Attachment is the tendency of human beings to create strong affective bonds towards specific figures, and has been described as a general vulnerability factor to diverse forms of psychopathology. Although attachment research has not tended to emphasize genetic contribution, heritability is estimated at 36–45 %. We explored the association between 5-HTTLPR, COMT Val158Met and BDNF Val66Met functional polymorphisms with attachment dimensions, as well as the gene-environment interaction, considering the perceived parental rearing styles, with both a vulnerability and a differential susceptibility approach.
Methods150 outpatients with a cluster B personality disorder participated in the study. Attachment was assessed using Experiences in Close Relationships-Revised and rearing styles by means of the Parental Bonding Instrument. Genotypes were analyzed using saliva samples. Statistical analyses were carried out with general linear models.
ResultsCOMT ValVal homozygotes and BDNF Met-carriers were associated with significantly higher scores in attachment anxiety (COMT: ValVal 4.95±1.25 vs Met-carriers 4.42±1.30, t=-2.096, p=0.038; BDNF: ValVal 4.38±1.31 vs Met-carriers 4.95±1.24, t = 2.833, p=0.005). From a differential susceptibility approach, plasticity genotypes were identified for the three functional polymorphisms, observing significant interactions with parental overprotection and differential outcomes in relation to attachment anxiety. No associations or interactions were found with regard to attachment avoidance and the care dimension.
ConclusionOur results suggest that there are individuals more susceptible to rearing experiences in terms of adult attachment outcomes, which probably also implies a greater potential to benefit from environmental and psychotherapeutic interventions.
Attachment has been defined as the tendency of human beings to create strong affective bonds towards specific figures and explains the various forms of emotional stress and personality pathology, including anxiety, anger, depression, and emotional detachment, which result from the unwanted separation or loss of such figures.1 Attachment behavior is innately present in human beings, with the presumed biological function of “protecting a person (especially during infancy and early childhood) from danger by assuring that he or she maintains proximity to caring and supportive others (attachment figures)”.2 During childhood, the relationship with the parent is the primary attachment bond, and this will be gradually replaced, during adolescence and adulthood, by social and romantic relationships.
Infants are thought to develop internal working models of attachment from early interaction patterns with caregivers. These are a set of mental representations of the self, the caregiver and the world, that will operate across the lifespan by guiding the individual's expectations and explorations of the social and physical environment.3 The development of these prototype working models of attachment is also influenced by the infant's temperament and the social context,4 and will be affected by attachment-relevant experiences that will occur during childhood, adolescence, and adulthood. In fact, the degree of stability in attachment patterns from infancy to adulthood has been described as moderate,5 which means that parental bonding, understood as the experiences of parental caregiving and protection, shapes but does not determine adult attachment style.
Broadly speaking, attachment can be considered insecure or secure and has two fundamental dimensions: anxiety and avoidance. Anxiety explores the concern regarding the availability and receptivity of the other, and is based on the hyperactivation of the attachment system. Avoidance, on the other hand, evaluates interindividual differences regarding the degree of comfort with closeness and interdependence, and is based on the deactivation of the attachment system. Higher scores in both dimensions would indicate greater insecurity in attachment relationships,2 and insecure attachment, especially high scores on attachment anxiety, has been described as a general vulnerability factor to diverse forms of psychopathology.6 Specifically, a disordered attachment system is a core feature of personality disorders (PDs).7 In fact, the persistent difficulty with interpersonal relations, which can be construed as constellations of insecure attachment strategies, is a central common feature of all PDs.2 Most research on attachment and PDs has been focused on borderline and antisocial PDs which are, at the same time, the personality disorders that have concentrated the bulk of genetic studies in the field of personality pathology.8,9
Attachment research tends not to emphasize genetic contribution. However, Bowlby suggested that there may be pre-existing temperamental differences in children, and that attachment experiences would modulate, interact, or overcome these differences.10 Independent twin studies conducted in adults reported that genetic effects account for 45 % of the variability in the anxiety dimension and 36–39 % in the avoidance dimension.11,12 They also described the existence of common genetic factors that determine characteristics of both personality and attachment style, and highlight that, unlike that observed in child attachment, the remaining variability is mainly explained by non-shared environmental factors.
Research to date has been based mainly on candidate gene studies and performed from two different perspectives: a vulnerability approach that seeks to identify genotypes associated with specific phenotypes, and a differential susceptibility or plasticity approach, whose objective is to identify genotypes that interact with the environment. The classic diathesis–stress model postulated that “some individuals are at heightened risk, due to their genetic make up, of succumbing to psychological disturbance when they encounter adversity, whereas others are not so affected even when exposed to the very same adversity”.13 The differential susceptibility approach, in contrast, claims that plasticity genotypes confer susceptibility both in a positive sense (a specific genotype associated with a favorable environment promotes a favorable outcome) and in a negative sense (the same genotype, in the presence of an unfavorable environment, associates with an unfavorable outcome).13 Mixed results have been obtained so far in relation to adult attachment, mainly on genes related to oxytocin, dopaminergic pathways, and serotonergic pathways, such that it continues to be a field with many unknowns and little evidence. Previous works conducted by our group have identified that COMT Val158Met and 5HTTLPR influence personality, attachment and other variables related to social interaction both in general and in clinical samples of borderline PDs,14,15 from a vulnerability approach.
Individuals with PDs, especially those of cluster B (which includes borderline and antisocial PDs), are a group of special interest when investigating genetic aspects of attachment and its interaction with the rearing environment. On one hand, and as we have already mentioned, because of the vast literature associating disordered attachment with PDs, and the existing body of research on the influence of genetics on personality features and disorders.7–9 On the other hand, there is also evidence from both cross-sectional and longitudinal studies that certain parenting styles may predispose to the development of PDs,16 as well as reports describing a gene-rearing environment interaction17–19 in the etiopathogenesis of PDs.
The candidate genes encoding the serotonin transporter (5-HTT), catechol-o-methyl transferase (COMT), and Brain-derived neurotrophic factor (BDNF) are some of the most commonly examined candidate genes in relation to human behavior and across major mental disorders,20,21 including personality. 5-HTT is responsible for the reuptake of serotonin from the synaptic space. Its gene has a 44 pb deletion/insertion polymorphism in the promoter region (5-HTTLPR). The long allele (L) in the promoter is associated with higher levels of 5-HTT mRNA, i.e., higher transcriptional activity, higher transporter density and, therefore, lower serotonin concentrations in the synaptic space. The short allele (S) would lead to the opposite effect, i.e., lower transcriptional activity, lower transporter density and, therefore, higher serotonin concentrations in the synaptic space. The S allele acts in a dominant manner; thus, SS homozygotes and LS heterozygotes associate with lower reuptake.22 The S alelle has been associated with antisocial behavior23 and personality traits such as neuroticism,24 but it has been mainly and consistently described as a plasticity genotype that interacts with the early environment in relation to diverse forms of human behavior and/or pathology,25–28 including attachment-related outcomes.29,30
COMT is an enzyme involved in the extraneuronal degradation of dopamine, adrenaline, and noradrenaline by adding a methyl group donated by S-adenosyl methionine. The COMT gene has a functional single nucleotide polymorphism, Val158Met, consisting of a guanine to adenosine substitution resulting in the substitution of valine (Val) for methionine (Met) in the protein. MetMet homozygous individuals have four times less enzyme activity than ValVal homozygotes, with heterozygotes having intermediate activity. Therefore, ValVal homozygotes would exhibit higher catabolic activity and lower concentrations of brain dopamine, especially in the prefrontal cortex, where COMT is responsible for more than 50 % of dopamine degradation.31 The Val allele has been associated with externalizing phenotypes, both in adults32,33 as in children,34 with some recent reports pointing to a differential susceptibility effect, with respect to internalizing and externalizing behavior, mainly in children.35,36
Finally, BDNF is a protein of the neurotrophin family. It acts by favoring the survival of existing neurons, contributing to the growth and differentiation of new neurons and synapses, and protecting against stress-derived neuronal damage.37 The gene encoding BDNF contains a functional single nucleotide polymorphism, BDNF Val66Met, where a guanine base is changed to adenosine resulting in the substitution of Val for Met in the protein. The Val allele is associated with greater secretory activity of neuronal BDNF than the Met allele, and heterozygotes are also described to have less efficient intracellular trafficking and, consequently, lower BDNF secretion.38 In this case, the most relevant findings are those associating rearing environment with differential psychopathological outcomes (including attachment-related ones) depending on the genotype, and point to the Met allele as the one that confers plasticity.29,39,40
A recent review on genetics of adult attachment emphasized that research in the field of attachment genetics should be preferably approached as an interaction between nature and nurture, that is, more with an approach of differential susceptibility than vulnerability.41 In this context, we propose a study that contemplates both approaches, conducted in a clinical sample of individuals diagnosed with a cluster B PD. On the one hand, we analyze the direct effect of functional genetic polymorphisms in the 5-HTT, COMT and BDNF genes on attachment dimensions, in line with a vulnerability approach. And on the other hand, the interaction of these polymorphisms with the perceived parenting style, using a differential susceptibility approach.
Our hypotheses are the following:
- -
The Val allele of COMT Val158Met polymorphism will be associated with a more externalizing attachment phenotype (higher attachment anxiety)
- -
The S allele of 5HTTLPR and the Met allele of BDNF Val66Met polymorphisms will act as plasticity genotypes, moderating attachment styles (scores in anxiety and avoidance) depending on the valence of rearing experiences
A total of 150 outpatients (38 males and 112 females, with a mean age of 33.31±10.36 years) with a main diagnosis of a cluster B personality disorder according to DSM-5 (Section II) criteria, were recruited from different outpatient settings (community mental health centers and a day hospital) from 2015 to 2019. Inclusion criteria were (a) aged 18 to 65 years, (b) Caucasian race, and (c) ability to communicate in Spanish. Exclusion criteria were (a) current or past comorbid diagnosis of any neurological disorder that could interfere with the performance of neuropsychological tasks, (b) current severe medical conditions, (c) current drug dependence, and (d) intellectual disability.
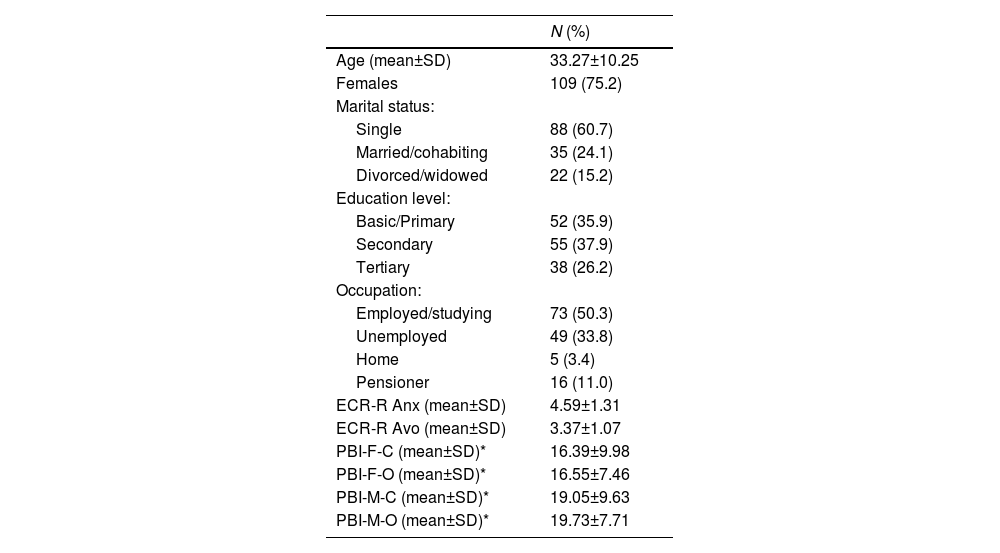
Patients were informed about the ongoing research by their treating psychiatrists or psychologists during regular follow-up appointments or regular visits to the day hospital. They were provided with detailed information about the study before signing the informed consent form. The instruments described below were administered by a trained researcher, who also gathered the sociodemographic data. Of the 150 patients, five left items blank for Experiences in Close Relationships-Revised (ECR-R) and were thus excluded from the analysis. The Parental Bonding Instrument (PBI) was completed by 75 of the 145 patients. Table 1 provides a sociodemographic description of the sample, including mean scores in the assessed psychological dimensions.
Description of the sample (N = 145).
| N (%) | |
|---|---|
| Age (mean±SD) | 33.27±10.25 |
| Females | 109 (75.2) |
| Marital status: | |
| Single | 88 (60.7) |
| Married/cohabiting | 35 (24.1) |
| Divorced/widowed | 22 (15.2) |
| Education level: | |
| Basic/Primary | 52 (35.9) |
| Secondary | 55 (37.9) |
| Tertiary | 38 (26.2) |
| Occupation: | |
| Employed/studying | 73 (50.3) |
| Unemployed | 49 (33.8) |
| Home | 5 (3.4) |
| Pensioner | 16 (11.0) |
| ECR-R Anx (mean±SD) | 4.59±1.31 |
| ECR-R Avo (mean±SD) | 3.37±1.07 |
| PBI-F-C (mean±SD)* | 16.39±9.98 |
| PBI-F-O (mean±SD)* | 16.55±7.46 |
| PBI-M-C (mean±SD)* | 19.05±9.63 |
| PBI-M-O (mean±SD)* | 19.73±7.71 |
SD: standard deviation; ECR-R: Experiences in Close Relationships-Revised; Anx: Anxiety; Avo: Avoidance; PBI: Parental Bonding Instrument; F: Father; M: Mother; C: Care; O: Overprotection.
Adult attachment style was explored by means of the ECR-R.42 It is a self-administered questionnaire of 36 items that are evaluated according to a 7-point Likert scale to assess attachment styles. Half of the items assess anxious attachment (e.g., “I'm afraid that I will lose my partner's love”) and the other half assess avoidant attachment (e.g., “I prefer not to show a partner how I feel deep down”). Scores in each dimension range from 1 to 7. Attachment anxiety is characterized by a fear of abandonment and anger at separation. Avoidance, on the other hand, is defined by a lack of closeness and by emotional repression. We used the Spanish validation of the questionnaire, which proved to have acceptable internal consistency (alpha coefficients of .83 and .86 for attachment anxiety and avoidance, respectively) and an interscale correlation of .18.43
Perceived rearing was assessed using the PBI.44 This is a self-administered questionnaire of 25 items that evaluates perceived care and overprotection dimensions in relation to the parental rearing style according to a 4-point Likert scale. Twelve items assess the Care dimension (e.g., “Spoke to me with a warm and friendly voice”) and 13 the Overprotection dimension (e.g., “Did not want me to grow up”), which provide a score from 0 to 36 and 0 to 39, respectively. The questionnaire is answered in relation to each parent, such that four scores are usually obtained in total. We used the Spanish validation of the instrument,45 which showed adequate internal consistency (alpha coefficients: .77–.93). Besides, the PBI has shown high long-term stability.46 We used both continuous scores as the established cut-offs according to the authors, which are the following: 27 and 24 for maternal and paternal care, respectively, and 13.5 and 12.5 for maternal and paternal overprotection, respectively.44
GenotypingDNA was obtained from saliva samples. These were collected using an ORAcollect DNA kit and subsequently DNA was extracted with prepIT-L2P (both from DNA GenoTek Inc., Ottawa, Canada). Once purified, the DNA samples were stored in aliquots at −20 °C. All the polymorphisms were assessed in duplicate on two different days. Details on genotyping are provided as supplementary data.
Statistical analysisDescriptive analyses were conducted using proportions in the case of categorical variables, and means and standard deviations in the case of continuous variables. Univariate general linear models were used to assess the effect of the functional polymorphisms studied on attachment anxiety and avoidance. Sex and age were included as covariates. 5-HTTLPR genotypes were analyzed based on their known activity (low-activity S carriers vs. high-activity LL homozygotes). As regards COMT Val158Met and BDNF Val66Met, both alleles are codominant; however, to dichotomize the sample, a Met dominant (ValVal vs. Met carriers) model was conducted in both cases based on previously reported phenotypic behavior (i.e., scores in attachment dimensions).39,34,47 Hence, genotypes and sex were introduced as fixed factors, age as covariate, and attachment anxiety and avoidance as dependent variables. The sex-polymorphism interaction term was also considered, yet was not maintained as it was not significant in any of the models (p < 0.05).
In a second step, univariate general linear models were also used to study the interaction between genotypes and perceived rearing (paternal and maternal care and overprotection). In these models, both the direct effect of the genotype and rearing dimensions, as the genotype-rearing interaction was analyzed in all models, including sex and age as covariates. In the face of significant interactions, post hoc analyses were performed with Bonferroni correction. To that end, PBI scores were dichotomized in high and low overprotection and care, and mean differences and adjusted p-values were calculated. All statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 29.0. Sample size and post hoc power calculations are provided as supplementary data.
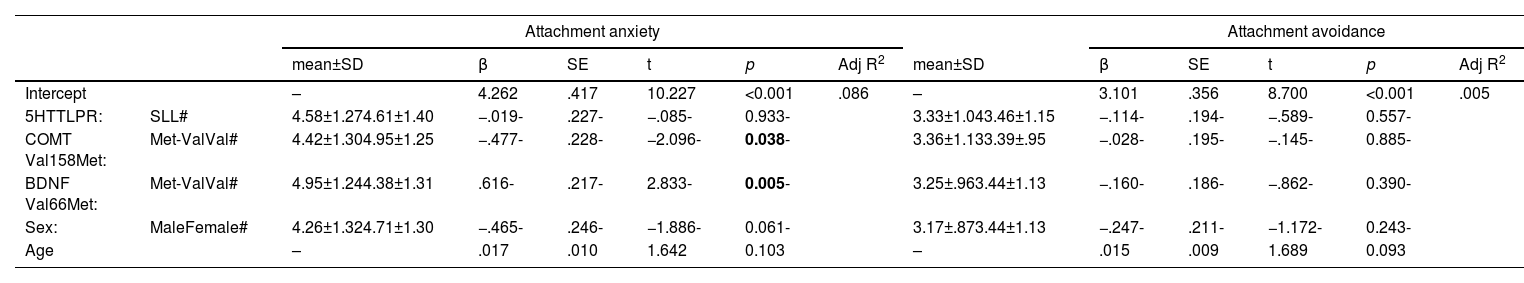
ResultsAll polymorphisms fulfilled the Hardy-Weinberg equilibrium (5-HTTLPR: X2=2.54, p=0.2804; COMT Val158Met: X2=0.09, p=09623; BDNF Val66Met: X2=.21, p=0.8992). The distribution of genotypes is shown in Supplementary Table 1. To answer the first objective, Table 2 shows the direct effect of the three polymorphisms on the dimensions of attachment anxiety and avoidance, controlled for age and sex. When assessing attachment anxiety, we observed that for COMT Val158Met, ValVal individuals scored significantly higher than Met-carriers, and for BDNF Val66Met, Met-carriers scored significantly higher than ValVal individuals. The COMT genotype accounted for 3.1 % of attachment anxiety variability and the BDNF genotype for 5.5 % (ɳ2=.031 and ɳ2=.055, respectively). As regards 5-HTTLPR, there were no differences in scores. We found no statistically significant effects of the polymorphisms studied on attachment avoidance.
Main effects of BDNF, COMT and 5HTT polymorphisms on attachment anxiety and avoidance (N = 145).
| Attachment anxiety | Attachment avoidance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean±SD | β | SE | t | p | Adj R2 | mean±SD | β | SE | t | p | Adj R2 | ||
| Intercept | – | 4.262 | .417 | 10.227 | <0.001 | .086 | – | 3.101 | .356 | 8.700 | <0.001 | .005 | |
| 5HTTLPR: | SLL# | 4.58±1.274.61±1.40 | −.019- | .227- | −.085- | 0.933- | 3.33±1.043.46±1.15 | −.114- | .194- | −.589- | 0.557- | ||
| COMT Val158Met: | Met-ValVal# | 4.42±1.304.95±1.25 | −.477- | .228- | −2.096- | 0.038- | 3.36±1.133.39±.95 | −.028- | .195- | −.145- | 0.885- | ||
| BDNF Val66Met: | Met-ValVal# | 4.95±1.244.38±1.31 | .616- | .217- | 2.833- | 0.005- | 3.25±.963.44±1.13 | −.160- | .186- | −.862- | 0.390- | ||
| Sex: | MaleFemale# | 4.26±1.324.71±1.30 | −.465- | .246- | −1.886- | 0.061- | 3.17±.873.44±1.13 | −.247- | .211- | −1.172- | 0.243- | ||
| Age | – | .017 | .010 | 1.642 | 0.103 | – | .015 | .009 | 1.689 | 0.093 | |||
5-HTTLPR, serotonin transporter linked polymorphic región; BDNF, Brain-derived Neurotrophic Factor; COMT, catechol-o-methyl transferase; SD, standard deviation; SE, standard error; Adj R2, Adjusted R squared; #, reference. Significant results are in bold.
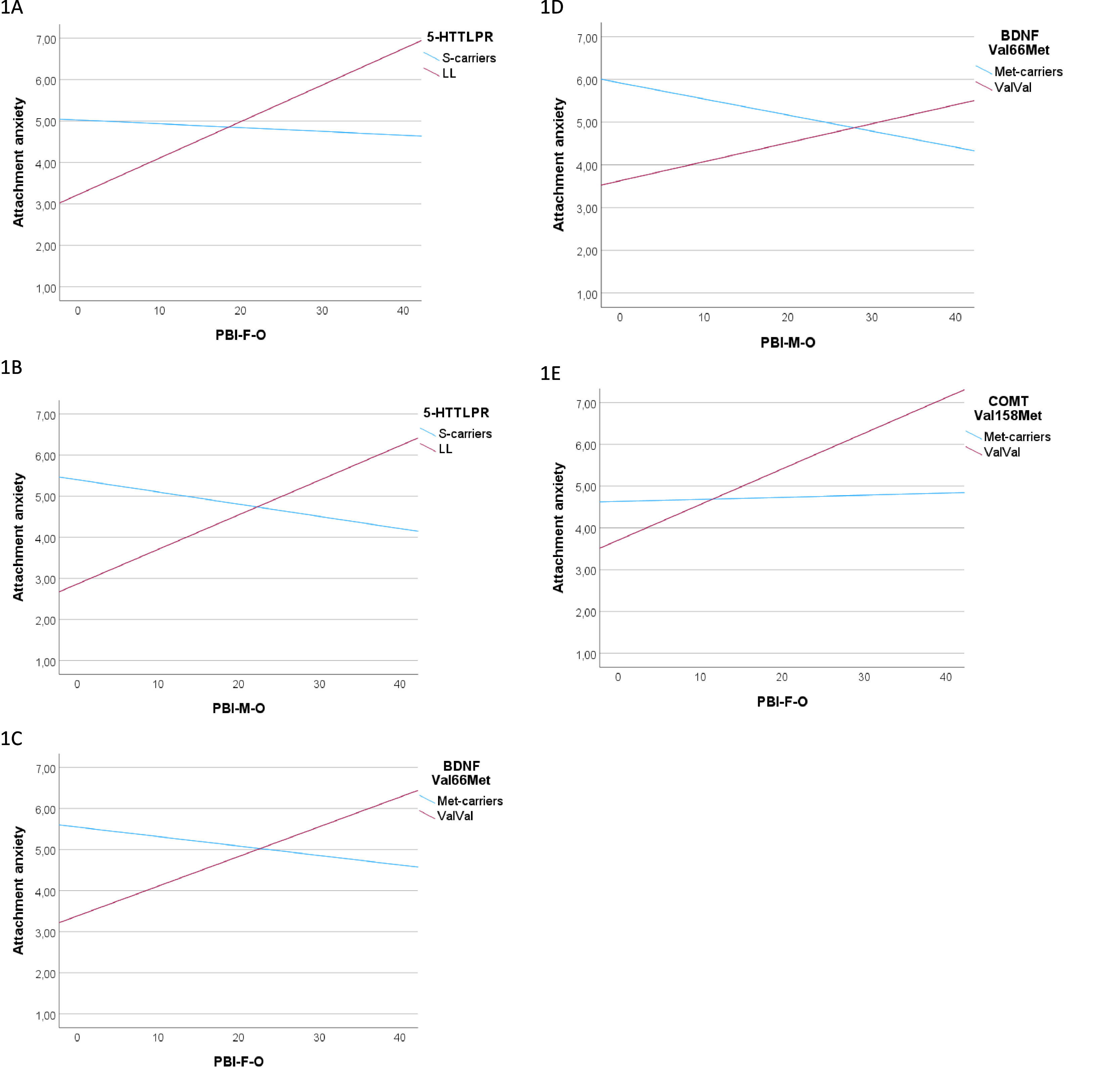
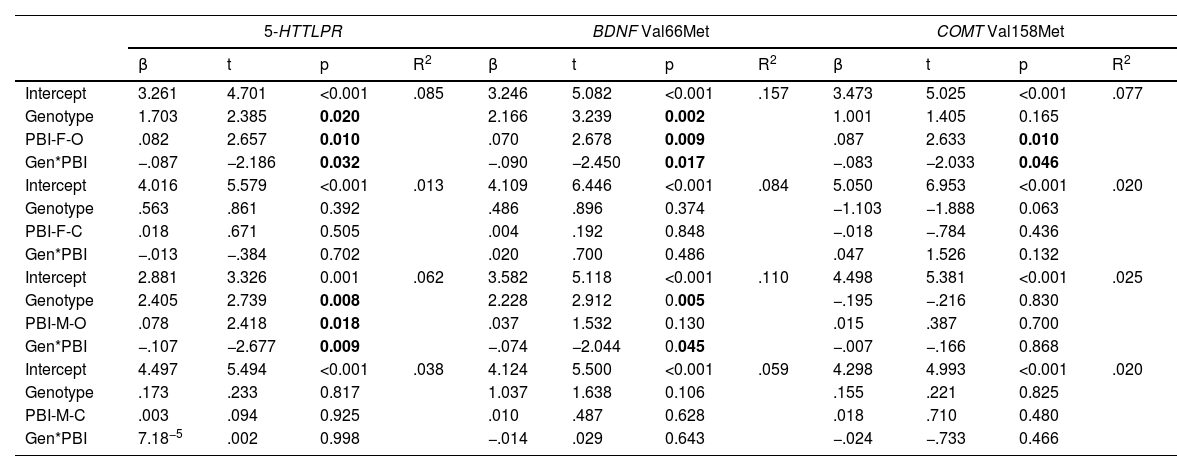
In response to the second objective, when studying the influence of the genotype-environment interaction on attachment anxiety (Table 3), we observed significant interactions between the three polymorphisms and parental overprotection (both paternal and maternal), as depicted in Fig. 1A-E. In the post hoc analyses, after dichotomizing the overprotection variable into high and low to quantify the differences evidenced in the interactions, we observed that the mean difference between L homozygotes of 5-HTTLPR was 2.366±.640 (p < 0.001) for paternal overprotection, and 1.706±.545 (p=0.003) for maternal overprotection; whereas no significant differences were detected between S-carriers regarding the level of parental overprotection. A similar pattern was obtained for BDNF Val66Met, so that the mean differences among ValVal individuals with high and low overprotection were .0958±.443 (p=0.034) for paternal scores and .868±.381 (p=0.026) for maternal scores, with no significant differences between Met allele carriers. Finally, the ValVal genotype for COMT Val158Met interacts only with paternal overprotection, with a mean difference in terms of high or low overprotection of 1.247±.494 (p=0.014), and no significant differences within Met allele carriers.
Effect of genotypes, rearing style and gene-rearing interaction on attachment anxiety, controlled by sex and age (N = 75).
| 5-HTTLPR | BDNF Val66Met | COMT Val158Met | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | t | p | R2 | β | t | p | R2 | β | t | p | R2 | |
| Intercept | 3.261 | 4.701 | <0.001 | .085 | 3.246 | 5.082 | <0.001 | .157 | 3.473 | 5.025 | <0.001 | .077 |
| Genotype | 1.703 | 2.385 | 0.020 | 2.166 | 3.239 | 0.002 | 1.001 | 1.405 | 0.165 | |||
| PBI-F-O | .082 | 2.657 | 0.010 | .070 | 2.678 | 0.009 | .087 | 2.633 | 0.010 | |||
| Gen*PBI | −.087 | −2.186 | 0.032 | −.090 | −2.450 | 0.017 | −.083 | −2.033 | 0.046 | |||
| Intercept | 4.016 | 5.579 | <0.001 | .013 | 4.109 | 6.446 | <0.001 | .084 | 5.050 | 6.953 | <0.001 | .020 |
| Genotype | .563 | .861 | 0.392 | .486 | .896 | 0.374 | −1.103 | −1.888 | 0.063 | |||
| PBI-F-C | .018 | .671 | 0.505 | .004 | .192 | 0.848 | −.018 | −.784 | 0.436 | |||
| Gen*PBI | −.013 | −.384 | 0.702 | .020 | .700 | 0.486 | .047 | 1.526 | 0.132 | |||
| Intercept | 2.881 | 3.326 | 0.001 | .062 | 3.582 | 5.118 | <0.001 | .110 | 4.498 | 5.381 | <0.001 | .025 |
| Genotype | 2.405 | 2.739 | 0.008 | 2.228 | 2.912 | 0.005 | −.195 | −.216 | 0.830 | |||
| PBI-M-O | .078 | 2.418 | 0.018 | .037 | 1.532 | 0.130 | .015 | .387 | 0.700 | |||
| Gen*PBI | −.107 | −2.677 | 0.009 | −.074 | −2.044 | 0.045 | −.007 | −.166 | 0.868 | |||
| Intercept | 4.497 | 5.494 | <0.001 | .038 | 4.124 | 5.500 | <0.001 | .059 | 4.298 | 4.993 | <0.001 | .020 |
| Genotype | .173 | .233 | 0.817 | 1.037 | 1.638 | 0.106 | .155 | .221 | 0.825 | |||
| PBI-M-C | .003 | .094 | 0.925 | .010 | .487 | 0.628 | .018 | .710 | 0.480 | |||
| Gen*PBI | 7.18−5 | .002 | 0.998 | −.014 | .029 | 0.643 | −.024 | −.733 | 0.466 | |||
5-HTTLPR, serotonin transporter linked polymorphic region; BDNF, Brain-derived Neurotrophic Factor; COMT, catechol-o-methyl transferase; PBI, Parental Bonding Instrument; F, father; M, mother; O, overprotection; C, care. Significant results are in bold.
It should be noted that these models also reflect a direct and independent effect of paternal overprotection on attachment anxiety, such that higher overprotection scores significantly and consistently associated with higher attachment anxiety scores, with no significant effects of the care dimension on attachment dimensions, nor any other significant association with respect to attachment avoidance.
DiscussionAs stated in the introduction, research on attachment has tended to emphasize the contribution of environmental factors over temperamental ones. There is consensus, also, on the influence of overprotective rearing on the development of psychopathology, and specifically PDs, especially when accompanied by low warmth and a harsh style.16,48 In our sample, paternal overprotection was associated with higher scores in attachment anxiety, which is the attachment dimension that has been associated in a nondeterministic manner with greater psychopathology in general,6 and again, with personality disorders in particular.49
In the first place, regarding the direct effect of the COMT Val158Met and BDNF Val66Met polymorphisms on the attachment anxiety dimension (COMT ValVal individuals and Met carriers of BDNF score higher in attachment anxiety), it should be noted that it is precisely the adult attachment dimension that has shown the greatest heritability in twin studies,11,12,50 specifically, between 37 and 45 %. In relation to the found association between COMT ValVal genotype and attachment anxiety, it is in line with our expectations. Different reviews have associated the Val allele of COMT Val158Met with more externalizing phenotypes, such as aggressiveness and extroversion.32,33 These are phenotypes more in line with attachment anxiety than avoidance and, in addition, characteristics shared by cluster B personality disorders, like those that make up our sample. It has been described that the overlap between adult attachment dimensions and personality traits is due to shared genetic influences, that is, that “they are capturing variance stemming from the same genetically influenced psychobiological traits”,11 which is in line with our observations.
The effect of BDNF on attachment has been studied mainly from a perspective of differential susceptibility, without, to our knowledge, previous studies on the effect on attachment anxiety or avoidance dimensions from a vulnerability perspective. Studies on the direct association of BDNF genotypes with personality traits are scarcer and more controversial, conducted mainly in the field of personality, and once again, from a perspective of interaction or plasticity, as we will discuss later. To our knowledge, this is the first publication reporting the effect of BDNF on the dimensions of adult attachment assessed by self-reports, and this positive association between Met carriers and greater attachment anxiety will have to be replicated in future works.
Secondly, we have explored whether some of the genotypes studied confer differential susceptibility to the environment, i.e., whether carriers of certain genotypes are more susceptible than others to both supportive and adverse experiences, in our case, parental rearing style. In relation to 5-HTTLPR, and in contrast to our findings, we expected the S allele of 5-HTTLPR to function as a plasticity allele. From the first description of the S allele's interaction with adverse experiences in the development of depressive symptomatology,25 numerous studies have replicated this differential susceptibility effect that moderates the relationship between rearing variables and different psychological outcomes.26,27,29,30 Nonetheless, other studies such as a meta-analysis of the 5-HTTLPR in relation to adverse environment and antisocial behavior has already reported that, despite the robust overall interaction effect found, it was not clear whether the significant interaction effect was driven by the S or L allele.51 In our case, it was the L homozygote that generated significant differences in attachment anxiety depending on whether there had been high or low overprotection. In a favorable environment (low paternal and maternal overprotection), homozygous L individuals scored significantly lower in attachment anxiety than those subjected to high overprotection or S-carrier individuals. Although most reports on differential susceptibility in the literature point to the S allele, recent works have also described that it is the L allele that confers this susceptibility, both with positive and negative valence.52,53
One of these investigations was carried out with the same instruments used in ours (ECR-R and PBI), indicating that the L allele interacted with maternal overprotection, in this case regarding attachment avoidance. Specifically, they described a positive and significant association between maternal overprotection and attachment avoidance in L allele carriers, while there was no significant relationship for S homozygotes.52 It is striking that this work, carried out in the general Italian population, found this effect in relation to avoidant attachment, while we found it in relation to anxious attachment, which, according to the literature, is also that most conditioned by genetics. The self-interpretation of the behaviors assessed in the study of attachment dimensions may vary due to social expectations and the culture of each country. On the other hand, with different outcomes and methodology, other recent research conducted with ecological momentary assessment also reported that L homozygotes present greater emotional reactivity to both uplifts and stressors, pointing to the L allele as a plasticity genotype, and describing greater affective inflexibility in S allele carriers.53 Some authors have proposed that epigenetic changes may vanish the functional differences between the S and L alleles and that it could explain the lack of consistent results regarding the plasticity of the S allele.54 They had previously observed that increased methylation of the L alleles made their carriers more vulnerable to attachment- and stress-related symptoms, and thus more similar to carriers of the S variants.55
Regarding the effect of BDNF Val66Met, results to date have been mixed. Previous work highlighted the BDNF Met allele as a genotype of plasticity in response to parental rearing in relation to constructs close to attachment, such as interpersonal sensitivity40 or indiscriminate behavior.29 It has also been described that Met-carriers were more sensitive to the effects of parental rearing in relation to anxiety and somatization.39 With this background in mind, our initial hypothesis was that Met carriers (who also associate with the highest attachment anxiety scores) would present a differential susceptibility with respect to attachment anxiety. However, this has not been the case. There are also other studies with results aligning with ours, highlighting ValVal homozygotes as more susceptible to the effect of adverse events in childhood,56,57 so it continues to be an area that requires further research and results.
Concerning the interaction observed with COMT Val158Met, no previous studies have assessed the moderating effect of COMT in the relationship between rearing and adult attachment, however, it has been described to confer differential susceptibility in the association between parenting and internalizing/externalizing symptomatology in children.35,36 In our case, there is a significant difference in attachment anxiety between ValVal individuals exposed to low and high paternal overprotection, being greater in those subjected to greater overprotection; however, there are no significant differences in the entire group exposed to low overprotection nor the entire group exposed to high overprotection. We could say, therefore, that in individuals homozygous for the Val allele, the effect of paternal overprotection is amplified, both in a positive and negative sense. However, as there are no significant differences within the high and low overprotection groups, we cannot clearly state that it confers differential susceptibility.
The main limitations of this study are the absence of a control group and the sample size, especially in the gene-rearing style interaction analysis, such that results should be considered exploratory and interpreted with due caution (see supplementary data on sample size and power calculations). Recent studies have begun to analyze the effect of candidate genes with polygenic scores to understand the differential susceptibility of children to environmental factors,26 which we believe is an approach to consider for the future with broader samples. On the other hand, we must point out that the individuals assessed in our study pertain to a heterogeneous clinical sample that includes different personality disorders and from different settings (outpatient clinic and day hospital), which probably associates with different burden of severity. The variables measured by the ECR-R and the PBI are dimensional, and thus, reproduce experiences and characteristics common to any individual on a continuous scale, whether from a clinical or general population sample. Nonetheless, we must bear in mind, in the first place, that the scores being discussed may differ from those that would be observed in a general population sample, both in relation to attachment and rearing styles. Also, studying a relatively heterogeneous group such as cluster B personality disorders, instead of a specific diagnosis, makes sense at the level of genetic study. There is increasing evidence that heritability is more related to transdiagnostic traits (neuroticism, externalization) than to specific categorical diagnoses which, as we know, present a high degree of overlap between the different categories. Finally, it is a cross-sectional study which, with regard to parental rearing experiences, may suffer from recall biases. The PBI has proved a long-term stability over a 20-year follow-up,46 although this does not prevent memory biases affecting the narrative about parental rearing style when assessed in early adulthood, which could be maintained over the years. However, the results observed in relation to the effect of parenting on the development of personality disorders, in both longitudinal and in cross-sectional studies conducted in adults, seem to converge to a large extent.16
Finally, we must also highlight the strengths of the study. Attachment, both as a concept of adult and child psychology, has received increasing interest in recent years from both society and the scientific community; however, research is mainly limited to descriptions (associations of dimensions with symptoms or syndromes) in the case of adult attachment, and the search for environmental determinants in child attachment. Our research aims to shed some light on the study of the biological basis of adult attachment, adding evidence, in the case of the vulnerability analysis, to increase the current body of knowledge, which is still scarce, and opening doors for future studies in the case of the analysis of the interaction with parental rearing styles. To know, in greater detail, profiles "plastic" to the environment is a way to identify individuals with greater potential to benefit from interventions.
Data availabilityThe data presented in this study are available on request from the corresponding author.
Ethical considerationsAll study procedures were carried out in accordance with the Declaration of Helsinki and approved by the Basque Clinical Research Ethics Committee.
FundingThe study was funded by the Health Department of the Basque Government under grant 2014111034. Edition was supported by the Research Unit of Galdakao-Usansolo Hospital. Open Access funding was provided by University of Basque Country UPV/EHU within the CRUE-CSIC Agreement.
We want to thank Mercedes Zumarraga and Maria Isabel Zamalloa for their support during the whole research and analysis process.