Two-stage exchange is the gold standard in the surgical management of prosthetic joint infection (PJI). However, perioperative reinfections (RePJI) can occur to newly inserted prosthesis, which highlights the importance of an adequate antibiotic prophylaxis, although there is scarce evidence in this field. Our objective was to evaluate the characteristics of RePJI, its prognosis and the antibiotic prophylaxis that is commonly used in second-stage surgery.
MethodsMulticentric retrospective observational study in Spanish hospitals including patients with RePJI between 2009 and 2018.
ResultsWe included 92 patients with RePJI from 12 hospitals. The most frequent isolated microorganism was Staphylococcus epidermidis in 35 cases (38.5%); 61.1% of staphylococci were methiciliin-resistant. In 12 cases (13%), the same microoganism causing the primary PJI was isolated in RePJI. When comparing with the microbiology of primary PJI, there were more cases caused by Gram-negative bacteria (the most frequent was Pseudomonas spp.) and less by Gram-positive bacteria. Failure occured in 69 cases (75%). There were 43 different courses of antibiotic prophylaxis after the second-stage surgery; the most frequent was a unique preoperative cefazolin dose, but most patients received prophylaxis before and after the second-stage surgery (61 cases).
ConclusionsThe most frequent microorganisms in RePJI are coagulase-negative staphylococci, although Gram-negative bacteria, especially Pseudomonas spp. are also common. There is a significant heterogeneity in antibiotic prophylaxis for a second-stage surgery. ReIPJI treatment has a high failure rate.
El recambio en 2 tiempos es un procedimiento habitual en el tratamiento de las infecciones de prótesis articular (IPA). Sin embargo, la prótesis que se coloca en el segundo tiempo (2T) puede reinfectarse de nuevo (ReIPA). Además, existe escasa evidencia sobre qué profilaxis antibiótica debe utilizarse en el 2T. Nuestro objetivo es describir las características de las ReIPA, su pronóstico y las profilaxis antibióticas que se emplean habitualmente en la cirugía del 2T.
MétodosEstudio observacional retrospectivo descriptivo multicéntrico en hospitales españoles de pacientes con ReIPA en el periodo 2009–2018.
ResultadosSe registraron 92 casos de 12 hospitales. El microorganismo más frecuentemente implicado fue Staphylococcus epidermidis con 35 casos (38,5%). El 61,1% de Staphylococcus spp. eran meticilin-resistentes. En 12 casos (13%), la ReIPA fue provocada por el mismo microrganismo responsable de la IPA primaria. En comparación con la IPA primaria hubo más casos producidos por gramnegativos (el más frecuente Pseudomonas spp.) y menos por grampositivos. En 69 casos (75%), la estrategia de tratamiento elegida fracasó. Se identificaron 43 pautas diferentes de profilaxis en la cirugía del 2T, la más frecuente cefazolina en dosis única preoperatoria, aunque lo más frecuente es que se administraran antibióticos antes y después del implante.
ConclusionesLos principales agentes causales de ReIPA son Staphylococcus spp. meticilin-resistentes, aunque los gramnegativos, particularmente Pseudomonas spp., también participan en una importante cuantía. Existe una notable heterogeneidad en la profilaxis antibiótica que se emplea en la cirugía del 2T. El tratamiento de la ReIPA tiene una alta tasa de fracasos.
One of the options in the treatment of prosthetic joint infection (PJI) is two-stage revision: in a first intervention the infected prosthesis is removed and after a course of antibiotics a new prosthesis is placed. However, it is known that the prosthesis that is implanted in the second stage (2S) has a high probability of suffering a new infection (RePJI), greater than that of the primary prosthesis, either due to recurrence of the original infection or, more frequently, due to an infection by another microorganism.1 Faced with the multitude of studies on primary PJI, there are not too many studies that specifically analyse this subgroup of infections. Some publications have observed a very high incidence of RePJI (between 5% and 29%) and, as might be expected, a worse prognosis than in primary PJI.1–12
On the other hand, given how well established antibiotic prophylaxis is in primary arthroplasty, there is controversy about the regimen to be used in 2S surgery. The few existing publications on the subject, with a heterogeneous methodology, show that maintaining antibiotic therapy for several weeks after 2S surgery could prevent the appearance of new infections.9–13 However, there are no studies on which specific antibiotics should be used, meaning that the PJI management guidelines establish recommendations with a low level of evidence.14
The objective of this study was to characterise RePJI after two-stage revision surgery due to a previous PJI, its microbiology and prognosis, in addition to describing the perioperative prophylaxis used at the hospitals performing 2S surgery.
MethodsPatientsWe designed a multicentre, retrospective, observational study in Spanish hospitals within the framework of the Grupo de Estudio de la Infección Osteoarticular (GEIO) [Osteoarticular Infection Study Group] of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) [Spanish Society of Infectious Diseases and Clinical Microbiology], in patients with RePJI after two-stage revision surgery for previous PJI, from 2009 to 2018.
We included patients older than 18 years of age with PJI treated with two-stage revision surgery in whom the intraoperative cultures from the 2S surgery were negative (or with isolates interpreted as contaminants and not treated with specific antibiotic therapy) who developed a new PJI, defined according to the latest diagnostic criteria established in the international consensus,15 in the two years after the 2S surgery. Those RePJIs where there was clear evidence of its haematogenous origin were excluded.
Data collectionThe data were collected anonymously from the databases of each participating hospital. The Príncipe de Asturias University Hospital (Alcalá de Henares, Madrid) was the coordinating site for the research. The clinical research ethics committee of this hospital approved the study before the data were collected (EPA protocol code 22/2019). All reported cases were collected and reviewed by the coordinating site between July 2021 and December 2022.
Clinical data and definitionsThe demographic and pre-existing conditions of the patients, the characteristics of the infected prosthesis, the classification of the infection and the microbiological findings were collected, both from the primary PJI and the RePJI. The following data were collected for each patient: age and gender, most important comorbidities and age-adjusted Charlson comorbidity index, characteristics of the primarily infected prosthesis, type of infection and microbiology of the primary infection, number of surgeries used to treat the primary PJI, American Society of Anesthesiologists (ASA) score of 2S surgery, antibiotic prophylaxis used in 2S surgery, number of positive cultures during 2S surgery, type of infection and microbiology of RePJI, therapeutic strategy initially used in RePJI, its failure rate, and functional status one year after completion of treatment.
Infections were classified as: early, those that appeared in the first three months after implantation of the prosthesis; late, those that occurred after three months; haematogenous, those secondary to bacteraemia from another source; and positive intraoperative culture, those in which the intraoperative cultures obtained during surgery performed without suspicion of infection on the prosthesis were compatible with infection.16 Identification of the responsible microorganisms and their sensitivity to antibiotics was carried out in the Microbiology laboratory of each participating hospital. Patients with RePJI were treated following the usual recommendations in the clinical practice guidelines for PJI.14,16
Failure of the treatment strategy was defined as: death of the patient, recurrence of infection during or in the year after completion of antibiotic treatment, need for unplanned surgical intervention in the initial therapeutic plan, or need for suppressive antibiotic treatment not initially planned.
Statistical analysisThe description of the quantitative variables was made using the mean and its confidence interval (CI) of 95%. The categorical variables were described using the percentage and its 95% CI. The differences in means were analysed using Student's t test and the analysis of the categorical variables by calculating the odds ratio (OR). Statistical significance was established at P < .05. All calculations were performed using the statistical package SPSS 15.0 (Chicago, USA).
ResultsA total of 92 cases of RePJI from 12 participating hospitals met the inclusion criteria. Table 1 describes the most important demographic data of the patients, comorbidities, characteristics of the prosthesis and the primary PJI, anaesthetic risk of the 2S surgery and RePJI classification.
Clinical characteristics of the patients and the infection.
| Characteristics | (n = 92) |
|---|---|
| Age (mean in years, 95% CI) | 68.1 (65.8–70.4) |
| Female gender (%) | 46 (50%) |
| Charlson Index score (mean, 95% CI) | 3.03 (2.6–3.5) |
| Comorbidities | |
| Obesity (%) | 41 (44.6%) |
| Diabetes mellitus (%) | 30 (32.6%) |
| Chronic kidney disease (%) | 8 (8.7%) |
| Cirrhosis (%) | 4 (4.3%) |
| HIV infection (%) | 2 (2.2%) |
| Immunosuppressive treatment (%) | 11 (11.9%) |
| Malignancy (%) | 9 (9.8%) |
| Location of the prosthesis | |
| Knee (%) | 49 (53.2%) |
| Hip (%) | 37 (40.2%) |
| Shoulder (%) | 6 (6.5%) |
| Original indication for the prosthesis | |
| Osteoarthritis (%) | 64 (69.6%) |
| Rheumatic arthropathy (%) | 4 (4.3%) |
| Fracture (%) | 15 (16.3%) |
| Malignancy (%) | 3 (3.3%) |
| Avascular necrosis (%) | 6 (6.5%) |
| Primary prostheses (%) | 54 (58.7%) |
| Revision prosthesis (%) | 38 (41.3%) |
| Primary PJI classification | |
| Early | 24 (26.1%) |
| Late | 60 (65.2%) |
| Haematogenous | 8 (8.7%) |
| Number of surgeries used in primary PJI (95% CI) | 2.7 (2.3–3.2) |
| ASA second-stage surgerya | |
| I | 4 (4.3%) |
| II | 33 (35.8%) |
| III | 50 (54.3%) |
| IV | 3 (3.3%) |
| RePJI classification | |
| Early | 58 (63%) |
| Late | 33 (35.7%) |
| Positive intraoperative culture type | 1 (1.1%) |
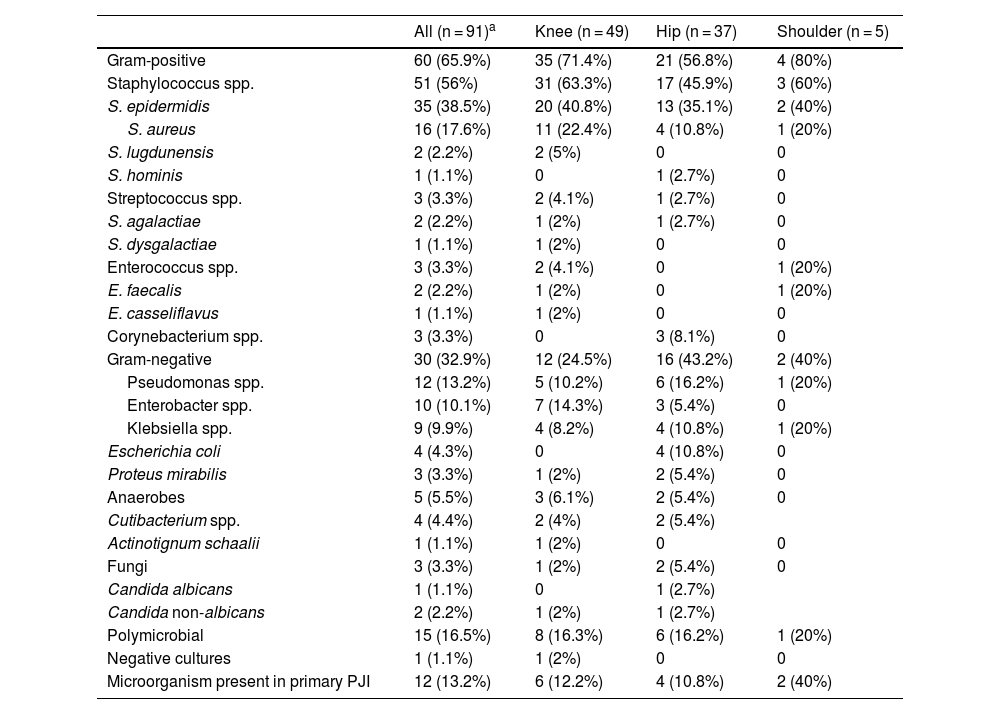
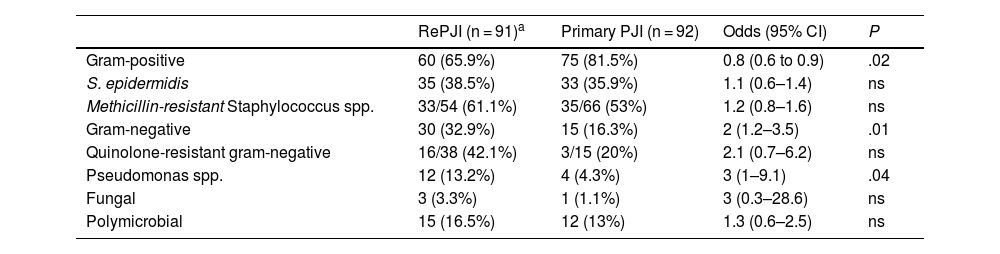
Table 2 shows the microorganisms involved in the cases of RePJI and Table 3 shows the differences with respect to the primary PJI. The most common causative agent, both in primary PJI and in RePJI, was Staphylococcus epidermidis. In RePJI, of the total Staphylococcus spp., including Staphylococcus aureus, S. epidermidis and other coagulase-negative staphylococci (CoNS), 33 of 54 were resistant to methicillin (61.1%). Compared with primary PJI, there was less involvement of gram-positive bacteria (although they were still the majority) and a higher percentage of cases caused by gram-negative bacilli, which was statistically significant. There was a higher rate of resistance to methicillin among staphylococci and greater resistance to fluoroquinolones among gram-negative bacilli, although without reaching statistical significance. In 12 cases (13.2%) of RePJI, one of the causative agents of the primary PJI was isolated, all of them Staphylococcus spp. (six S. aureus and five S. epidermidis), except for one case due to Streptococcus spp. In 14 cases (15.2%), some of the cultures that were taken routinely in the 2S surgery were positive, the vast majority CoNS; four of 11 cases (36.4%) with positive culture for CoNS in the 2S surgery developed RePJI due to S. epidermidis.
Microbiological results of the RePJI.
| All (n = 91)a | Knee (n = 49) | Hip (n = 37) | Shoulder (n = 5) | |
|---|---|---|---|---|
| Gram-positive | 60 (65.9%) | 35 (71.4%) | 21 (56.8%) | 4 (80%) |
| Staphylococcus spp. | 51 (56%) | 31 (63.3%) | 17 (45.9%) | 3 (60%) |
| S. epidermidis | 35 (38.5%) | 20 (40.8%) | 13 (35.1%) | 2 (40%) |
| S. aureus | 16 (17.6%) | 11 (22.4%) | 4 (10.8%) | 1 (20%) |
| S. lugdunensis | 2 (2.2%) | 2 (5%) | 0 | 0 |
| S. hominis | 1 (1.1%) | 0 | 1 (2.7%) | 0 |
| Streptococcus spp. | 3 (3.3%) | 2 (4.1%) | 1 (2.7%) | 0 |
| S. agalactiae | 2 (2.2%) | 1 (2%) | 1 (2.7%) | 0 |
| S. dysgalactiae | 1 (1.1%) | 1 (2%) | 0 | 0 |
| Enterococcus spp. | 3 (3.3%) | 2 (4.1%) | 0 | 1 (20%) |
| E. faecalis | 2 (2.2%) | 1 (2%) | 0 | 1 (20%) |
| E. casseliflavus | 1 (1.1%) | 1 (2%) | 0 | 0 |
| Corynebacterium spp. | 3 (3.3%) | 0 | 3 (8.1%) | 0 |
| Gram-negative | 30 (32.9%) | 12 (24.5%) | 16 (43.2%) | 2 (40%) |
| Pseudomonas spp. | 12 (13.2%) | 5 (10.2%) | 6 (16.2%) | 1 (20%) |
| Enterobacter spp. | 10 (10.1%) | 7 (14.3%) | 3 (5.4%) | 0 |
| Klebsiella spp. | 9 (9.9%) | 4 (8.2%) | 4 (10.8%) | 1 (20%) |
| Escherichia coli | 4 (4.3%) | 0 | 4 (10.8%) | 0 |
| Proteus mirabilis | 3 (3.3%) | 1 (2%) | 2 (5.4%) | 0 |
| Anaerobes | 5 (5.5%) | 3 (6.1%) | 2 (5.4%) | 0 |
| Cutibacterium spp. | 4 (4.4%) | 2 (4%) | 2 (5.4%) | |
| Actinotignum schaalii | 1 (1.1%) | 1 (2%) | 0 | 0 |
| Fungi | 3 (3.3%) | 1 (2%) | 2 (5.4%) | 0 |
| Candida albicans | 1 (1.1%) | 0 | 1 (2.7%) | |
| Candida non-albicans | 2 (2.2%) | 1 (2%) | 1 (2.7%) | |
| Polymicrobial | 15 (16.5%) | 8 (16.3%) | 6 (16.2%) | 1 (20%) |
| Negative cultures | 1 (1.1%) | 1 (2%) | 0 | 0 |
| Microorganism present in primary PJI | 12 (13.2%) | 6 (12.2%) | 4 (10.8%) | 2 (40%) |
Most relevant microbiological differences between primary PJI and RePJI.
| RePJI (n = 91)a | Primary PJI (n = 92) | Odds (95% CI) | P | |
|---|---|---|---|---|
| Gram-positive | 60 (65.9%) | 75 (81.5%) | 0.8 (0.6 to 0.9) | .02 |
| S. epidermidis | 35 (38.5%) | 33 (35.9%) | 1.1 (0.6–1.4) | ns |
| Methicillin-resistant Staphylococcus spp. | 33/54 (61.1%) | 35/66 (53%) | 1.2 (0.8–1.6) | ns |
| Gram-negative | 30 (32.9%) | 15 (16.3%) | 2 (1.2–3.5) | .01 |
| Quinolone-resistant gram-negative | 16/38 (42.1%) | 3/15 (20%) | 2.1 (0.7–6.2) | ns |
| Pseudomonas spp. | 12 (13.2%) | 4 (4.3%) | 3 (1–9.1) | .04 |
| Fungal | 3 (3.3%) | 1 (1.1%) | 3 (0.3–28.6) | ns |
| Polymicrobial | 15 (16.5%) | 12 (13%) | 1.3 (0.6–2.5) | ns |
In the 2S surgery, 43 different antibiotic regimens were used, with different durations (Table 4). The most common regimen was a preoperative single-dose cephalosporin (19 cases, 21.1%), but overall it was more common for antibiotic prophylaxis to be administered both before and after implantation of the prosthesis. A multitude of antibiotic combinations were used, on different occasions before and after the intervention, but the most commonly used was a glycopeptide with an antipseudomonal cephalosporin. The mean duration of postoperative prophylaxis was 8.1 days (95% CI 5.2–13.1). In three cases, prophylaxis was only administered after surgery, and in two cases there was no record in the clinical history of any prophylaxis administered. Additionally, in 10 cases prophylaxis was prolonged with oral antibiotics.
Antibiotic prophylaxis regimens used in the second-stage surgery.
| Regimen | n = 88a |
|---|---|
| Preimplantation only (n = 24) | |
| Cefazolin | 16 |
| Cefuroxime | 3 |
| Cefazolin + teicoplanin | 2 |
| Vancomycin | 1 |
| Vancomycin + ceftriaxone | 1 |
| Teicoplanin | 1 |
| Preimplantation and postoperative (n = 61) | |
| Vancomycin + ceftazidime | 9 |
| Teicoplanin + preimplantation ceftazidime and vancomycin + postoperative ceftazidime | 7 |
| Cefazolin | 7 |
| Preimplantation cefazolin and postoperative levofloxacin | 3 |
| Teicoplanin + preimplantation gentamicin and postoperative teicoplanin | 3 |
| Teicoplanin + preimplantation meropenem and vancomycin + postoperative meropenem | 2 |
| Vancomycin | 2 |
| Teicoplanin + cefepime | 2 |
| Preimplantation cefazolin and postoperative linezolid | 1 |
| Teicoplanin | 1 |
| Teicoplanin + ertapenem | 1 |
| Teicoplanin + preimplantation aztreonam and vancomycin + postoperative aztreonam | 1 |
| Teicoplanin + ceftazidime | 1 |
| Cloxacillin | 1 |
| Preimplantation and postoperative cloxacillin and oral cefadroxil | 1 |
| Preimplantation cefazolin and postoperative cotrimoxazole | 1 |
| Preimplantation cefazolin and postoperative meropenem | 1 |
| Preimplantation cefazolin and postoperative vancomycin | 1 |
| Preimplantation cefazolin and vancomycin + postoperative ceftazidime | 1 |
| Cefazolin + amikacin | 1 |
| Cefazolin + preimplantation teicoplanin, postoperative teicoplanin and cotrimoxazole and oral linezolid | 1 |
| Preimplantation cefuroxime, postoperative levofloxacin and oral doxycycline | |
| Ceftazidime + amikacin | 1 |
| Preimplantation and postoperative vancomycin and oral minocycline | 1 |
| Preimplantation vancomycin, postoperative vancomycin and rifampicin and oral cotrimoxazole | 1 |
| Vancomycin + ertapenem | 1 |
| Teicoplanin + preimplantation levofloxacin and vancomycin + postoperative levofloxacin | 1 |
| Teicoplanin + preimplantation meropenem and vancomycin + postoperative meropenem | 1 |
| Teicoplanin + preimplantation amikacin and teicoplanin + postoperative ceftazidime | 1 |
| Teicoplanin + preimplantation ceftazidime, postoperative teicoplanin and oral linezolid | 1 |
| Teicoplanin + rifampicin | 1 |
| Daptomycin + preimplantation ertapenem + postoperative linezolid | 1 |
| Clindamycin + preimplantation gentamicin and postoperative clindamycin | 1 |
| Preimplantation and postoperative linezolid and oral clindamycin | 1 |
| Postoperative only (n = 3) | |
| Vancomycin + ceftazidime | 1 |
| Teicoplanin + ceftazidime | 1 |
| Teicoplanin + aztreonam | 1 |
In the treatment of RePJI, different treatment strategies were established with an overall failure rate of 75% (Table 5). The most frequently used strategy was surgical debridement, antibiotics and implant retention (DAIR), with a success rate of 33.8%, while the most successful strategy was resection arthroplasty (without reimplantation). Six patients died during the year after the infection (6.5%), 48 patients ended up losing the prosthesis implanted in two-stage revision (52.2%), and among the patients with knee and hip infection who did not die, only 19 out of 81 (23.5%) were able to walk without assistance at one year.
Failure rate of treatments used in prosthetic joint reinfection.
| Treatment used | Failures (%) |
|---|---|
| Overall (n = 92) | 69 (75%) |
| Debridement with implant retention (n = 65) | 43 (66.2%) |
| Two-stage revision (n = 19) | 8 (42.1%) |
| Single-stage revision (n = 6) | 5 (83.3%) |
| Removal of the prosthesis without reimplantation (n = 4) | 0 |
| Suppressive antibiotic treatment (n = 10) | 7 (70%) |
In our study, the most frequent causative microorganism of RePJI was, as in the primary PJIs, S. epidermidis, but there was a higher proportion of cases caused by gram-negative bacilli, particularly Pseudomonas spp., and a higher rate of antibiotic resistance. We found great heterogeneity in the antibiotic prophylaxis used in the second stage of the two-stage revision. In addition, the RePJI treatment had poor results, both in microbiological failures and in functional results.
Compared to the abundant literature on the characteristics of primary PJI, its microbiology, prognosis and prophylaxis measures to prevent it, there are far fewer publications on PJIs after a two-stage revision due to a previous PJI. In the studies where the incidence has been evaluated, this is much higher than that of primary prostheses, in most above 10%, and in some it is even close to 30%.1–12 The factors that have been associated with a higher risk of developing RePJI are staphylococcal infections,1–3,17–19 delay in 2S surgery,3,7,8,20 rheumatoid arthritis,19 previous joint surgeries,19 obesity and smoking.5 In our study, we found that the patients who suffered from these infections had numerous comorbidities, the most common being obesity and a high anaesthetic risk (more than 50% had an ASA score greater than 2), and a significant proportion had previously had their prostheses replaced, factors already previously described as risk factors for RePJI.5,19 The original PJIs were mainly late infections treated with 2S revision surgery, but also in a significant percentage early infections with failed DAIR that had ended up requiring revision surgery.
In the 2S surgery, microbiological samples are routinely taken to confirm that the original PJI has resolved. However, its interpretation can sometimes be difficult, since it is not uncommon for some of them to be positive. If these cultures are positive, it is not clear whether this represents a risk factor for RePJI; while some studies have shown their relationship,1,13 others have not done so and, furthermore, there is usually no correlation between the microorganisms found in these cultures with those that are later identified as causing the RePJI in those patients who end up developing it.5–7,21 Usually, when they are positive, they are interpreted as contaminants, particularly when they are CoNS and only appear in a single culture. In our series, in 14 cases (15.2%) one of these cultures was positive, and 36% of the patients who had a positive culture for CoNS developed RePJI due to S. epidermidis. We cannot conclude that this CoNS from the culture from the 2S surgery was the causal microorganism of the RePJI, because in most cases the CoNS was not identified as it was interpreted as a contaminant, but this high percentage raises the possibility that the result of these cultures cannot always be downplayed.
Regarding the microbiology of RePJI, compared to primary PJI, we found less involvement of gram-positive bacilli (although they were still the majority) and more of gram-negative bacilli. In addition, the rate of resistance to antibiotics was higher: to methicillin, in the case of Staphylococcus spp., and to fluoroquinolones among gram-negative bacilli (although it did not reach statistical significance), which could be explained by prior exposure to antibiotics to treat the primary PJI. Among the gram-negative bacilli, the species most frequently involved was Pseudomonas spp., a phenomenon that has already been described in other publications.7
The vast majority of RePJI cases were caused by microorganisms other than those of the primary infection, although 13.2% were found to be the same as in the original PJI, almost all of them Staphylococcus spp. (only one exception, one case of Streptococcus agalactiae), in line with other publications.1 In our study, given its retrospective nature, it was not possible to determine whether these supposed recurrences were due to the original strain, which had not been completely eradicated (although in all these cases the intraoperative cultures from the 2S surgery had been negative), or if it was a reinfection by a different strain acquired during the perioperative period.
Unlike in primary arthroplasty surgery, there are few studies evaluating the antibiotic prophylaxis that should be used in 2S surgery after a previous infection. So much so that most guidelines or review articles on PJI do not mention which antibiotics to administer when the new prosthesis is implanted, when to administer them, and for how long. The few studies carried out in this regard have focused on evaluating whether maintaining this prolonged prophylaxis with oral antibiotic therapy can be useful to prevent RePJI, with favourable results,9–13 but there are no trials with any specific antibiotic therapy for this type of surgery.
Probably this absence of formal recommendation in clinical guidelines could explain the great diversity of prophylaxis regimens used in the usual clinical care of patients included in our series. The regimen most commonly used was a first or second generation cephalosporin, in a single dose prior to surgery, presumably by analogy with the primary arthroplasty surgery. However, it was more common to use antibiotics both before and after implanting the final prosthesis, albeit with multiple regimens. The two most widely used regimens, which included a glycopeptide and an antipseudomonal cephalosporin, each one used at one hospital, as a protocol, in accordance with the recommendation of the SEIMC guide, which advises including broad-spectrum antibiotics against microorganisms of nosocomial origin, with special attention to methicillin-resistant staphylococci,14 but in the rest of the hospitals the regimens were more heterogeneous. The microbiological results of our series would support this recommendation.
On the other hand, the very long duration of antibiotic prophylaxis is striking. This is due to the fact that it is usual practice to maintain the antibiotics until the results of the intraoperative cultures from the 2S surgery are available, in anticipation that these continue to be positive and reveal the presence of active infection, in which case early treatment would already be being carried out.
The treatments initially planned for RePJI had a high percentage of failure, particularly those aimed at maintaining joint functionality, either with preservation of the prosthesis (both with debridement and retention of the prosthesis and with suppressive antibiotic treatment), or with a new reimplantation (in one or two stages). This fact is understandable as it is a joint that has previously been operated on numerous occasions, at least three (primary prosthesis implantation, removal after infection, 2S implantation), but often more (primary PJI on revision prosthesis, failed DAIR, surgeries to clean after the first surgical period due to poor progression…). When resection arthroplasty was initially chosen, the success rate from the microbiological point of view was better, but at the cost of poorer functionality. Overall, more than half of the patients who developed RePJI ended up losing the prosthesis implanted in the second stage, and only a small percentage recovered full functionality.
The most important limitation of our study was its retrospective nature, which did not allow us to answer any of the questions raised in the discussion, such as whether the microorganisms isolated in 2S surgery were the real causes of subsequent RePJI or if the cases with the same microorganism in the primary PJI and the RePJI were true recurrences or different reinfections. It would be necessary to plan prospective studies to clarify these issues.
In conclusion, RePJI is a not uncommon complication after 2S revision surgery, with serious repercussions for patients. The most appropriate antibiotic prophylaxis in 2S surgery remains to be defined, but given that the microbiology is different from the primary PJIs, this should probably be the same, particularly aimed at the most commonly involved microorganisms. It is possible that this prophylaxis should include antibiotics active against methicillin-resistant staphylococci and gram-negative bacilli, including Pseudomonas spp., although this should be supported by prospective studies.
FundingThis study received no specific funding from public, private or non-profit organisations.
Conflicts of interestJosé M. Barbero Allende has received speaker fees from Pfizer and Angelini.
Álex Soriano has received speaker and consulting fees from Pfizer, MSD, Menarini, Gilead, Shionogi, and Angelini.
Laura Morata Ruiz has received speaker and consulting fees from Pfizer, MSD and Angelini.
Dolors Rodríguez-Pardo has received speaker and consulting fees from Pfizer, MSD and Angelini.
All the other authors declare that they have no conflicts of interest.
Pablo S. Corona. Septic and Reconstructive Surgery Unit (UCSO), Department of Orthopaedic Surgery. Hospital Universitario Vall d'Hebron [Vall d'Hebron University Hospital], Barcelona.
Mayli Lung. Department of Microbiology. Hospital Universitario Vall d'Hebron, Barcelona.
Júlia Sellarès-Nadal. Department of Infectious Diseases. Hospital Universitario Vall d’Hebron, Barcelona.
Carlos Garcés Zarzalejo. Department of Trauma and Orthopaedics. Hospital Universitario Marqués de Valdecilla [Marqués de Valdecilla University Hospital], Santander.
Daniel Pablo Marcos. Department of Microbiology. Hospital Universitario Marqués de Valdecilla, Santander.
Laura Soldevila-Boixader. Department of Infectious Diseases. Hospital Universitari de Bellvitge [Bellvitge University Hospital], Barcelona.
Eva Benavent. Department of Infectious Diseases. Hospital Universitari de Bellvitge, Barcelona.
Josu Merino Pérez. Department of Orthopaedic Surgery and Trauma. Hospital Universitario de Cruces [Cruces University Hospital], Barakaldo.
Ernesto Muñoz-Mahamud. Department of Orthopaedic Surgery and Trauma. Hospital Clínic de Barcelona [Barcelona Hospital Clinic], Barcelona.
Juan C Martínez-Pastor. Department of Orthopaedic Surgery and Trauma. Hospital Clínic de Barcelona, Barcelona.