Sustained virological response (SVR) 12 weeks after the end-of-therapy (EOT) has been correlated with SVR24 for HCV-monoinfection. We aim to validate SVR12 as criterion for definition of HCV cure in HIV-coinfected patients treated with all-oral direct-acting antivirals (DAA).
MethodsProspectively observational study including HIV/HCV-coinfected subjects who received DAA and had HCV-RNA measures at weeks 12 and 24 after EOT. Every patient who took ≥1 drug dose was analyzed.
ResultsDAA were prescribed to 423 patients, of whom 387 had HCV-RNA measures both at weeks 12 and 24 after EOT. SVR12 was confirmed in 379/387 patients, while SVR24 was confirmed in 377/387 subjects. The positive-predictive-value (PPV) of SVR12 for SVR24 was 99.5% (95%CI: 98.1–99.9). One of the recurrences was clinically suspected to be a late relapse.
ConclusionsSVR12 has a high PPV for HCV cure in HIV/HCV-coinfection, though further follow-up could be necessary for those with deeper immunosuppression.
En monoinfección por VHC, se ha demostrado correlación entre la respuesta viral sostenida (RVS) 12 semanas posterapia antiviral con la RVS24. Proponemos validar la RVS12 como criterio de curación en sujetos coinfectados por VIH/VHC tratados con antivirales de acción directa (AAD).
MétodosEstudio observacional prospectivo con pacientes coinfectados VIH/VHC, tratados con AAD y con determinación de ARN-VHC en semanas 12 y 24 posterapia. Se analizó todo sujeto que tomó ≥1 dosis de AAD.
ResultadosSe prescribieron AAD a 423 sujetos: 387 tenían determinación de ARN-VHC en semanas 12 y 24 posterapia. Se confirmó RVS12 en 379/387 pacientes y RVS24 en 377/387. El valor predictivo positivo (VPP) de RVS12 para RVS24 fue del 99,5% (IC 95%: 98,1-99,9). Una recurrencia se interpretó clínicamente como recidiva tardía.
ConclusionesLa RVS12 tiene un elevado VPP para predecir curación de la infección por VHC en pacientes VIH-positivo, aunque podrían ser necesarios más controles en aquéllos más inmunosuprimidos.
Throughout the first years of treatment for HCV infection with interferon-based regimens, confirmation of the HCV eradication required an undetectable HCV-RNA measure performed 24 weeks after the end of therapy (EOT) (the so-called sustained virological response 24, SVR24).
Afterwards, the maintenance of undetectable HCV-RNA 12 weeks after EOT (sustained virological response 12, SVR12) was correlated with SVR24 in HCV-monoinfection.1–4 Thereafter, SVR12 has been used as criterion to define HCV cure in clinical trials and observational studies, and current Spanish guidelines recommend it.5
Nevertheless, despite the evidence for immunological participation in HCV eradication,6,7 there is no study validating the use of this criterion in HIV-coinfected patients, for whom immune response can be both quantitative and qualitatively impaired. Therefore, we aim to validate the SVR12 criterion in HIV/HCV-coinfected subjects treated with all-oral direct-acting antivirals (AAD).
MethodsPatients and designProspective observational study within the VIH-DOC Cohort (Hospital 12 de Octubre, Madrid, Spain). HIV/HCV-coinfected patients treated with DAA between 9 January 2015 and 31 August 2016 and with available data for HCV-RNA measures at weeks 12 and 24 after EOT were included.
ObjectiveTo evaluate the proportion of patients without SVR24 among those with SVR12 (positive predictive value, PPV).
VariablesSVR12 and SVR24 were confirmed by undetectable HCV-RNA 12 and 24 weeks after EOT, respectively.
Recurrence was defined by undetectable HCV-RNA at EOT with a detectable measure after EOT. It could be either a relapse (no change in HCV genotype before and after therapy, and no risk exposure for infection) or a reinfection (confirmed when there was a difference between the HCV genotypes before and after therapy, and suspected when there was no difference in the genotype study but the subject described a clear risk exposure).
Statistical analysisQualitative data are described as absolute numbers (percentage), and quantitative as medians (interquartile range – IQR). Each SVR proportion is accompanied by the binomially estimated 95% confidence interval (95%CI).
Every patient who took at least one drug dose was analyzed (intention-to-treat exposed approach).
The PPV of SVR12 for SVR24 was calculated as the proportion of patients with SVR24 among those with SVR12. The negative predictive value (NPV) of non-SVR12 for non-SVR24, as the proportion of patients without SVR24 among those without SVR12.
EthicsEvery patient belonging to the the VIH-DOC Cohort had signed a written consent which had been approved by the Local Ethical Committee. Clinical y laboratory data were collected anonymously.
ResultsA total of 423 HIV/HCV-coinfected patients received DAA in the study period (data published elsewhere).8 Out of them, HCV-RNA measures both at weeks 12 and 24 after EOT were available in 387/423 subjects.
The remaining 36 patients did not have this information due to the following causes: eight subjects had been diagnosed with HCV recurrence at week 12 after EOT and they were being retreated or their follow-up was lost at week 24 after the end of the first HCV treatment. Three patients achieved SVR12, but two of them died before SVR24 could be confirmed and the third one was lost for follow-up. The rest of the subjects (n=25) did not have HCV-RNA determination at week 12 after EOT owing to death (n=4) or lost for follow-up (n=21) at that moment.
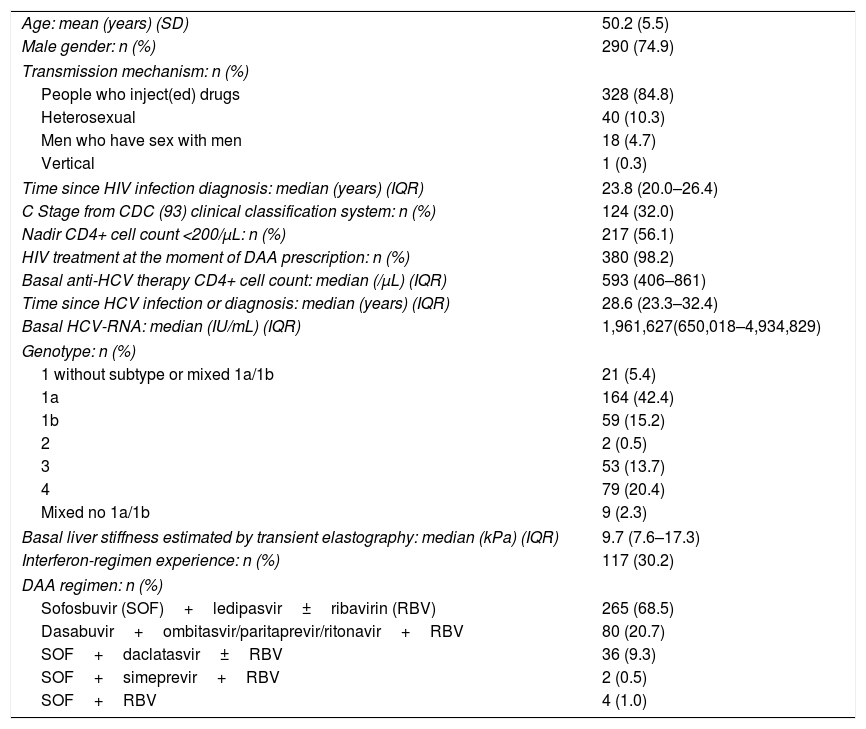
The characteristics of those 387 patients included in this study are shown in Table 1. Briefly, they were mainly men (74.9%), and the most frequent acquisition mechanism of the infection was drug injection (84.8%).
Baseline characteristics (n=387).
| Age: mean (years) (SD) | 50.2 (5.5) |
| Male gender: n (%) | 290 (74.9) |
| Transmission mechanism: n (%) | |
| People who inject(ed) drugs | 328 (84.8) |
| Heterosexual | 40 (10.3) |
| Men who have sex with men | 18 (4.7) |
| Vertical | 1 (0.3) |
| Time since HIV infection diagnosis: median (years) (IQR) | 23.8 (20.0–26.4) |
| C Stage from CDC (93) clinical classification system: n (%) | 124 (32.0) |
| Nadir CD4+ cell count <200/μL: n (%) | 217 (56.1) |
| HIV treatment at the moment of DAA prescription: n (%) | 380 (98.2) |
| Basal anti-HCV therapy CD4+ cell count: median (/μL) (IQR) | 593 (406–861) |
| Time since HCV infection or diagnosis: median (years) (IQR) | 28.6 (23.3–32.4) |
| Basal HCV-RNA: median (IU/mL) (IQR) | 1,961,627(650,018–4,934,829) |
| Genotype: n (%) | |
| 1 without subtype or mixed 1a/1b | 21 (5.4) |
| 1a | 164 (42.4) |
| 1b | 59 (15.2) |
| 2 | 2 (0.5) |
| 3 | 53 (13.7) |
| 4 | 79 (20.4) |
| Mixed no 1a/1b | 9 (2.3) |
| Basal liver stiffness estimated by transient elastography: median (kPa) (IQR) | 9.7 (7.6–17.3) |
| Interferon-regimen experience: n (%) | 117 (30.2) |
| DAA regimen: n (%) | |
| Sofosbuvir (SOF)+ledipasvir±ribavirin (RBV) | 265 (68.5) |
| Dasabuvir+ombitasvir/paritaprevir/ritonavir+RBV | 80 (20.7) |
| SOF+daclatasvir±RBV | 36 (9.3) |
| SOF+simeprevir+RBV | 2 (0.5) |
| SOF+RBV | 4 (1.0) |
SVR12 was observed in 379/387 patients (97.9%; 95%CI: 96.0–99.1), while SVR24 was confirmed in 377/387 (97.4%; 95%CI: 95.3–98.8). Therefore, the PPV of SVR12 for SVR24 was 99.5% (95%CI: 98.1–99.9); the NPV of non-SVR12 for non-SVR24 was 100% (95%CI: 63.1–100.0).
The two patients for whom SVR12 but not SVR24 was confirmed were both men with undetectable HIV-RNA under antiretroviral therapy before, while on and after DAA treatment, and DAA adherence >95%. In both cases, the same HCV genotype was found in the pre-therapy and post-therapy samples.
One of the patients had acquired the HIV/HCV-coinfection by intravenous drug use >20 years ago. This subject was treated with sofosbuvir/ledipasvir for 12 weeks (HCV-genotype 4, baseline liver stiffness 10.0kPa). His CD4+ cell count was 321/mm3 (17%) when the recurrence was detected. There was no clinical evidence of re-exposure. Thus, this recurrence was clinically considered as late HCV relapse.
The second man with HCV recurrence acquired the first HCV infection by homosexual contact. He received dasabuvir plus ombitasvir/paritaprevir/ritonavir plus ribavirin for 12 weeks (HCV-genotype 1a, baseline liver stiffness 8.8kPa). The CD4+ cell count was 1226/mm3 (45%) at the recurrence time. He reported unprotected receptive anal intercourse with another man (unknown sero-status) between weeks 12 and 24 after EOT. Though he denied both drug use while on sex and fisting, it was clinically interpreted as HCV reinfection.
No phylogenetic study could be performed to confirm the actual nature of these recurrences.
Both subjects have been successfully treated thereafter with sofosbuvir/velpatasvir/voxilaprevir for 12 weeks.
DiscussionWe corroborate the use of the SRV12 criterion to assume HCV cure after DAA therapy in HIV-coinfection.
Some studies have previously correlated the SVR12 measure with the SVR24 one. They analyzed HCV eradication with interferon plus ribavirin plus boceprevir or telaprevir,1 regimens based on sofosbuvir,2 or the combination of dasabuvir plus ombitasvir plus ritonavir-boosted paritaprevir.3 Moreover, Burgess et al. revised every data on interferon-free therapy reported in different conferences, with similar high correlation between SVR12 and SVR24.4
Nevertheless, those studies focus on HCV-monoinfected patients treated within clinical trials. Regarding HIV-coinfection, Sarrazin et al.9 studied the HCV-viremia situation at week 24 after EOT in patients who achieved SVR12 within the PHOTON trials without validating purposes and combining data with other trials on HCV-monoinfection. To the best of our knowledge, no other study has validated this widely used criterion for HIV/HCV-coinfected patients treated with these new regimens in a real-life setting.
Since one of the recurrences was probably a reinfection, the PPV could be even higher. Here, our data suggest the importance of frequent screening for early HCV reinfection in patients at risk, mainly men who have sex with men (MSM). A systematic review showed higher HCV recurrence rate in HIV/HCV-coinfected patients treated with interferon-based regimens when comparing with HCV-monoinfected subjects, with the highest number of cases in a cohort composed only with HIV-positive MSM.10 More recently, the Madrid-Core Cohort found a rate of HCV reinfection 28-fold higher among MSM treated with DAA compared to drug users.11 Therefore, a closer reinfection surveillance is widely recommended in this population after SVR12 is confirmed.12
Regarding the recurrence interpreted as late relapse, the phylogenetic tests performed in different sofosbuvir-trials demonstrated that 42% of the detected recurrences between weeks 12 and 24 after EOT were due to the emerge of the primary HCV infection.9
Certainly, there are data about HCV-RNA persistence after SVR in peripheral blood mononuclear cells,13 and a recent research evinced HCV replication in gastrointestinal mucosa.14 Nevertheless, since HCV-RNA does not integrate within the host DNA, the explanation for very late relapses remains uncertain. Anyway, it is postulated that the immune system is involved in the eradication of those remaining infected cells.6,7 In fact, a large cohort study on predictive factors of HCV eradication with DAA in HIV/HCV-coinfection shows that CD4+ cell count <200/mm3 predicts lower response.15 Larger studies are needed to state whether SVR12 criterion has no high PPV enough to confirm HCV eradication in HIV/HCV-coinfected patients with poor immunological situation.
This study has some limitations. First of all, concerning the probability of early HCV reinfection mainly in MSM with risk behavior, the predominance of injected drug users in our cohort could make these results not generalizable. Nevertheless, the reinfection is considered a different event from treatment failure, so its exclusion when estimating SVR12 PPV in other populations would allow a better accuracy of this parameter. Secondly, we could not perform phylogenetic analysis in case of HCV recurrence to confirm relapse vs. reinfection, but they are hardly available in other centers, too. Third, new pangenotypic DAA regimens with even higher efficacy have been approved since this cohort was treated. However, insofar as the reinfection and, probably, the late relapse events are related to patients’ behavior and immunological characteristics, respectively, SVR12 PPV in this new therapy era is expected to be similar.
In conclusion, SVR12 accurately predicts HCV cure in HIV-coinfected subjects, thought the need for further measurements in immunocompromised patients should be addressed in larger studies.
FundingThis study was conducted without any financial support from either public or private entities. All the reported payments and honoraria have been received by every author outside the submitted work.
Conflict of interest- -
LDD, ML, OB and MM have received payment for lectures and financial support for expert courses and Congress from Abbvie, Gilead, Janssen, Merck Sharp and Dome, and ViiV Healthcare.
- -
RR reports payment for lectures from Abbott, Boehringer Ingelheim, Bristol-Myers-Squibb, Gilead, Janssen, Merck Sharp and Dome and ViiV.
- -
FP has received honoraria for lectures and advisory boards from Abbvie, Gilead, Janssen, Merck Sharp and Dome and ViiV.
Previous presentation of work: These results were partially presented as poster in the XXII National Congress of the SEIMC (“Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica” – Spanish Society of Infectious Diseases and Clinical Microbiology), Bilbao, Spain (Abstract Number 0549).