Moderate to severe Crohn's disease (CD) treatment was revolutionized by introducing anti-tumor necrosis factor (TNF) agents, which is still a cornerstone of the treatment. It is speculated that adipose tissue may influence treatment response, especially for non-weight-adjusted agents.
Patients and methodsResearch comparing the effectiveness of anti-TNFs between eutrophic and overweight patients may impact clinical management. We performed a retrospective analysis of a CD patient database. The primary endpoint was loss of response (LOR) after 54 weeks with infliximab (IFX) and adalimumab (ADA) in patients with body mass index (BMI) <25 and ≥25. Secondary endpoints were steroid-free remission and endoscopic remission rate.
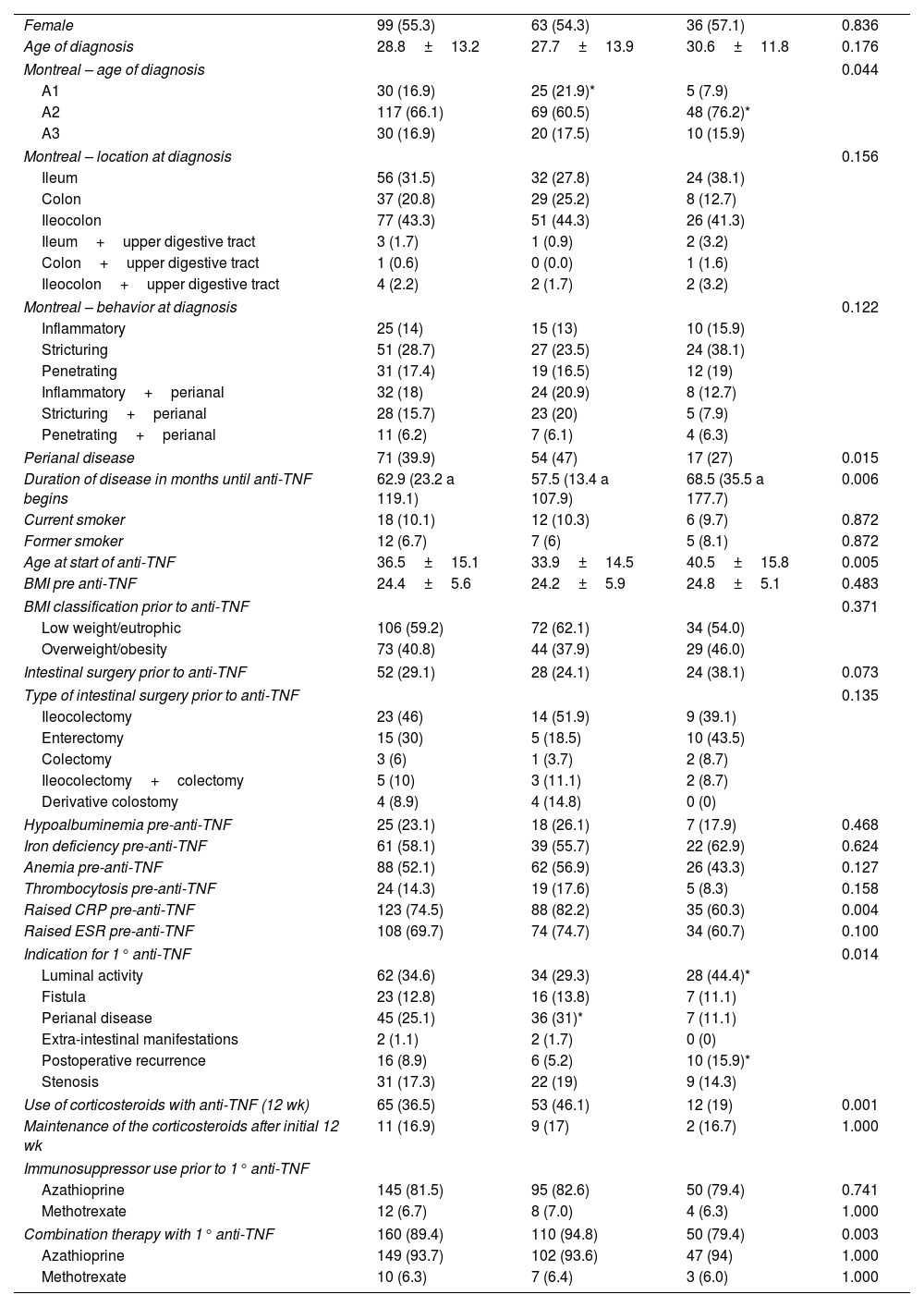
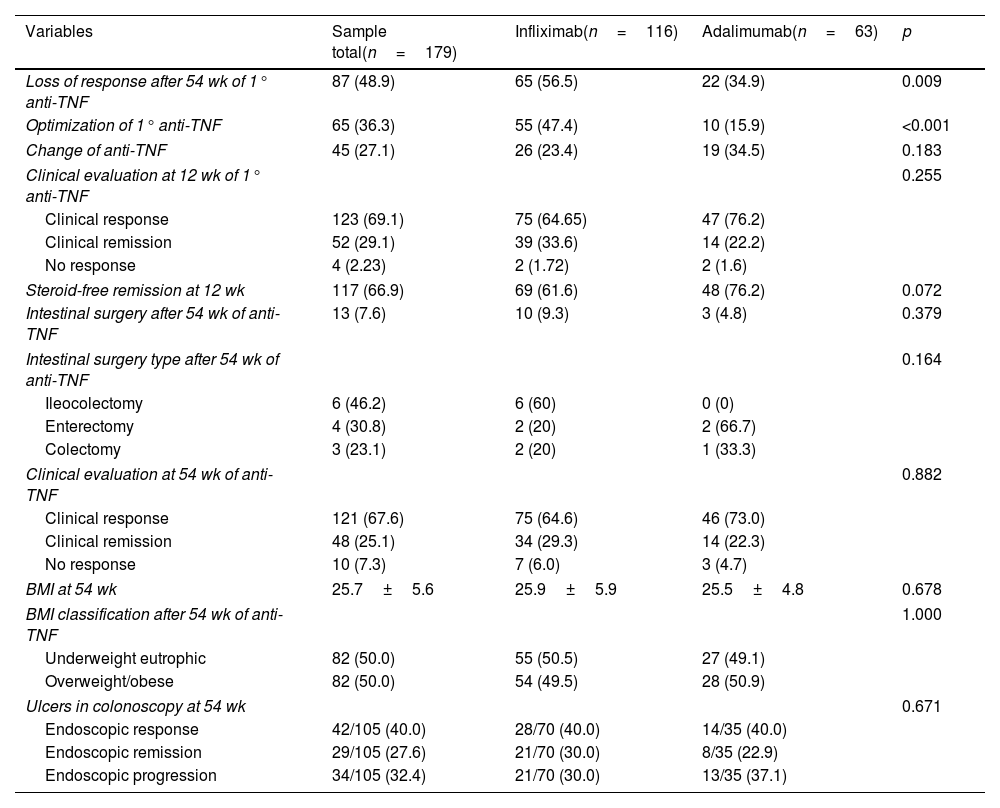
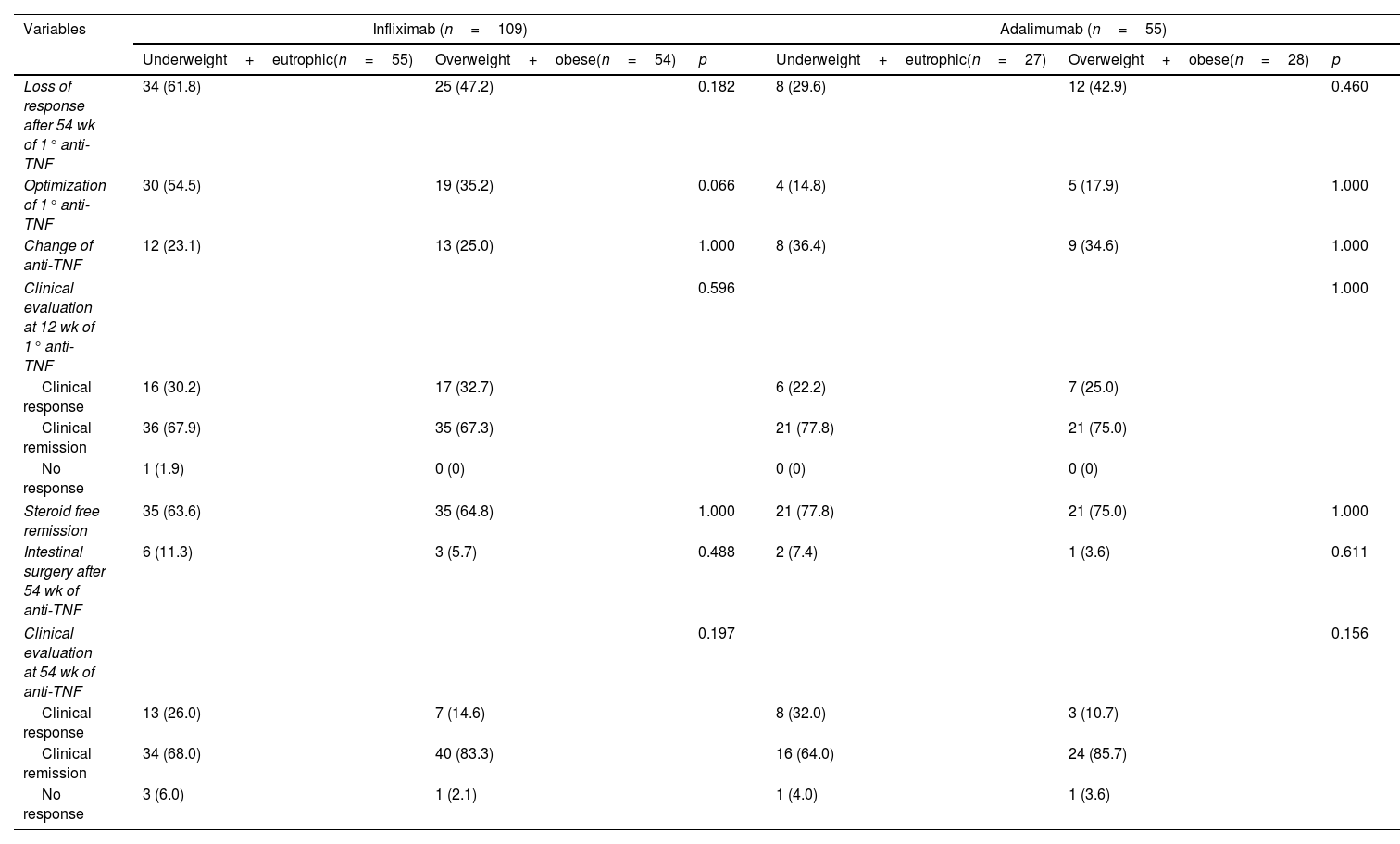
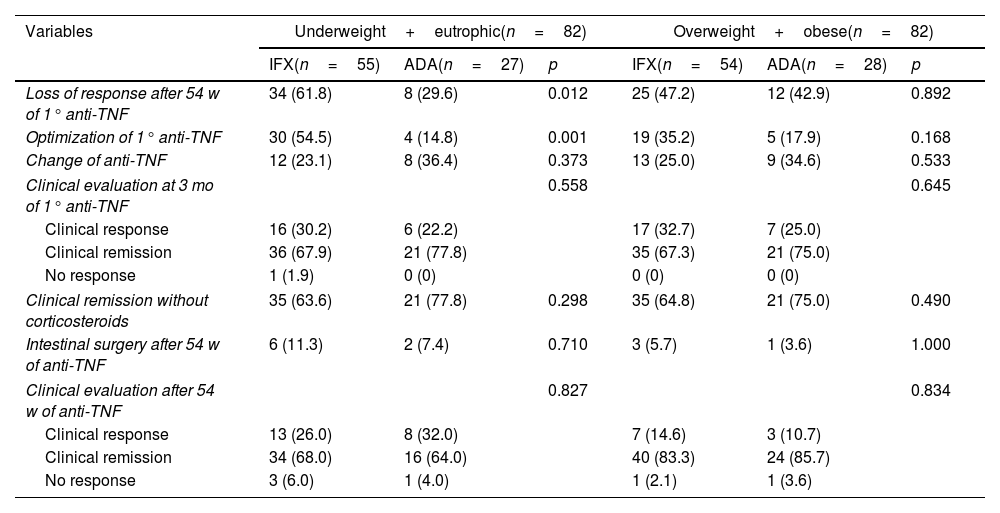
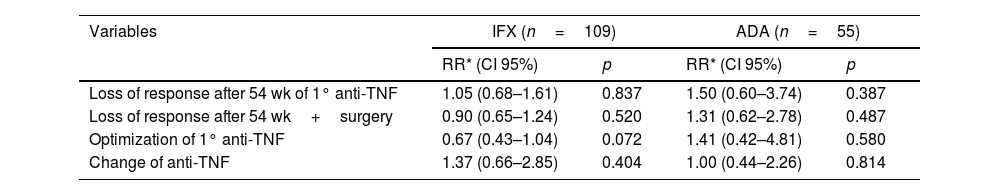
ResultsOne hundred seventy-nine CD patients were evaluated; 48.9% had LOR after 54 weeks of anti-TNF therapy. Fifty-four patients had a BMI ≥25, with 51 receiving IFX and 28 receiving ADA. The univariate analysis identified LOR in 56.5% of the patients with IFX and 34.9% in the ADA group (p=0.009). In the 54-week multivariate analysis, loss of response in the IFX group with BMI ≥25 had a relative risk of 1.04 [CI 0.60–1.80 (p=0.891)] compared to patients with BMI <25. Being overweight or obese led to a risk of 1.50 for LOR for ADA at 54-week time point [CI 0.60–3.74 (p=0.0387)]. Clinical remission at 54 weeks was similar between BMI groups.
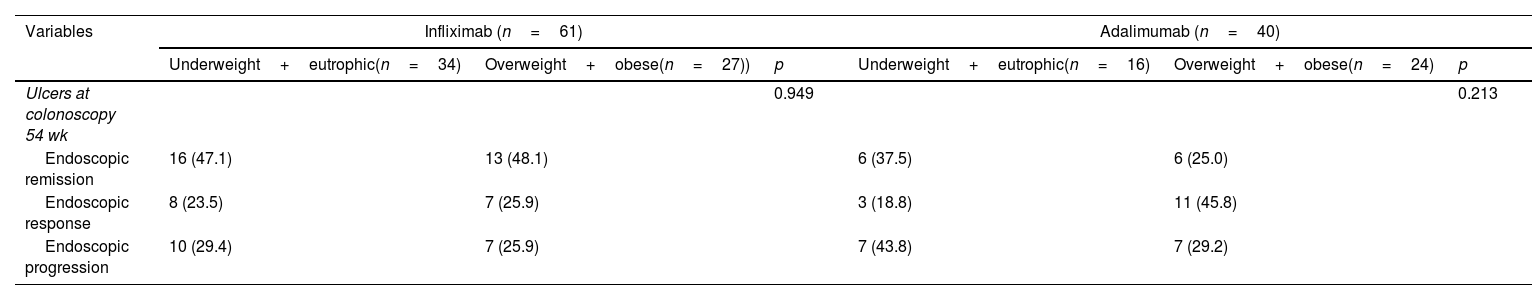
ConclusionsBeing overweight did not influence the LOR to treatment when IFX and ADA were compared, nor did it affect clinical and endoscopic remission after 54 weeks.
El tratamiento de la enfermedad de Crohn (EC) fue revolucionado por los agentes antifactor de necrosis tumoral (TNF) y estos agentes siguen siendo una piedra angular del tratamiento. Se especula que el tejido adiposo puede influir en la respuesta al tratamiento, especialmente para agentes no ajustados al peso. Las investigaciones que comparan la eficacia de los anti-TNF entre pacientes eutróficos y con sobrepeso pueden afectar el manejo clínico.
MétodosRealizamos un análisis retrospectivo de una base de datos de pacientes con EC. El criterio de valoración principal fue la pérdida de respuesta (LOR) después de 54 semanas con infliximab (IFX) y adalimumab (ADA) en pacientes con IMC (índice de masa corporal) <25 y ≥ 25. Los criterios de valoración secundarios fueron la remisión clínica sin esteroides y la tasa de remission endoscópica.
ResultadosSe evaluaron un total de 179 pacientes con EC; El 48.9% tuvo LOR después de 54 semanas de terapia anti-TNF. En total, 79 pacientes tenían un IMC ≥ 25, 51 recibieron IFX y 28 recibieron ADA. En el análisis univariado, se identificó LOR en el 56.5% de los pacientes con IFX y en el 34,9% en el grupo ADA (p=0,009). En el análisis multivariado de 54 semanas, la perdida de respuesta en el grupo IFX con IMC ≥ 25 tuvo un riesgo relativo (RR) de 1.04 [CI 0,60-1.80 (p=0.891)] em comparación con los pacientes con IMC <25. El sobrepeso e la obesidad generó un riesgo de 1,50 para LOR para ADA al cabo de 54 semanas [CI 0,60-3,74 (p=0.0387)]. La remisión clínica a las 54 semanas fue similar entre los grupos de IMC.
ConclusionesEl sobrepeso no influyó en la LOR al tratamiento cuando se compararon IFX y ADA, ni influyó en la remisión clínica y endoscópica después de 54 semanas de tratamiento.