The generalization of treatment with dexamethasone or other immunosuppressants in patients with SARS-CoV-2 infection may increase the risk of occurrence of severe forms of strongyloidiasis. A nationwide survey was conducted to better understand the diagnostic and therapeutic situation of strongyloidiasis in SARS-CoV-2 co-infected patients in Spain.
Materials and methodsA survey was designed and sent to all SEIMC members during February and March 2021. Responses were exported for computer processing to Microsoft Excel 2017 and statistically processed with the free software PSPP.
Results189 responses were received, of which 121 (64%) were selected for further processing. Eighty-four centers (69.5%) had no specific strongyloidiasis screening protocol. Forty-two centers (34.7%) had serological techniques available in their laboratories and the rest were sent to a reference laboratory. Only 22 centers (18%) screened for strongyloidiasis in SARS-CoV-2 infected patients. A total of 227 cases of strongyloidiasis were diagnosed in patients with SARS-CoV-2 infection. In four cases patients developed a massive hyperinfestation syndrome leading to the death of one patient.
ConclusionCOVID-19 has highlighted the need to unify screening and treatment protocols for imported pathologies such as strongyloidiosis. Efforts to disseminate knowledge are needed to ensure that this potentially fatal disease is adequately treated in patients with the highest risk of complications, such as those with COVID-19.
La generalización del tratamiento con dexametasona u otros inmunosupresores en pacientes con infección SARS-CoV-2 puede aumentar el riesgo de aparición de formas graves de estrongiloidiosis. Se realizó una encuesta a nivel nacional para conocer mejor de la situación diagnóstica y terapéutica de la estrongiloidiasis en España en pacientes coinfectados por SARS-CoV-2.
Materiales y métodosSe diseñó una encuesta que fue enviada a todos los miembros de SEIMC durante los meses de febrero y marzo de 2021. Las respuestas se exportaron para su procesamiento informático al programa Microsoft Excel 2017 y se procesaron estadísticamente con el software libre PSPP.
ResultadosSe recibieron 189 respuestas de las cuales se seleccionaron 121(64%) para su procesamiento posterior. En ochenta y cuatro centros (69.5%) no existía ningún protocolo de cribado específico de estrongiloidiosis. Cuarenta y dos centros (34.7%) disponían de técnicas serológicas en sus laboratorios y en el resto se enviaban a un laboratorio de referencia. Solo 22 centros (18%) realizaron cribado de estrongiloidiasis en pacientes infectados por SARS-CoV-2. Se diagnosticaron 227 casos de estrongiloidiasis en pacientes con infección por el SARS-CoV-2. En cuatro casos los pacientes desarrollaron un síndrome de hiperinfestación masiva que condujo al fallecimiento de uno.
ConclusiónLa COVID-19 ha puesto de manifiesto la necesidad de unificar protocolos de cribado y tratamiento de patologías importadas como la estrongiloidiosis. Es necesario realizar un esfuerzo de difusión del conocimiento para que esta patología potencialmente mortal sea tratada adecuadamente en los pacientes con mayor riesgo de complicaciones como son aquellos con COVID-19.
Strongyloidiasis is an infection caused by Strongyloides stercoralis, a globally prevalent nematode. It is estimated that at least 370 million people are infected worldwide, with a high degree of endemic burden found in tropical areas of Southeast Asia, sub-Saharan Africa and Latin America1. Its distinctive characteristics include its ability to persist and replicate in the host through an auto-infection cycle that allows it to survive over the years and its capacity to produce severe clinical symptoms, mainly in immunosuppressed patients, such as hyperinfection syndrome or disseminated strongyloidiasis associated with high mortality rates2,3. The factors favouring these disseminated forms include the use of steroids or other immunosuppressive treatments, the presence of solid or haematological neoplasms, human immunodeficiency virus (HIV) infection or being a transplant recipient4–7. Moreover, the disseminated forms can produce symptoms similar to those of septic shock or another infection with severity criteria such as meningitis, making it difficult to distinguish in the absence of adequate diagnostic suspicion. In the case of patients with COVID-19, the emergence of new pulmonary infiltrates accompanied by clinical and blood gas deterioration may be indistinguishable from the evolution of the SARS-CoV-2 infection, complicating prognosis even further.
The outbreak of the SARS-CoV-2 pandemic posed an unprecedented challenge from the standpoint of infectious diseases, not only because of the appearance of the actual virus but also because of its influence on the reactivation of other concomitant infections. In the case of parasites, this influence is poorly characterised8, but the generalisation of treatment with dexamethasone or other immunosuppressants9,10 in patients with SARS-CoV-2 infection is expected to increase the risk of the appearance of severe forms of strongyloidiasis in the absence of adequate and early diagnostic suspicion11–13. In addition, the combination of dexamethasone with tocilizumab, an IL-6 inhibitor known to improve survival in patients with COVID-19 with hyperinflammation10, could be an additional risk factor for disseminated strongyloidiasis14 due to an decreased Th2 immune response conducive to nematode infections15,16.
We decided to conduct a nationwide survey in order to glean a better understanding of the diagnostic and therapeutic situation of strongyloidiasis in Spain and more specifically in patients co-infected with SARS-CoV-2.
Materials and methodsFor the purposes of the study, a survey was designed based on the opinion of a panel of experts from the Grupo de Patología Importada [Imported Pathology Group] (GEPI) of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [Spanish Society of Infectious Diseases and Clinical Microbiology] (SEIMC). The SEIMC is the main Spanish society for infectious diseases and has 4001 members, 1946 of whom practice clinical microbiology, whereas 2055 are infectious disease specialists. The society's excellent implantation in Spain made it possible to obtain the high level of participation shown in the results, covering practically all the Autonomous Communities and a wide array of centres, which was fundamental in guaranteeing a representative sample.
The questions posed included: a) general characteristics of the centre (number of hospital beds, existence of an imported diseases specialised unit, a specific unit for parasitology in the microbiology laboratory, activity of the microbiology laboratory 24/7; b) staffing and equipment of the microbiology laboratory, including the techniques and protocols used for screening and diagnosis of strongyloidiasis; c) access to screening for strongyloidiasis in patients with SARS-CoV-2; d) availability of treatment for strongyloidiasis.
The survey was designed using the Google Forms platform and was sent to all SEIMC members in February and March 2021. If responses were received from several members of the same hospital, a single response was selected. The responses were exported to Microsoft Excel 2017 for computer processing and were statistically processed with the free PSPP software.
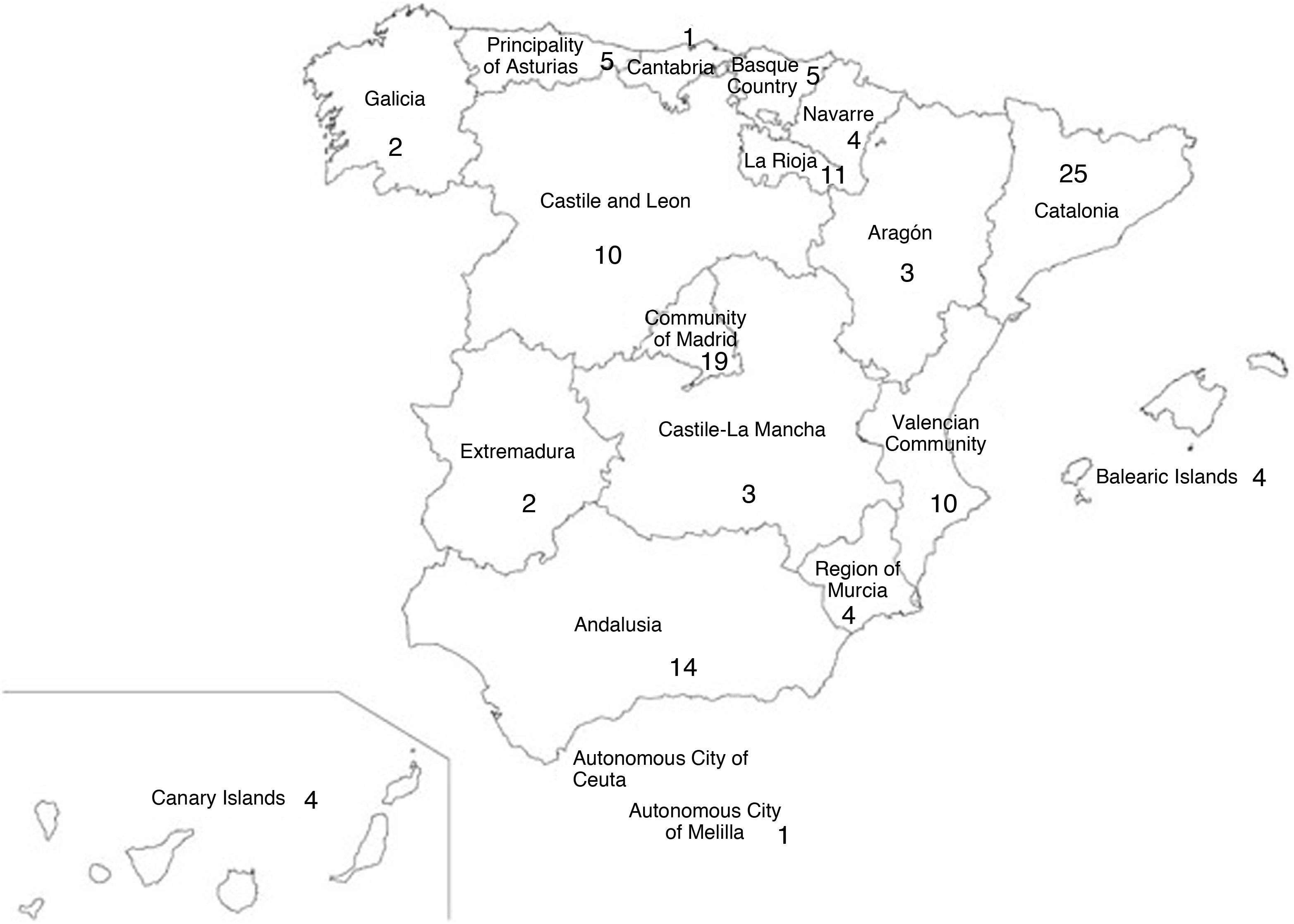
ResultsInitially, 189 responses were received, 121 (64%) of which were selected for subsequent processing after duplicate centres had been removed. The responses covered all the autonomous communities in Spain (Fig. 1).
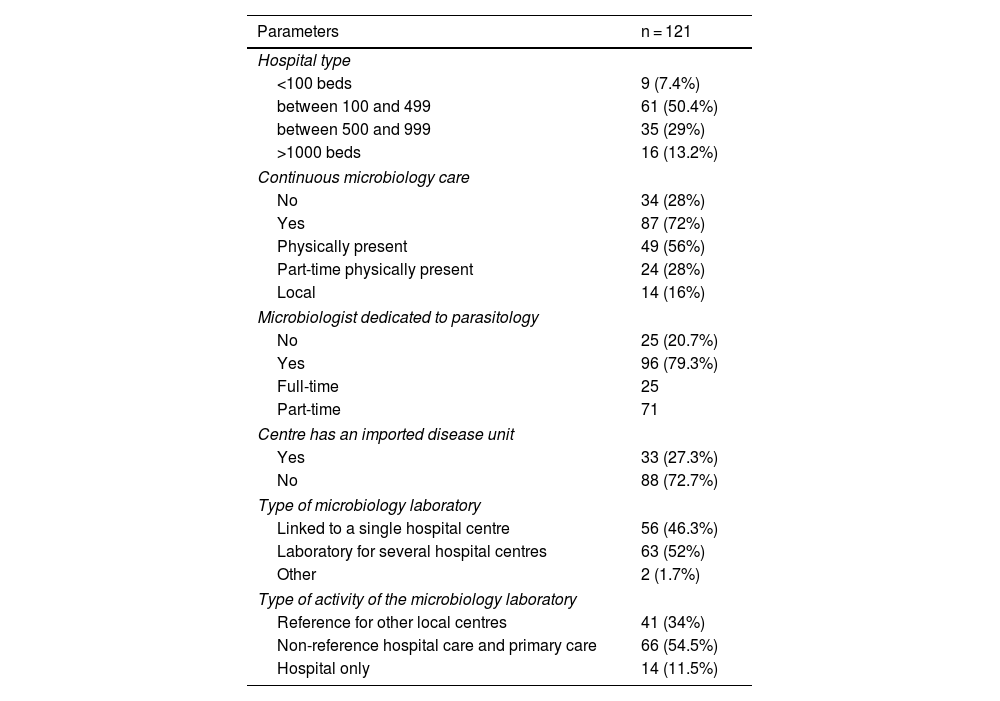
General characteristicsRegarding the number of hospital beds, most of the completed surveys corresponded to centres with 100–499 beds (50.4%), followed by hospitals with 500–999 (29%), those with more than 1000 beds (13.2%) and those with less than 100 beds (7.4%). With regard to the type of microbiology laboratory service, 66 served both the hospital and the health centres under the authority of their area, 44 were reference laboratories and the rest provided only hospital care. The general characteristics of the centre are shown in Table 1.
General characteristics of the participating centres.
| Parameters | n = 121 |
|---|---|
| Hospital type | |
| <100 beds | 9 (7.4%) |
| between 100 and 499 | 61 (50.4%) |
| between 500 and 999 | 35 (29%) |
| >1000 beds | 16 (13.2%) |
| Continuous microbiology care | |
| No | 34 (28%) |
| Yes | 87 (72%) |
| Physically present | 49 (56%) |
| Part-time physically present | 24 (28%) |
| Local | 14 (16%) |
| Microbiologist dedicated to parasitology | |
| No | 25 (20.7%) |
| Yes | 96 (79.3%) |
| Full-time | 25 |
| Part-time | 71 |
| Centre has an imported disease unit | |
| Yes | 33 (27.3%) |
| No | 88 (72.7%) |
| Type of microbiology laboratory | |
| Linked to a single hospital centre | 56 (46.3%) |
| Laboratory for several hospital centres | 63 (52%) |
| Other | 2 (1.7%) |
| Type of activity of the microbiology laboratory | |
| Reference for other local centres | 41 (34%) |
| Non-reference hospital care and primary care | 66 (54.5%) |
| Hospital only | 14 (11.5%) |
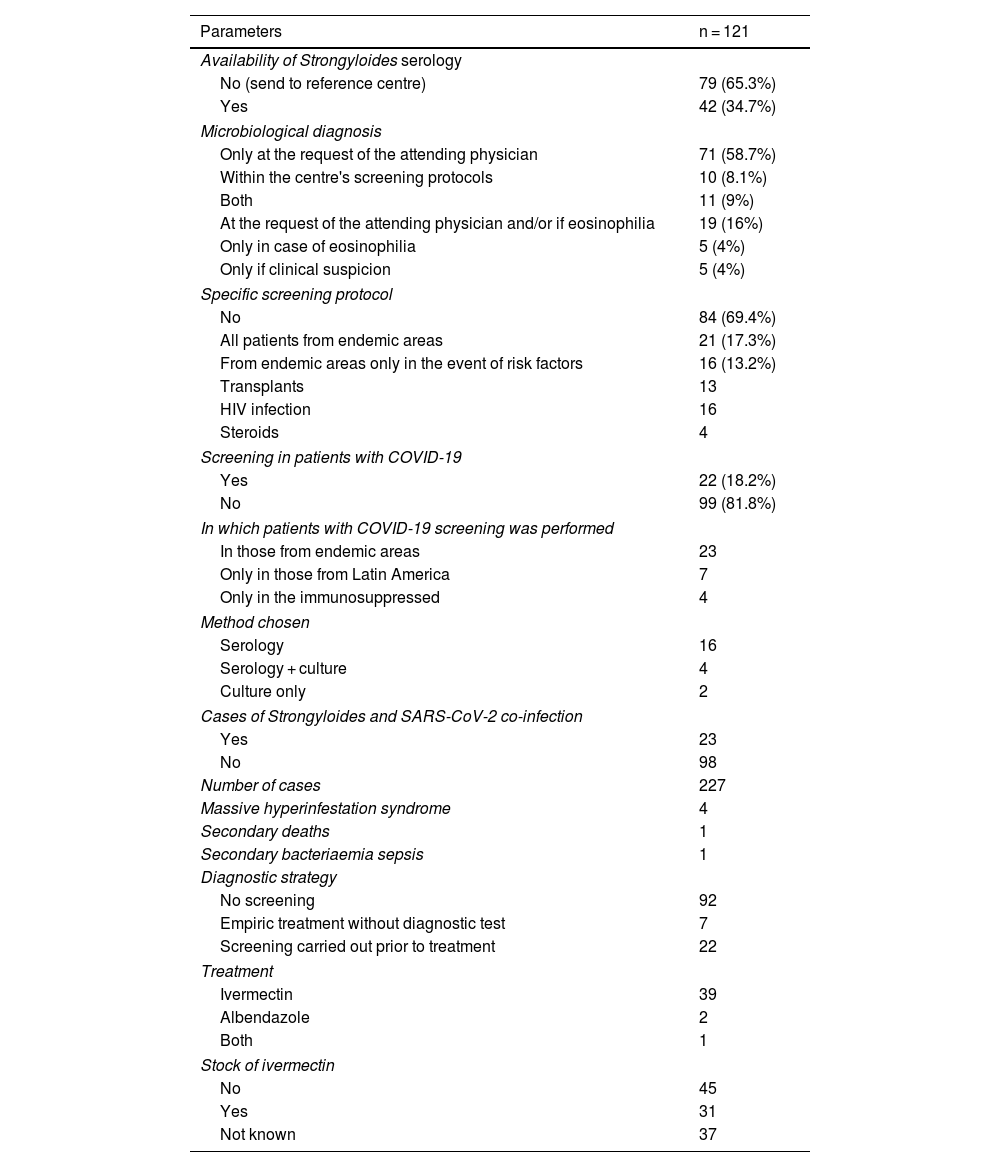
In 84 of the 121 centres (69.5%) there was no specific screening protocol for strongyloidiasis. Of the remaining centres, in 21 (17.3%) screening was performed in all patients from endemic areas and in the other 16 (13.2%) screening was only performed in the presence of risk factors that included HIV infection in all of them, transplant in 13, although only 4 were screened in the case of steroid treatment (Table 2).
Situation regarding the clinical and microbiological diagnosis.
| Parameters | n = 121 |
|---|---|
| Availability of Strongyloides serology | |
| No (send to reference centre) | 79 (65.3%) |
| Yes | 42 (34.7%) |
| Microbiological diagnosis | |
| Only at the request of the attending physician | 71 (58.7%) |
| Within the centre's screening protocols | 10 (8.1%) |
| Both | 11 (9%) |
| At the request of the attending physician and/or if eosinophilia | 19 (16%) |
| Only in case of eosinophilia | 5 (4%) |
| Only if clinical suspicion | 5 (4%) |
| Specific screening protocol | |
| No | 84 (69.4%) |
| All patients from endemic areas | 21 (17.3%) |
| From endemic areas only in the event of risk factors | 16 (13.2%) |
| Transplants | 13 |
| HIV infection | 16 |
| Steroids | 4 |
| Screening in patients with COVID-19 | |
| Yes | 22 (18.2%) |
| No | 99 (81.8%) |
| In which patients with COVID-19 screening was performed | |
| In those from endemic areas | 23 |
| Only in those from Latin America | 7 |
| Only in the immunosuppressed | 4 |
| Method chosen | |
| Serology | 16 |
| Serology + culture | 4 |
| Culture only | 2 |
| Cases of Strongyloides and SARS-CoV-2 co-infection | |
| Yes | 23 |
| No | 98 |
| Number of cases | 227 |
| Massive hyperinfestation syndrome | 4 |
| Secondary deaths | 1 |
| Secondary bacteriaemia sepsis | 1 |
| Diagnostic strategy | |
| No screening | 92 |
| Empiric treatment without diagnostic test | 7 |
| Screening carried out prior to treatment | 22 |
| Treatment | |
| Ivermectin | 39 |
| Albendazole | 2 |
| Both | 1 |
| Stock of ivermectin | |
| No | 45 |
| Yes | 31 |
| Not known | 37 |
HIV: human immunodeficiency virus.
In the microbiology laboratory, the diagnosis of strongyloidiasis was made predominantly only at the request of the attending physician (71 centres, 59%). In 19 (16%) centres, in addition to the physician's request, diagnosis was added in the case of eosinophilia. In 11 (9%) centres it was performed according to both the laboratory's own protocols and at the request of the attending physician. Five centres (4%) did so only in the case of eosinophilia, and another 5 only in the case of compatible symptoms.
Forty-two (42) centres (34.7%) had serological techniques available in their laboratories, whereas they were sent to a reference laboratory in the other cases.
SARS-CoV-2 and strongyloidiasis coinfectionOnly 22 centres (18%) screened for strongyloidiasis in patients infected with SARS-CoV-2. Among the techniques used for screening, 90.9% of the centres used serology as the only diagnostic test. In two centres, the serology was complemented with culture in blood agar and culture was performed exclusively in two centres. Seven centres chose to start empiric treatment with ivermectin in the case of high clinical suspicion without performing a prior microbiological diagnosis.
A total of 227 cases of strongyloidiasis were diagnosed in patients with SARS-CoV-2 infection, corresponding to 23 centres. In 4 cases, the patients developed a massive hyperinfestation syndrome that led to the death of one of them. One case developed bacterial-origin sepsis. The most utilised treatment was ivermectin, although albendazole was used in 2 cases and both of them in 1 patient. Ivermectin was available in the hospital only in 31 centres (25.6%), in 37 centres it proved impossible to ascertain the situation and in the rest it was not available.
DiscussionThe generalisation of steroid and immunomodulatory treatment for SARS-CoV-2 infection has heightened concerns about the emergence of severe forms of strongyloidiasis in at-risk patients. Our survey revealed a high number, and possibly underdiagnosed, of patients with S. stercoralis and SARS-CoV-2 co-infection, which is not surprising given the high prevalence of infection in the immigrant population. Previous studies report global seroprevalence rates of S. stercoralis of 12.2% in this group, reaching 17.3% in the case of immigrants from Asia, 14.6% for immigrants from sub-Saharan Africa and 11.4% for those from the Caribbean and Latin America11. Studies conducted in Spain on 12,796 patients found similar prevalences (9.7%), although in this case the main area of provenance was South America17–19.
As was to be expected, given the high number of immigrants residing in Spain, they have frequently been infected by SARS-CoV-2, with some studies observing a higher risk of infection in both Latin American and sub-Saharan patients20.
Previous works21 conducted in Spain show that up to 11% of patients hospitalised for COVID-19 had been born outside the country, were mostly of Latin American origin (5.9%) and they accounted for 3.3% of deaths.
In this context of high prevalence and nonspecific, or even absent, symptoms, most experts and international organisations concur on the need to screen and treat the population from high prevalence areas22,23. The Canadian clinical guidelines for immigrants and refugees consider screening for strongyloidiasis in immigrants from endemic areas with (i) signs and/or symptoms consistent with infection and/or (ii) eosinophilia22. The presence of eosinophilia is a sign that is classically associated with strongyloidiasis. However, there is little correlation between positive serology and the presence of eosinophilia in the blood. Although it is true that serology is more frequently positive in patients with eosinophilia, serology can also be positive in its absence, perhaps because it generally occurs in response to tissue invasion by a parasite, which is why it occurs intermittently and may be missed if just a single complete blood count is examined. Even lower rates of eosinophilia have been found in immunocompromised patients, and the sensitivity, specificity, and positive predictive value in cases of massive hyperinfestation syndrome are considered to be even lower3,19. For these reasons, other organisations consider that screening should be based fundamentally on the patients' area of origin23. In this regard, the European Centre for Disease Control recommends that serology screening be carried out in all immigrants with a high risk of exposure to S. stercoralis, regardless of the eosinophilia value (evidence level III D), with immigrants from Central and South America, sub-Saharan Africa and Southeast Asia being understood to be high-risk23. In the specific case of patients co-infected with SARS-CoV-2, there is a significant change in immunity, and the most severe cases are also associated with eosinopenia, which can mask the suspicion of this parasitic disease if only this marker is taken into account24.
Despite these institutional recommendations, our results show that there was only a specific screening protocol for strongyloidiasis in just over one third of the cases at the centre and that in those where it existed it only covered the immunocompromised population, in many cases without steroid treatment being included in this group, despite it being the main risk factor. This lack of clinical protocols correlates with the lack of protocols in microbiology laboratories, and in most cases the diagnosis is based solely on the opinion of the attending clinician and is completed with other criteria such as the presence of eosinophilia. As we already pointed out, the chronic forms of strongyloidiasis frequently course with non-specific symptoms and even without symptoms, hence the existence of both clinical and microbiological protocols is essential to reach an early diagnosis.
The choice of techniques used in screening varies depending on their availability and the experience of each laboratory. The definitive diagnosis of strongyloidiasis is made by detecting larvae in faeces. However, due to the intermittent shedding of larvae, the sensitivity of the microscopic examination of a single sample is low and is negative in up to 70% of cases. Different techniques have been used to improve the sensitivity of microscopic techniques, such as the Baermann technique, the formalin-ethyl acetate concentration technique, the Harada-Mori method with culture on filter paper moistened with hot water and cultures on blood agar plates25. Studies using stool microscopy have reported lower prevalence rates (0.8%–4.3%) than those obtained using serologic enzyme immunoassays (9%–77%)25,26, so serology is currently considered the method of choice for screening. Our study reveals that despite this, only 42 (34.7%) of the centres surveyed had serological techniques available at their laboratories and in the rest of the cases they were sent to a reference laboratory, substantially delaying the arrival of results.
Perhaps for this reason our results show that screening in patients with COVID-19 was scant and was only carried out in 18% of the centres surveyed. Despite this scant coverage, 227 cases of Strongyloides infection were diagnosed, giving some idea of the magnitude the infection could reach if screening were mainstreamed to cover the rest of the centres.
Since the responses to this study were voluntary, the real figure regarding the availability of serological techniques and centres with a screening protocol in all Spanish hospitals could be even lower due to the possibility of obtaining a lower response rate to the survey in centres that are not aware of the problem and therefore do not carry out screening.
In the case of patients with COVID-19, this screening is especially important due to the ability of S. stercoralis to persist and replicate in a host for long periods of time, as well as its potential to spread and cause life-threatening infection in an immunocompromised host. In the presence of some predisposing conditions such as immunosuppression due to the use of steroids or other drugs, transplant recipients or haematological patients, the disease can lead to severe forms such as hyperinfection syndrome or disseminated strongyloidiasis with variable prevalence and associated high rates of mortality3,4,11,19. The use of steroids has been shown to be the main predisposing factor for severe forms of strongyloidiasis regardless of the dose of the steroid2,19. In the study by Salvador et al.4, 83% of the patients had received a mean of 40 mg of prednisone, but the appearance of disseminated strongyloidiasis with doses of 20 mg of prednisone has been described. It also seems to be independent of treatment time and appears both in the case of single doses and in prolonged regimens19. The facilitating role played by other immunomodulators such as tocilizumab has been less studied, although it could be associated with reactivations of both this and other parasites15,16,27. Isolated cases of reactivation of strongyloidiasis have been described in patients on treatment with etanercept16.
The publication of the RECOVERY9 study data, demonstrating the benefit of steroid treatment in patients hospitalised for COVID-19 who required supplemental oxygen, has led to the generalised and worldwide use of dexamethasone. After the generalisation of steroid treatment for COVID-19, many of these patients from areas of high prevalence of S. stercoralis have received dexamethasone alone or in combination with tocilizumab. Rodríguez-Baño et al.28 describe the results of a cohort of patients treated with steroids at conventional and bolus doses, and/or tocilizumab. It is striking that 8% of the patients treated with tocilizumab were non-Caucasian, as were 2.7% of those treated with moderate-dose corticosteroids and 3.8% of those who received pulse steroids. This percentage rose to 10.2% in those who received combined treatment.
In this scenario, in which the generalisation of steroid treatment in the SARS-CoV-2 pandemic and the high prevalence of S. stercoralis in the immigrant population is combined, the possibility of a reactivation of strongyloidiasis becomes a significant and possibly underdiagnosed problem, since the symptoms of the disseminated form (appearance of pulmonary infiltrates, adult respiratory distress syndrome, digestive symptoms, etc.) can be indistinguishable from those presented by patients with COVID-19. Occasional cases of disseminated infection that required specific treatment with good evolution have been described in the literature12,13,29,30. All the cases had previously received steroids and one of them tocilizumab as well.
A specific strategy31 based on epidemiological risk stratification22 has recently been proposed to prevent Strongyloides hyperinfection/disseminated infection in patients with COVID-19 on treatment with steroids. This strategy proposes empiric treatment with ivermectin for at-risk patients who are starting or are candidates for steroids while awaiting the results of diagnostic tests. Even in the outpatient setting, in the presence of a risk factor for strongyloidiasis, empiric treatment (usually one dose) should be considered if it is not contraindicated and serology is not available. However, during the study period, ivermectin was only available as a foreign medication in Spain, which complicated this approach, as the results show. The recent marketing of ivermectin in Spain could facilitate compliance with this strategy. Another problem with this strategy would be the lack of screening, which would prevent real data being available on the magnitude of the problem of this infection.
Although it brings to light the situation regarding diagnosis of strongyloidiasis in Spain, the study is not without its limitations. On the one hand, the participants were not asked about the number of cases screened, which does not allow an estimation of the real prevalence. Furthermore, the survey did not elicit data on the characteristics of patients with hyperinfestation and it would have been useful to define this group's characteristics better.
In conclusion, COVID-19 has brought to light the need to harmonise screening and treatment protocols for other imported pathologies such as strongyloidiasis. As with other neglected diseases, an effort should be made to spread awareness to ensure that this potentially fatal pathology is adequately treated, particularly in patients with a higher risk of complications, such as those with COVID-19.
The authors wish to thank Javier Ávila and the other members of the SEIMC secretariat for their technical support in carrying out the work.