Blood culture contamination can occur from extraction to processing; its rate should not exceed 3%.
ObjectiveTo evaluate the impact of a training programme on the rate of contaminated blood cultures after the implementation of sample extraction recommendations based on the best evidence.
MethodProspective before-after study in a polyvalent intensive care unit with 18 beds. Two phases were established (January–June 2012, October 2012–October 2015) with a training period between them. Main recommendations: sterile technique, surgical mask, double skin disinfection (70° alcohol and 2% alcoholic chlorhexidine), 70° alcohol disinfection of culture flasks and injection of samples without changing needles. Including all blood cultures of patients with extraction request. Variables: demographic, severity, pathology, reason for admission, stay and results of blood cultures (negative, positive and contaminated). Basic descriptive statistics: mean (standard deviation), median (interquartile range) and percentage (95% confidence interval). Calculated contamination rates per 100 blood cultures extracted. Bivariate analysis between periods.
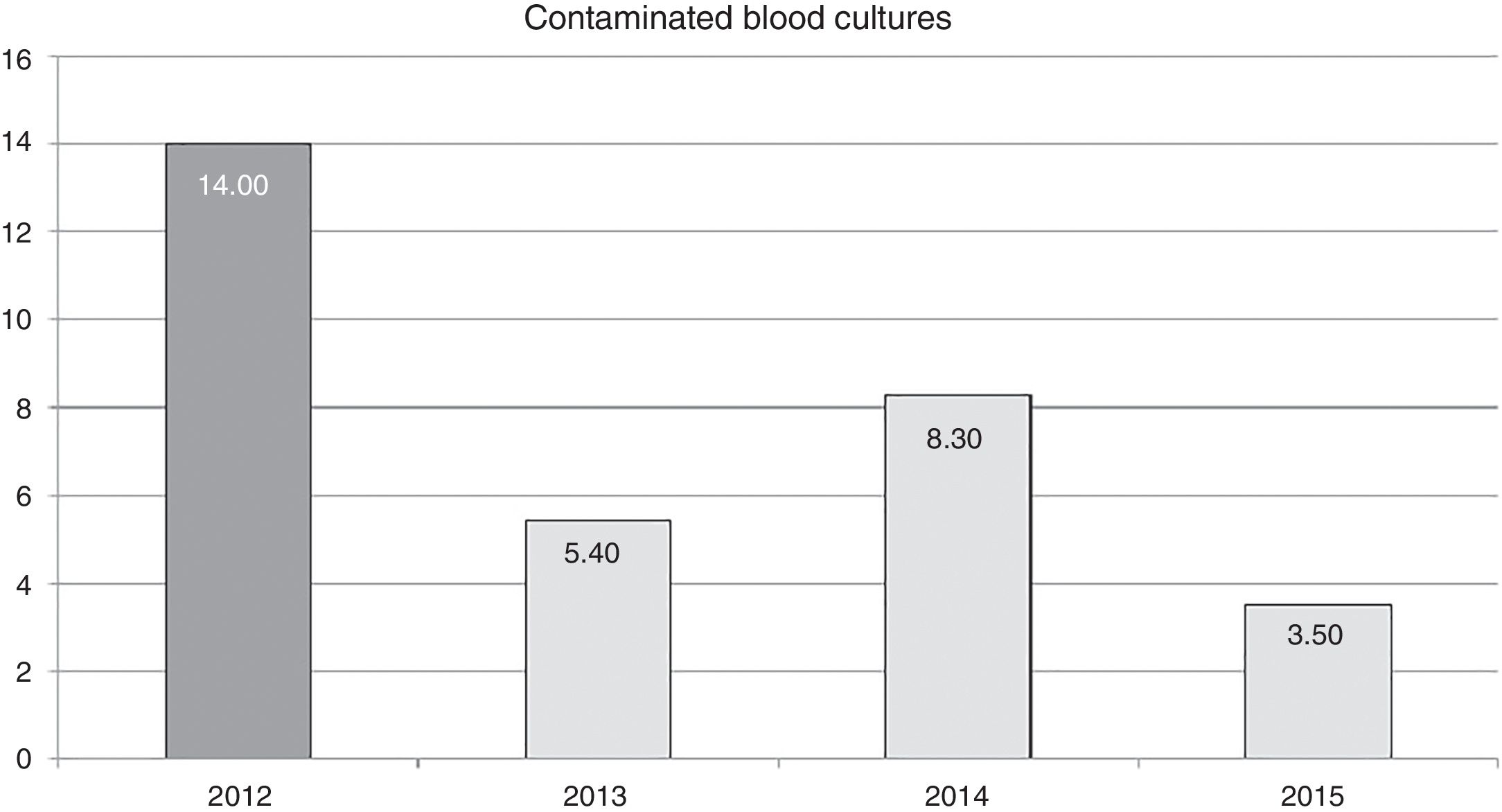
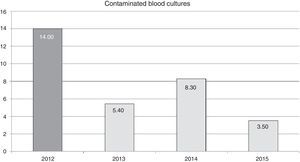
ResultsFour hundred and eight patients were included. Eight hundred and forty-one blood cultures were taken, 33 of which were contaminated. In the demographic variables, severity, diagnosis and stay of patients with contaminated samples, no differences were observed from those with uncontaminated samples. Pre-training vs post-training contamination rates: 14 vs 5.6 per 100 blood cultures extracted (p=.00003).
ConclusionAn evidence-based training programme reduced the contamination of samples. It is necessary to continue working on the planning of activities and care to improve the detection of pollutants and prevent contamination of samples.
La contaminación de hemocultivos puede ocurrir desde la extracción al procesamiento, y su tasa no debería exceder del 3%.
ObjetivoEvaluar el impacto de una acción formativa sobre la tasa de hemocultivos contaminados tras la instauración de recomendaciones de extracción de muestras basadas en la mejor evidencia.
MétodoEstudio prospectivo antes-después en una unidad de cuidados intensivos polivalente de 18 camas. Se establecieron dos fases (enero-junio 2012, octubre 2012-octubre 2015) con un período formativo entre ellas. Principales recomendaciones: técnica estéril, mascarilla quirúrgica, doble desinfección de piel (alcohol 70° y clorhexidina alcohólica 2%), desinfección con alcohol 70° de tapones de frascos de cultivo e inyección de muestras sin cambiar aguja. Incluidos todos los hemocultivos de pacientes con solicitud facultativa de extracción. Variables: demográficas, gravedad, patología, motivo de ingreso, estancia y resultados de hemocultivos (negativo, positivo y contaminado). Estadística descriptiva básica: media (desviación estándar), mediana (rango intercuartílico) o porcentaje (intervalo de confianza del 95%). Calculadas tasas de contaminación por 100 hemocultivos extraídos. Análisis bivariado entre períodos.
ResultadosIncluidos 458 pacientes. Extraídos 841 hemocultivos, 33 de ellos contaminados. En las variables demográficas, gravedad, diagnóstico y estancia en pacientes con contaminación de la muestra, no se observaron diferencias con no contaminados. Tasas de contaminación pre-formación vs post-formación: 14 vs 5,6 por 100 hemocultivos extraídos (p=0,00003).
ConclusiónUna acción formativa basada en la evidencia ha reducido la contaminación de las muestras. Es necesario seguir trabajando en la planificación de actividades y cuidados para mejorar la detección de contaminantes y prevenir la contaminación de las mismas.
The existence of false positives in blood culture due to contamination when extraction takes place is a common problem. This is associated with an increase in costs, confusion for medical professionals, inappropriate administration of antibiotics, additional trials and prolonged hospital stay.
Contamination of samples may be due to several factors involving incorrect extraction technique, an inappropriate time for extraction and even an altered mental state of the patient who is not collaborative and who makes simple obtainment difficult.
What this article contributesRecommendations based on the best available evidence for extraction of this type of samples. Their dissemination and the consequent training of nursing staff has been successful in lowering the rate of false positives in blood cultures in our patient cohorts.
Implications of the studyTo enrich research on the effect of training actions for nurses and how they directly or indirectly influence the administration of quality care.
The extraction of blood cultures in febrile patients has been a consolidated practice since the 1940s.1 The presence of bacteria or fungi in the blood is detected from isolation in blood cultures, which comprise the samples that are most frequently processed in microbiology laboratories. In our service, in 2015, they accounted for the highest percentage of extracted samples (31%). The objective of their extraction, in addition to the diagnosis of bacteraemia or fungemia, is to determine a specific therapy and provide prognostic value.2 One of the main limitations for diagnosis is that no available appropriate gold standard blood culture exists from the false positives or contaminated blood cultures.3–9
Contamination may occur at any stage of the process, but inappropriate disinfection and poor technique is highlighted as the primary reason for the false positive.10 Other causes could be an unrestrictive request for the test, the wrong time for extraction, the altered mental state of the patient or haste in administrating initial doses of antibiotics.3–10 Contamination is associated with an increase in costs,11–16 is a contributing factor of confusion for the practitioners, involves incorrect administration of antibiotics, further testing, and prolonged hospital stay.17,18 According to the Spanish Society of Infectious Diseases and Clinical Microbiology,19 the American Society for Microbiology and the Clinical Laboratory Standards Institute,20,21 the rate of contamination should not exceed 3%.
A study was conducted in 2003 in our intensive care unit22 on the use and performance of blood cultures where the level of contamination was also assessed, which at that time was 6%. In 2012, during the months from January to June, we observed that contamination was 8% in 158 blood cultures, data which surpassed our rate of 2003 and the accepted standard for this type of sample.
Due to the variability observed in blood culture extraction technique, a guide was designed with recommendations based on the best available evidence for this procedure. Once the guide had been designed, we decided to carry out a study aimed at assessing the impact of training on the rate of contaminated blood cultures, based on these recommendations.
MethodologyA quasi-experimental before-and-after study was conducted in an all-purpose intensive care unit (ICU) with 18 beds, at a third tier university hospital.
The research team comprised 8 nurses from the specialist microbiology unit, two internal medicine resident doctors and one intensive care specialist.
Two study phases were established, with a training period between them. Phase 1 (pre-training) took place from January to June 2012. Training was from July to September 2012. Phase II (post-training) was from October 2012 to October 2015.
All extracted blood cultures were included, by medical referral, from patients who had been admitted to the ICU during the study periods. Minors and pregnant patients were not included. The doctors requesting the blood cultures were not involved in the data collection and the decision for extraction of blood cultures was solely and exclusively due to the clinical status of the patient. The extraction of blood cultures was always made by direct puncture.
The classification of the blood cultures was made independently by intensive care practitioners from the research team, establishing the rate of positive blood cultures and contaminated cultures during the whole study. When there was a discrepancy a consensus was reached.
Phase i. Pre-trainingThe variables relating to the extraction of blood cultures were collected (those referred to in the section “variables collected in both phases”), along with their results, and the rate of false positive incidence was calculated.
A guide was simultaneously designed (Appendix 1) for the extraction of blood cultures based on the “Sample Collection Manual of the Hospital Microbiology Laboratory” and the contributions made by the research team based on the best evidence found.
A search of references was performed using the Pubmed and Ovid platforms, in addition to general non-specific search engines. The search was made with the following key words (in English and Spanish): blood cultures, intensive care, blood culture extraction, contamination of blood cultures, false bacteremia, bacteremia associated with catheters, cost, effectiveness and blood cultures performance. Among the selected articles, reviews were found with meta-analysis and articles of “good practices”.
Following phase i, once the guide had been designed, the nurses from the research team performed the necessary session, in morning, afternoon and evening shifts, explaining the extraction procedure and the processing of the blood cultures. A registry of all the nurses and nursing auxiliaries involved in training was made to ensure that they all received training. In addition to the nursing sessions, posters were made and were placed in the units, with the main points to be taken into consideration in sample extraction. The guide was also inserted into the service computers so that it was accessible to all workers in the unit.
The primary modifications concerning the previous extraction guide corresponded to how the procedure was to be carried out with a sterile technique and field, the use of a surgical mask, double disinfection of the skin with 70° alcohol and alcoholic chlorhexidine at 2%, disinfection with 70° alcohol of cork caps of the culture recipients and injection of the samples into the culture phials without changing needle and by the same person who made the puncture (extractor), whilst the assistant removed the compressor, when necessary, and pressed onto the puncture point.
Phase ii. Post-trainingThe second stage of training was initiated after the training of staff and introduction of the recommendations.
During this phase and up until study finalisation, the research team informed the whole nursing team of the contamination rates on a monthly basis.
Variables collected in both phasesPatient demographic variables were collected (age and gender), severity on admittance in the ICU estimated by the Simplified Acute Physiologic Score II (SAPS II), the pathology which had led to admittance, hospital stay and the results of the blood cultures (negative, positive, contaminated blood culture).
Variable definitions23- •
Positive blood cultures: isolation in at least one set, of any of the following microorganisms: gram positive coccus which was different from coagulase-negative Staphylococcus, gram negative bacilli or fungi. Also considered positive was when in the two sets coagulase-negative Staphylococcus was isolated and the patient presented clinical symptoms compatible with bacteremia.
- •
Contaminated blood culture: when in a single set coagulase-negative Staphylococcus, Bacillus spp. (except Bacillus anthracis), Propionebacterium spp., Streptococcus from the viridans group, Aerococcus spp., Micrococcus spp. or Corynebacterium spp. were isolated.
When blood cultures extraction was indicated, two sets per patient (4 tubes) were collected. The recommended sample volume, depending on the technical characteristics of the product was of 8–10ml, although according to the manufacturer's indications it was possible to use sample volumes of just 3ml.24 Each blood sample was inoculated into two aerobic and anaerobic BACTEC recipients and sent to the microbiology laboratory for incubation in the BD BACTEC™ FX (Becton Dickinson Diagnostics, Sparks, MD) system for a period of 5 days.
The sets used contained the following reactive ingredients in specific compositions for aerobic and anaerobic samples: treated water, soybean casein digest medium, yeast extract, animal tissue digest, amino acids, sugar, sodium citrate, sodium polyanethole sulfonate, vitamins, antioxidants/reducing agents, non-ionic absorbent resins (13.4% for aerobics and 16% for anaerobics) and cation-exchange resins (.9% aerobics and 1% for anaerobics). All BACTEC media were supplied with CO2 which was added to the anaerobic media, pre-reduced with and dispensed with CO2 and N2.
All blood cultures phials detected as positive were subjected to the Gram stain of tissue test, were inserted into the blood agar culture media, chocolate agar, McConckey agar and Brucella (bioMérieux) agar and incubated at 35°C. The positive blood cultures with ram negative stain were reinserted into the equipment to complete their 5 days of incubation.
Direct positive blood culture microorganism identification was carried out using standard methods: MicroScan panels (Micro-Scan Systems, Siemens, Renton, WA, USA), API strips (bioMérieux, Marcy l’Etoile, France) and biochemical testing. From September 2015 identification of the isolated samples was carried out with Maldi-Tof (Bruker Daltonics, Bremen, Germany). Sensitivity tests were also made using a diffusion disk method with selected antibiotics depending on the type of microorganisms. The inoculum used for all of them was drops of liquid extracted from the blood culture recipient.
AnalysisA basic descriptive statistical analysis was performed, expressing the outcome as a mean (standard deviation), median (interquartile range) or percentage, as appropriate. Contamination rates were estimated per 100 blood cultures extracted. The comparisons between periods were made using the Student's t-test or the Mann–Whitney U test as standard. A statistically significant level was considered to be p<.5. The statistical programmes SPSS 17.0 and Stata 14.0 were used.
Ethical considerationsThe study was approved by the hospital ethics and clinical research committee and it was not necessary to request informed consent. The criteria of respect for the dignity of the patient and protection of their data, rights and wellbeing prevailed.
The procedure followed complied with the committee's ethical standards on responsible human experimentation and was in keeping with the World Medical Association and the Declaration of Helsinki.25
The recommendations contained in the 1997 Agreement Oviedo were followed, as were those of Law 15/1999 regulating the automated treatment of personal data and Law 41/2002 regulating patient autonomy.
ResultsA total of 126 nursing professionals were trained, of whom 73 were nurses and 53 were nursing auxiliaries. These 126 professionals comprised the whole permanent and the temporary staff.
540 patients were included in the study which generated 584 hospital admittances: 82 patients in the pre/training phase and 458 patients in the post/training phase.
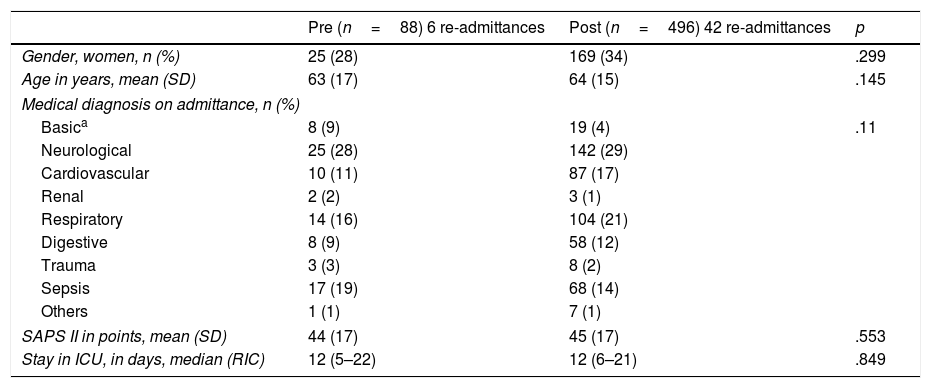
No significant differences were found in the demographics, severity, reason for admittance and length of stay in the ICU between the two periods (Table 1).
Patient characteristics during both periods.
| Pre (n=88) 6 re-admittances | Post (n=496) 42 re-admittances | p | |
|---|---|---|---|
| Gender, women, n (%) | 25 (28) | 169 (34) | .299 |
| Age in years, mean (SD) | 63 (17) | 64 (15) | .145 |
| Medical diagnosis on admittance, n (%) | |||
| Basica | 8 (9) | 19 (4) | .11 |
| Neurological | 25 (28) | 142 (29) | |
| Cardiovascular | 10 (11) | 87 (17) | |
| Renal | 2 (2) | 3 (1) | |
| Respiratory | 14 (16) | 104 (21) | |
| Digestive | 8 (9) | 58 (12) | |
| Trauma | 3 (3) | 8 (2) | |
| Sepsis | 17 (19) | 68 (14) | |
| Others | 1 (1) | 7 (1) | |
| SAPS II in points, mean (SD) | 44 (17) | 45 (17) | .553 |
| Stay in ICU, in days, median (RIC) | 12 (5–22) | 12 (6–21) | .849 |
SD: standard deviation; IQR: interquartile range; SAPS II: Simplified Acute Physiologic Score II.
Regarding the samples extracted, a total of 999 blood cultures were obtained, of which 158 were extracted in the pre-training phase and 841 in the post-training phase. The contamination rate (false positives) from the pre-training phase was of 14 out of every 100 blood cultures extracted vs 5.6 out of every 100 blood cultures extracted during the post-training phase (p=.00003).
The severity of the patients with false positives in both periods was not significant (SAPSII, mean [SD]: 42 [14] vs 42 [15], p=.96). The length of stay in the ICU was not significant either (median [IQR]: 13 [5–37] vs 17 [9–31], p=.44).
The effect of training in annual contamination rates may be observed in Fig. 1. We may observe that after training activity, the rates dropped by 14 for each blood cultures extracted, to 5.4 for each 100 blood cultures extracted in 2013. This rate increases the following year but drops in 2015 to 3.5 for every 100 blood cultures extracted.
DiscussionIn our study the primary finding was the reduction of the rate of false positive blood cultures or contaminated blood cultures subsequent to training based on the best possible evidence.
The reduction of the rate of blood culture contamination, after training activity, was 62% in the first year (2013). This rate increased in 2014, compared with the previous year, although it did not surpass the pre-training rate (Fig. 1). It is possible that this was due to relaxation in the application of the recommendations since they were corrected (up to 3.5 per 100 blood cultures in 2015) after informing the nursing staff of the increase in the rate and insisting on the importance of compliance with recommendations. This could prove that continual monitoring of the rates could alert us to correct applications of recommended guidelines and correct any undesirable deviation from them.
It is difficult to compare our results with those found in the literature, due to the heterogeneity of the extraction procedure and the origin of the sample. The samples of this study were always obtained by direct puncture, whilst in other studies26,27 the origin of the sample was not specified (puncture or catheter).
Our values were above those obtained by Mimoz et al.26 (1.4%). In their study comparison was made between two antiseptics: povidone iodine and an alcoholic solution of chlorhexidine gluconate at .5%. More favourable values were raised with the latter antiseptic. In this study the extraction procedure was not specified. Septimus et al.27 introduced the chlorhexidine bath as the most important variable for reducing sample contamination, obtaining a rate of 3.3% in one of the groups under consideration. This was most similar to our cohort. However, in this study it was not specified if the sample had been obtained by puncture or extracted through a catheter, which we would consider a serious limitation.
Compared with other studies14,15,28–31 on critically ill patients, the results are similar to ours, showing contamination rates of between 3.5%31 and 4%.14,15 Alahmadi et al.15 specifically deal with a training procedure on the contamination rate of blood cultures which remained above the standard, but still induced a reduction from 9% to 4%.
If we compare our study22 carried out in our unit in 2003, which did not assess the effect of training but estimated the rate of false positives, the reduction in the contamination rate was considerable in 2015 (6% vs 3.5%), but still above the standard established by scientific associations.
Furthermore, both the direct and indirect the financial cost of sample contamination calculated by Alahmadi et al.14 in 2011, was above 5000 pounds (approximately 5777 euros) per contaminated blood culture. If we take this figure as a reference, bearing in mind the two healthcare systems differ from one another, by reducing contamination from 8% to 3.5% in our service we would hypothetically have made a saving of 218,630 Euros.
In addition to the financial cost, we could mention qualitative improvements, such as the reduction of patient suffering and the increase in care excellence, neither of which were taken into account in this study and which could be considered a limitation to the same.
Among the limitations associated with training, the skills acquired during training were not assessed, but the drop in rates could be considered an indirect indicator of training as confirmed by other studies.32,33 that showed the positive effect of an educational intervention on the drop in contamination of this type of samples.
In their review of “good practices” Garcia et al.,34 included studies which considered the introduction of feedback processes, either associated or not with the retraining procedures of staff responsible for sample extraction. In our study training did not take into account this feedback action and the staff responsible for extraction were not told whether the sample extracted was contaminated or not. This should be taken into consideration for further studies as it could be relevant, as is indicated by published evidence.
Finally, we consider that the most salient limitation is in the volume of sample extraction, which was not recorded because it did not form part of standard practice. Studies like those of Cockerill et al.,35 Bouza et al.,36 Lee et al.37 and Patel et al.38 determined that the greater the sample volume the higher the test sensitivity, and this was confirmed in the latest review made by Lamy et al.39 in 2016. Notwithstanding, Bekeris et al.40 and the Clinical and Laboratory Standards Institute41 recommend that the manufacturer's indications be followed with regards to the sample volume required. In any case, this would be a variable to consider in future related studies.
ConclusionFrom these results we may conclude that appropriate training in recommendations based on evidence has reduced the contamination of blood cultures in our patient cohort. Further work is still required so as to plan activities and healthcare for reduce this rate. The activities would be aimed at carrying out a technique based on evidence, continuous training of staff and the suitability of requests for blood culture samples in patients with a very low probability of bacteremia based on clinical criteria.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Sánchez-Sánchez MM, Arias-Rivera S, Fraile-Gamo P, Jareño-Collado R, López-Román S, Vadillo-Obesso P, et al. Efecto de una acción formativa en cuidados intensivos sobre la tasa de contaminación de hemocultivos. Enferm Intensiva. 2018;29:121–127.