There is discussion about the frequency of STI screening among pre-exposure prophylaxis (PrEP) users. The aim of this study was to analyse the incidence of STIs and to evaluate different screening models in order to optimise the follow-up.
MethodologyA prospective study was conducted between 2017 and 2023, including 138 PrEP users in a STI clinic. Participants were tested for STIs every three months. Unscheduled visits were performed for those with STI-related symptoms or for people who were notified for an STI by a sexual partner. We performed a survival analysis of repeated events, estimating the cumulative incidence (CI) and incidence rate (IR).
ResultsThe overall CI by quarterly screening was 8.3 (95% CI: 7.6–9.1) infections per person over six years, with a decreasing trend. The most frequently diagnosed pathogen was Neisseria gonorrhoeae, with a IR of 0.76 (95% CI: 0.68–0.84). If the frequency of screening is reduced to every six months, the IR of STIs is reduced by (95% CI: 0.5–0.66) infections per user per year, and at 12 months by 0.82 (95% CI: 0.73–0.89). In the case of no pharyngeal or urethral screening, IR is reduced by 0.37 (95% CI: 0.32–0.42) infections per person per year and in those over 35 years of age by 0.33 (95% CI: 0.25–0.4). Eliminating unscheduled visits, the reduction in IR is 0.33 (95% CI: 0.24–0.42).
ConclusionsThe incidence of STIs among PrEP users is high, especially in the rectum, but it does not increase over time. STI screening could be optimised reducing the frequency of pharyngeal and urethral testing, particularly in those over 35 years of age. It is essential to redistribute health resources for unscheduled visits, which have been shown to be the most cost-effective screening.
Existe un debate sobre la frecuencia del cribado de ITS entre los usuarios de profilaxis pre-exposición (PrEP). El objetivo de este estudio fue conocer la incidencia de ITS entre usuarios de PrEP y evaluar distintos modelos de cribado para optimizarlo.
MetodologíaEstudio descriptivo prospectivo de una cohorte de usuarios de PrEP en seguimiento desde 2017–2023 en una clínica de ITS. Se incluyeron 138 participantes, a quienes se realizó un cribado de ITS trimestralmente y visitas no programadas ante sospecha clínica o epidemiológica de ITS. Se realizó un análisis de supervivencia para eventos repetidos, calculando la incidencia acumulada (IA) y la tasa de incidencia (TI).
ResultadosLa IA global mediante el cribado trimestral fue de 8,3 (IC 95%: 7,6–9,1) infecciones por persona en 6 años, con una tendencia descendente. El patógeno más frecuentemente diagnosticado fue la Neisseria gonorrhoeae, TI de 0,76 (IC 95%: 0,68−0,84). En caso de reducir la frecuencia de cribado semestralmente, la TI de ITS se reduce en 0,58 (IC 95%: 0,5−0,66) infecciones por usuario y año, y a 12 meses en 0,82 (IC 95%: 0,73−0,89). En caso de no realizar cribado en localización faríngea ni uretral la TI se reduce en 0,37 (IC 95%: 0,32−0,42) infecciones por persona y año y en aquellos usuarios con más de 35 años en 0,33 (IC 95%: 0,25−0,4). Eliminando las visitas no programadas la reducción en la TI es de 0,33 (IC 95%: 0,24−0,42).
ConclusionesLa incidencia de ITS entre los usuarios de PrEP es elevada, especialmente en el recto, pero no se incrementa con el paso del tiempo. Se podría optimizar el cribado de ITS reduciendo la frecuencia de búsqueda en localización faríngea y uretral, especialmente en mayores de 35 años. Es fundamental disponer de recursos para las visitas no programadas, que han demostrado ser un cribado con mayor rentabilidad diagnóstica.
A number of preventive measures have been used to prevent HIV transmission, including promotion of consistent condom use, needle exchange programmes, HIV screening for early diagnosis, immediate antiretroviral therapy, post-exposure prophylaxis, sex education and harm reduction on recreational drug use.1 In 2012, the FDA approved the use of TDF/FTC as another preventive measure under the umbrella term pre-exposure prophylaxis (PrEP).2 PrEP is the use of antiretroviral drugs targeted at HIV-negative people with high-risk practices with the aim of preventing HIV acquisition.3,4 It has shown great preventive efficacy and is recommended by various health agencies and scientific societies such as CDC, WHO and GESIDA (Grupo de Estudio de Sida [AIDS Study Group]).5–7 The FDA has also approved other drugs such as TAF/FTC or cabotegravir for PrEP.8,9
In November 2019, the Spanish Ministry of Health announced funding for TDF/FTC with the indication of PrEP in Spain within the National Health System, as an additional HIV prevention measure.10 As has been the case in other developed countries, the number of PrEP users has been steadily increasing. However, according to UNAIDS data, expectations have not yet been met in terms of the number of candidates who could benefit from it.11 PrEP programmes must be accessible and facilitate correct adherence to achieve the greatest benefit.
On the other hand, the implementation of this programme has been associated with lower condom use in sexual intercourse and higher diagnosis of other sexually transmitted infections (STIs).12 Given that PrEP is indicated for people at high risk for HIV, they are also exposed to other STIs. STI screening is an essential part of a PrEP programme.10 Its aim is to diagnose STIs early, provide targeted treatment and break chains of transmission. However, there is a debate on the frequency of screening, taking into account the need to optimise healthcare resources and concerns about increasing antibiotic resistance.13 One study reported that quarterly STI screening would diagnose 18,250 more STIs than screening six-monthly over 10 years of follow-up in more than 36,000 men who have sex with men (MSM), although this effort would not be cost-effective.14 Moreover, a very high number of visits may be a barrier to user adherence to the programme. The updated PrEP guidelines have been more flexible in their recommendations for STI screening.7
The aim of this study was to gain an understanding of the incidence of STIs among PrEP users, with a sub-analysis taking into account age and sampling methodology, and to evaluate different screening models in order to optimise screening.
MethodologyStudy designThis was a retrospective descriptive study of a cohort of PrEP users from 2017 to 2023, conducted at a national referral STI clinic. The first 138 users who had early access to PrEP were included in the study and were screened for STIs on a quarterly basis. At each of these scheduled visits, HIV and syphilis serology, collection of pharyngeal and rectal swabs, and urine sampling for Neisseria gonorrhoeae (NG) and Chlamydia trachomatis (CT) by polymerase chain reaction (PCR) were performed. All incidences of CT detected in the pharynx and rectum were retested for lymphogranuloma venereum (LGV). HCV serology was also performed every six months. In addition to the quarterly visits, all unscheduled visits with clinical or epidemiological suspicion of STIs were included. STI screening was performed at these visits at the clinicians' discretion. Ulcer studies for CT and Treponema pallidum (TP) and urethral exudates for NG, CT and LGV were also performed. In cases of primary syphilis, the study was completed with serology. Samples for STI screening were collected by healthcare professionals at both scheduled and unscheduled visits. From 1 July 2022, self-collection of pharyngeal and rectal samples at scheduled visits was implemented.
Diagnostic techniquesTwo real-time PCRs (Allplex™ STI Essential Assay and Allplex™ Genital ulcer Assay, Seegene) were used to detect NG, CT and LGV. Serological diagnosis of syphilis was performed using a chemiluminescent microparticle immunoassay (Alinity s Syphilis TP, Abbott Laboratories), an RPR technique (Macro-Vue RPR Card Test, Becton Dickinson) and a TP-PA technique (Serodia TP-PA, Fujirebio INC). HCV serology was determined by chemiluminescent microparticle immunoassay (Alinity s Anti-HCV, Abbott Laboratories).
Statistical analysisThe qualitative variables are summarised by the distribution of absolute and relative frequencies. Quantitative variables are summarised with the mean and standard deviation (SD) or with the median and interquartile range (IQR) if they do not fit a normal distribution. A recurrent event modelling methodology was used to estimate the cumulative incidence (CIn). The time of screening was taken as T = 0 and for each sample the time in days to screening was calculated. A negative sample was considered a negative event; a positive sample, a positive event; and censored in the case of loss to follow-up. In cases where the last record was positive, a negative record was added one day after the last record. The Nelson-Aalen mean cumulative function estimator was used to estimate the CIn of recurrent events, in accordance with the methodology proposed by Lawless. Simply put, the mean cumulative function enables the estimation of the average number of recurrent events per subject at time "t", taking into account the censored data. Cumulative incidences are shown using the point estimate and 95% confidence interval every three months until the end of the total follow-up. CIns are expressed in infections per patient, with isolation of the same microorganism in different anatomical niches not being considered different infections. Alpha values less than 0.05 were considered statistically significant. In the simulation of the different scenarios, the CIn was calculated according to the methodology presented above, eliminating the corresponding visits in each case. CIns are all expressed for the full follow-up period (number of new infections per person over six years), and incidence rates (IRs) are shown on an annual basis (number of new infections per person per year). Statistical analysis was performed using the R statistical package (R version 4.3.1) and RStudio (version 2023.06.0 + 421). The reda and reReg packages were used for the analysis of recurrent events. The ggplot2, gridExtra, dplyr, reshape and tidyr packages were used for the graphical representation and ordering of the data.
Ethical aspectsAll data were collected through clinical records from routine clinical practice. All the information analysed was previously anonymised. The study protocol was approved by the Hospital Clínico San Carlos Ethics Committee: 20/214-E.
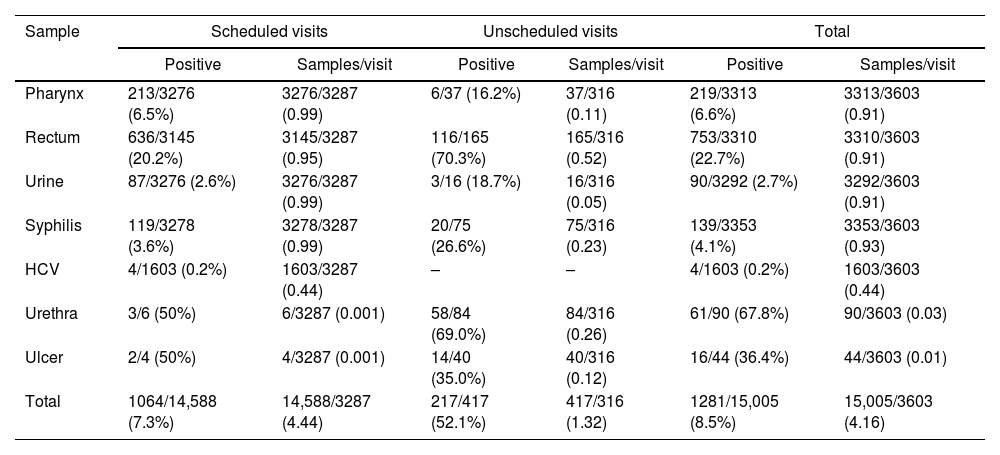
ResultsA total of 138 daily PrEP users were included in the study. Some 98.6% (136) were MSM and 1.4% (2) were transgender women. The median age was 33 years (IQR 30–38). In total, 79.7% (110) were Spanish, 10.1% (14) were Latin American and the rest were of different nationalities. The median follow-up time was 5.98 years (IQR 3.84–6.03), with a minimum follow-up time of 41 days and a maximum of 6.23 years. Over these six years, a total of 3313 pharyngeal swabs (219 positive; 6.6%), 3310 rectal swabs (753 positive; 22.7%), 3292 urine samples (90 positive; 2.7%), 3353 syphilis serologies (139 positive; 4.1%), 1603 HCV serologies (4 positive; 0.17%), 90 urethral exudates (61 positive; 67.8%) and 44 ulcer samples (16 positive; 36.4%) were carried out. In total, 15,005 samples were available, of which 14,588 were from scheduled visits and 417 were unscheduled (Table 1). Some 82.7% of infections were diagnosed at scheduled visits, although unscheduled visits had a higher diagnostic yield.
Samples collected for STI screening at scheduled and unscheduled visits and positive detection from 2017 to 2023.
| Sample | Scheduled visits | Unscheduled visits | Total | |||
|---|---|---|---|---|---|---|
| Positive | Samples/visit | Positive | Samples/visit | Positive | Samples/visit | |
| Pharynx | 213/3276 (6.5%) | 3276/3287 (0.99) | 6/37 (16.2%) | 37/316 (0.11) | 219/3313 (6.6%) | 3313/3603 (0.91) |
| Rectum | 636/3145 (20.2%) | 3145/3287 (0.95) | 116/165 (70.3%) | 165/316 (0.52) | 753/3310 (22.7%) | 3310/3603 (0.91) |
| Urine | 87/3276 (2.6%) | 3276/3287 (0.99) | 3/16 (18.7%) | 16/316 (0.05) | 90/3292 (2.7%) | 3292/3603 (0.91) |
| Syphilis | 119/3278 (3.6%) | 3278/3287 (0.99) | 20/75 (26.6%) | 75/316 (0.23) | 139/3353 (4.1%) | 3353/3603 (0.93) |
| HCV | 4/1603 (0.2%) | 1603/3287 (0.44) | – | – | 4/1603 (0.2%) | 1603/3603 (0.44) |
| Urethra | 3/6 (50%) | 6/3287 (0.001) | 58/84 (69.0%) | 84/316 (0.26) | 61/90 (67.8%) | 90/3603 (0.03) |
| Ulcer | 2/4 (50%) | 4/3287 (0.001) | 14/40 (35.0%) | 40/316 (0.12) | 16/44 (36.4%) | 44/3603 (0.01) |
| Total | 1064/14,588 (7.3%) | 14,588/3287 (4.44) | 217/417 (52.1%) | 417/316 (1.32) | 1281/15,005 (8.5%) | 15,005/3603 (4.16) |
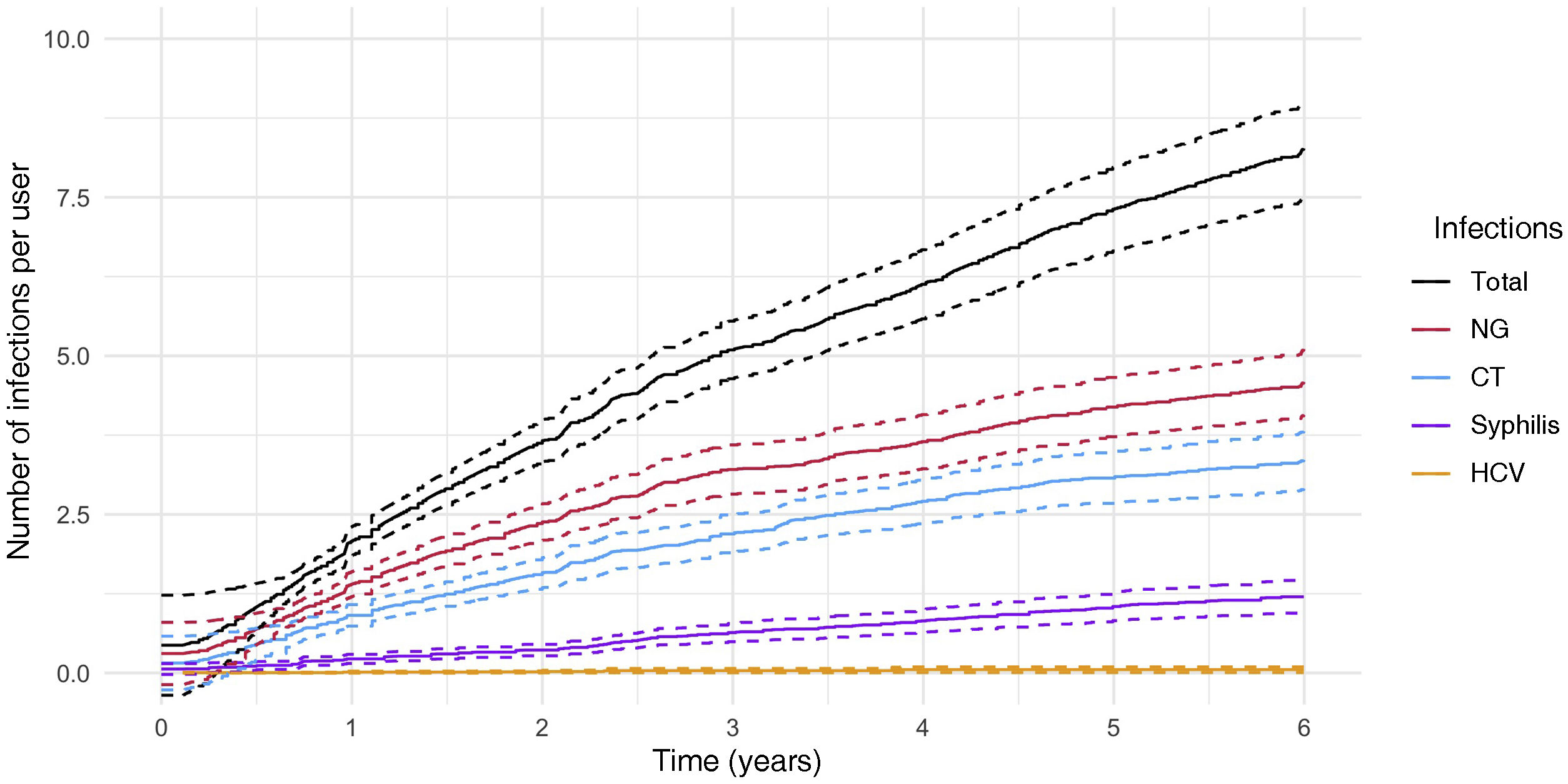
Fig. 1 shows the CIn of NG, CT, syphilis and HCV during the six years of follow-up. The overall CIn was 8.3 (95% CI: 7.6–9.1) infections per person from the start of follow-up to year six (overall incidence rate 1.38 [95% CI: 1.27–1.51] infections per person per year). The six-monthly CIns according to microorganism are set out in Appendix A, Table 1 of the Supplementary material. The most frequently diagnosed pathogen was NG, with an IR of 0.76 (95% CI: 0.68−0.84), followed by CT with an IR of 0.56 (95% CI: 0.49−0.63) and syphilis with an IR of 0.21 (95% CI: 0.17−0.26).
A total of 9222 CT tests were performed, of which 474 (5.1%) were positive. LGV subtyping was performed on 361 of these samples and was positive in 79 cases (21.8%). Of these, 87.3% (69) were from rectal samples, 11.4% (9) from ulcers and one case from the urethra.
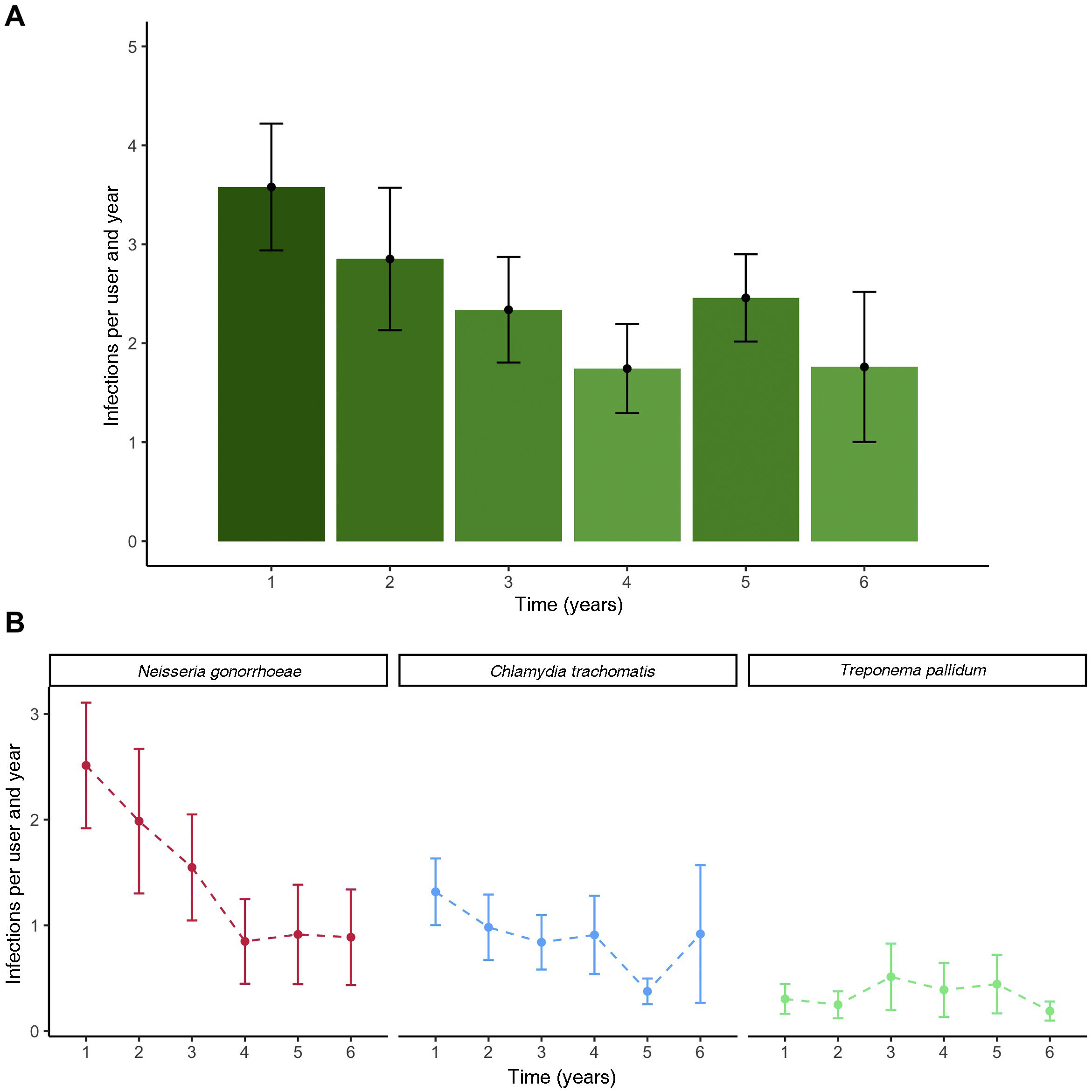
The IR, taking into account all STIs, decreased over the years (Fig. 2). The most significant difference was found between the third and fourth year, coinciding with the COVID-19 pandemic. The IR for 0–3 years was 1.87 (95% CI: 1.7–2.03) infections per person per year, and for 3–6 years 0.91 (95% CI: 0.76–1.03), consistent with a reduction of 0.97 (95% CI: 0.73–1.16) infections per person per year (51.2% reduction [95% CI: 42.9–57.1]). Fig. 2 shows a significant reduction in the IR of NG and CT, while syphilis remains stable during follow-up.
There were significant differences in STI CIn by age. Among users over 35 years of age, STI CIn was significantly lower (1.7 [95% CI: 1.53–1.86] in under 35 s vs 1.28 [95% CI: 1.06–1.51] infections per person per year in over 35 s, representing a 24.7% [95% CI: 18.8–30.7] reduction [p < 0.001]). An analysis was also conducted comparing STI CIn between the period after 1 July 2022, coinciding with the implementation of self-testing, and the period between 1 July 2021 and 1 July 2022, with sampling by a healthcare professional. There was no significant difference between the two periods: 1.32 (95% CI: 1.01–1.61) and 1.77 (95% CI: 0.98–2.57) infections per person per year, respectively (p = 0.91).
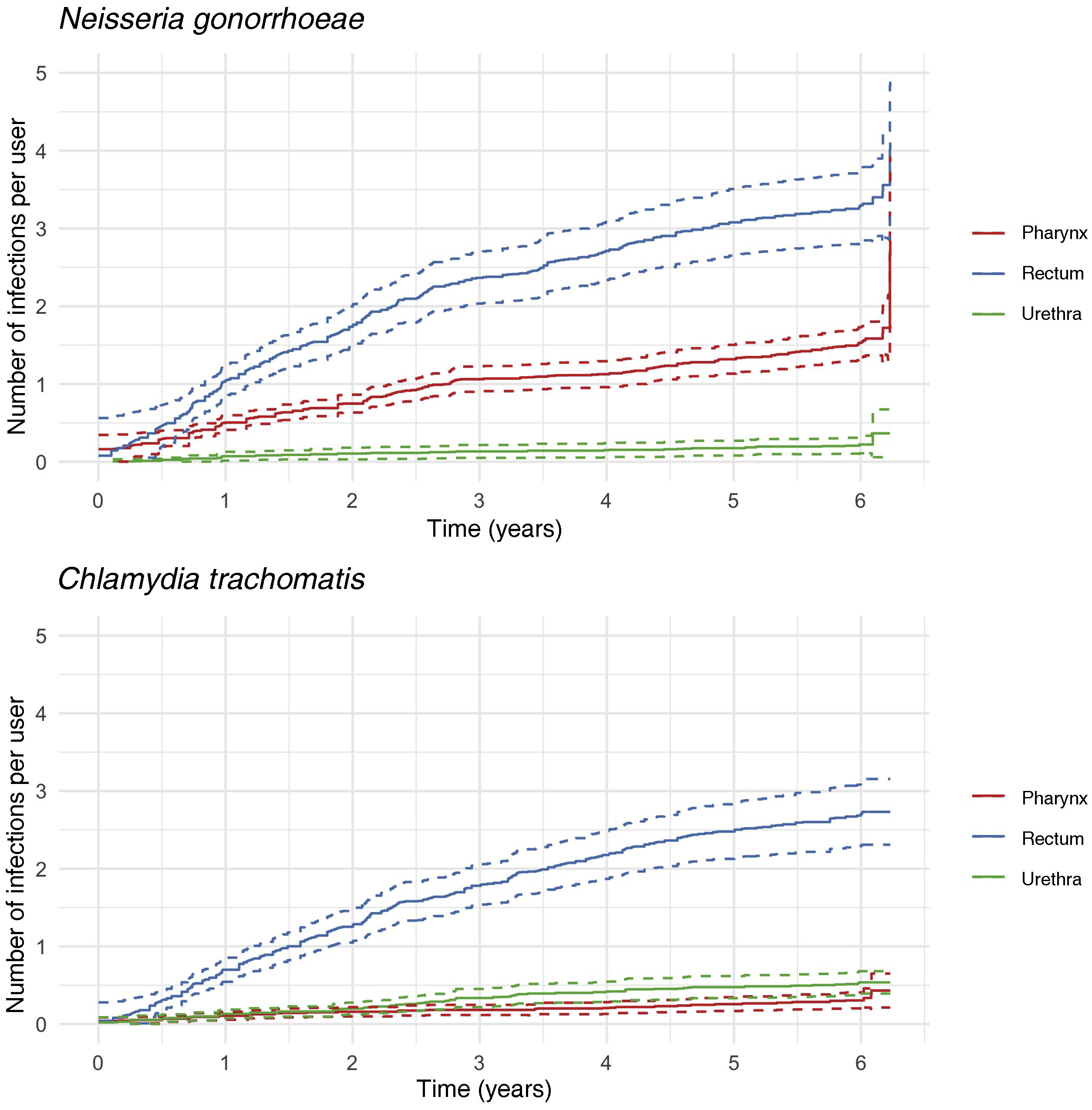
Fig. 3 shows the CIn, analysed by each pathogen and sample location, taking into account all visits, both scheduled and unscheduled. Most NG and CT cases were detected in the rectum. The majority of STIs detected in the pharynx were for NG, and a minority for CT. There were very few NG or CT cases diagnosed in the urethra. Syphilis was diagnosed by PCR and serology, with incidence rates of 0.05 (95% CI: 0.03−0.07) and 0.17 (95% CI: 0.13−0.21) infections per person per year, respectively.
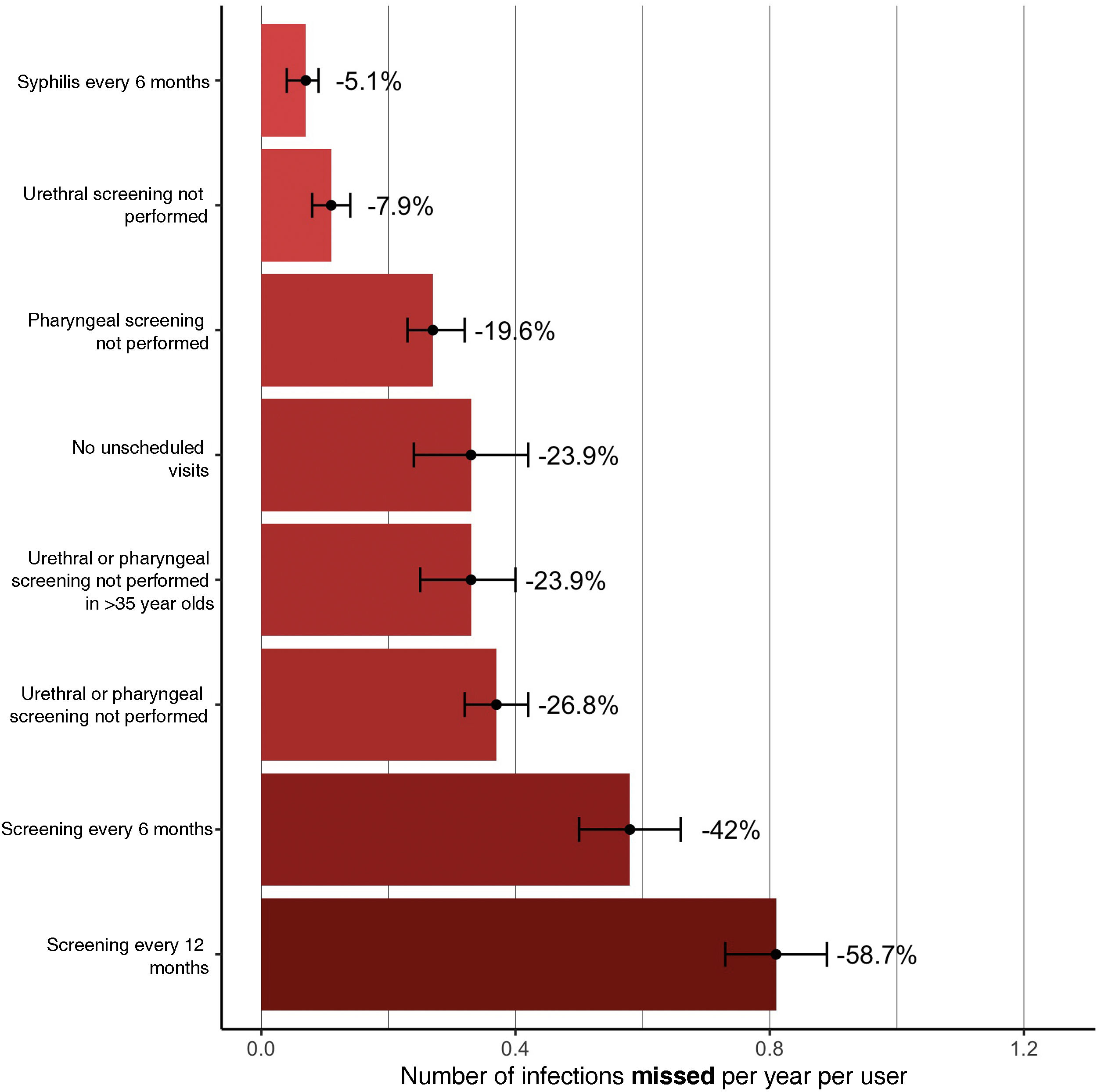
An analysis of the IR of STIs was performed by generating different screening models, compared to the quarterly regimen established in the study (Fig. 4 and Appendix A, Table 2 of the Supplementary material). The difference between quarterly full screening and the reduction of each method indicates the number of missed diagnoses. If screening frequency is reduced to six months, the IR of STIs reduces by 0.58 (95% CI: 0.5−0.66) infections per user per year, and to 12 months by 0.82 (95% CI: 0.73−0.89) (42.0% [95% CI: 39.4–43.7], 58.7% [IQR: 57.4–58.9] reduction in IR). For syphilis, the incidence remains similar in six-monthly screening, with a difference of 0.07 (95% CI: 0.04−0.09) infections per user per year (5.1% [95% CI: 3.1–5.9]).
Differences in IR for any STI were also analysed by the techniques used in screening at scheduled visits. If urethral and pharyngeal screening is not performed, infections per user per year are reduced by 0.11 (95% CI: 0.08−0.14) (7.9% [95% CI: 6.3–9.3]) and 0.27 (95% CI: 0.23−0.32) (19.6% [18.1; 21.2]). In the case of no STI screening in either pharyngeal or urethral sites, the IR reduces by 0.37 (95% CI: 0.32−0.42) (26.8% [95% CI: 25.2–27.8]) infections per person per year, and in users over 35 years of age by 0.33 (95% CI: 0.25−0.4) (23.9% [95% CI: 19.7–26.5]). In the case of eliminating unscheduled visits, the reduction in IR is 0.33 (95% CI: 0.24−0.42). Appendix A, Fig. 1 of the Supplementary material shows the evolution of cumulative incidences according to each screening strategy.
DiscussionThe incidence of STIs among PrEP users was high, as reported in other publications.15 However, despite much debate about the continuing rise of STIs among PrEP users, in this study the IR of STIs declined over the years.16,17 There may be several factors behind this decline. On the one hand, increasing age among users in the cohort could be associated with a lower rate of STIs.18 On the other hand, the onset of the COVID-19 pandemic coincides with the most significant decline in STIs during the study. In the general population in Spain, according to the surveillance registry, in the year following the COVID-19 pandemic, the incidence of STIs increased and even surpassed pre-2020 data.19 However, these results are not congruent with those found among the participants in our study. As in the Schmidt et al. publication, the incidence of STIs among PrEP users remained lower than in the pre-pandemic era.20
The majority of STIs detected in our study were NG followed by CT and, as in other publications, the most frequent location was rectal.17 NG infections declined significantly, with incidence rates in recent years approaching those of CT, as in other PrEP cohorts.21 Despite six-monthly screening, cumulative HCV incidence remained close to zero. According to the meta-analysis by Traeger et al. cases of hepatitis C are very rare among PrEP users and are associated with specific epidemiological characteristics.22 Although some studies look at mycoplasma genitalium (MG) among PrEP users,23 the CDC does not recommend routine screening for this emerging pathogen in asymptomatic PrEP users, so no data were included in this study.24
In a cohort of PrEP users in France, 18.7% of rectal cases of CT were LGV.25 In our study, 21.8% of all cases of CT were LGV, the vast majority of which were rectal. Detecting LGV seems to be essential when it comes to the rectum, but less so in the pharynx or urethra, especially without strong suspicion.
The implementation of PrEP, which is increasingly in demand, poses additional challenges such as the continuity of the programme to adapt to the resources and the user. Self-testing could be an effective and expeditious method for STI screening among asymptomatic users. No significant differences in STI incidence were found when self-testing was implemented, as in previous studies.26 In this study, alternatives to quarterly STI screening were analysed by modifying the frequency and tests to be performed. If STI screening were to be carried out every six months, 45% of STIs would remain undiagnosed. These data are superimposable to those described in the AmPrEP cohort, where the diagnostic delay would have been 52% and 30% if unscheduled visits were included.21 Therefore, according to our study, reducing the frequency does not seem to be the best option to achieve early diagnosis and avoid chains of transmission. However, in the case of syphilis, screening every six months did not significantly reduce the incidence of STIs. There was also no significant reduction in incidence if pharyngeal or urethral swabs were not collected, especially among those over 35 years of age. These results support the possibility of optimising the frequency of screening by taking into account the tests to be performed at each visit.
In addition, it should be borne in mind that not everyone will derive the same benefit from a given STI screening. A PrEP cohort in Australia reported that 76% of STIs recurred in the same 25% of users.27 This demonstrates the great heterogeneity that exists among PrEP users. Risk factors for STIs among PrEP users include being under 35 years of age, having multiple sexual partners and engaging in chemsex.28 This study provides data to optimise STI screening among PrEP users. However, it should be individualised according to each user's practices, so it is essential to conduct and update a detailed sexual history. In addition, optimising STI screening at scheduled visits would allow resources to be distributed and directed to those unscheduled visits associated with clinical or epidemiological suspicion, where a higher diagnostic yield has been observed.21
This study had some limitations: information collected on sexual practices and clinical manifestations were not taken into account in the analysis; relevant information to be considered in future analyses. However, since this was a cohort of PrEP users with long follow-up and close monitoring, it allowed for extensive data collection.
ConclusionsThe incidence of STIs among PrEP users, particularly regarding the rectal location, is high, but does not increase over the years. Scheduled STI screening could be optimised through self-testing, six-monthly syphilis serology and reducing the frequency of STI testing in pharyngeal and urethral sites, especially in asymptomatic individuals over 35 years of age. It is essential to have resources available for unscheduled visits, which have been shown to be a more cost-effective screening tool.
Conflicts of interestThe authors declare that they have no conflicts of interest.