (75) | 4 (50) | 7 (38.8) |
| Specific PCR for Staphylococcus aureus | 3 (10) | 0 (0) | 1 (12.5) | 2 (11.1) |
BHI: brain heart infusion; PCR: polymerase chain reaction.
For the diagnosis of prosthetic joint infections, the majority of the hospitals surveyed in our study collected at least five intraoperative samples from different locations. Atkins et al. were the first to evaluate the sensitivity and performance of the number of samples for correct diagnosis of prosthetic joint infection. They conducted a prospective evaluation of 297 joint replacement procedures and found five to six cultures to yield the best diagnostic sensitivity.3 The American guidelines (Infectious Diseases Society of America [IDSA], 2013) recommended obtaining at least three and ideally five to six intraoperative samples from different periprosthetic locations.4 Bémer et al. subsequently concluded that four samples processed on three different culture media represented a highly sensitive and affordable method for diagnosing osteoarticular infections.5 Their findings were consistent with those of Peel et al. and the latest International Consensus Meeting (ICM) consensus document (Philadelphia, 2018), both of which advocated for at least three samples with additional blood culture bottles.6,7
In addition, samples of bone and prosthesis material were mostly processed by vortex mixing, and to a lesser extent by sonication mainly at tertiary hospitals, probably as an effect of available hospital resources. Survey results varied with regard to using a combination of liquid and solid culture media, though they were uniform in relation to incubation time. Liquid media (preferably blood culture bottles) should be combined with solid media to improve sensitivity.6,7 At most of the different hospitals, incubation times were six to 10 days; this was consistent with the SEIMC recommendation that the incubation time for a conventional culture should be no less than seven days and should be prolonged when slow-growing micro-organisms are suspected.1
Finally, molecular biology techniques were used at a significant proportion of the hospitals surveyed, mainly the tertiary hospitals, and universal (16S rRNA) PCR testing was the most commonly used technique, probably due to available hospital resources as mentioned above. With certain overlap, they also used universal 16S PCR testing along with other available in-house and commercial techniques. In general, universal PCR, while less sensitive than conventional culture, is very useful in negative cultures, patients with prior antibiotic therapy and infections caused by fastidious, difficult-to-grow micro-organisms.1 In specific cases of strong suspicion, targeted PCR testing for specific pathogens such as S. aureus, with greater sensitivity than universal PCR, could be used.1,8 The molecular technique selected, whatever it may be, should always be used in combination with the rest of the microbiological and non-microbiological diagnostic methodology available to optimise diagnosis.
In conclusion, we found mixed results with respect to the use of liquid media, sonication and molecular diagnosis. The use of latter two was likely directly tied to the resources available at the hospital. It is recommended that working methodologies be standardised to the extent possible, according to national and international guidelines, to minimise this inter-hospital variability and thus prevent potential discrepancies in the results obtained.
FundingThe authors declare that they did not receive any funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank the SEIMC for their help and support. We would also like to thank the participants at the various hospitals who completed this survey.
Please cite this article as: Falces-Romero I, Rico-Nieto A. Procesamiento de muestras osteoarticulares para diagnóstico microbiológico: resultados de una encuesta multicéntrica nacional. Enferm Infecc Microbiol Clin. 2022;40:317–321.