This document summarizes the contents of the Clinical Guidelines for the Endoscopic Mucosal Resection of Non-Pedunculated Colorectal Lesions that was developed by the working group of the Spanish Society of Digestive Endoscopy (GSEED of Endoscopic Resection). This document presents recommendations for the endoscopic management of superficial colorectal neoplastic lesions.
Este documento resume el contenido de la Guía de resección mucosa endoscópica elaborada por el grupo de trabajo de la Sociedad Española de Endoscopia Digestiva (GSEED de Resección Endoscópica) y expone las recomendaciones sobre el manejo endoscópico de las lesiones neoplásicas colorrectales superficiales.
Endoscopic mucosal resection (EMR) is one of several endoscopic techniques that have modified the therapeutic approach of superficial and neoplastic lesions of the gastrointestinal tract. It constitutes an effective and safe alternative to conventional surgical treatment. Despite the fact that this procedure is performed routinely in all digestive endoscopy units, its efficacy and technical aspects vary significantly. Incomplete resections/recurrences and delayed bleeding are the main complications related to this procedure in the colon. The technique must be standardized in order to improve the rate of curative resections and minimize complications. The Spanish Endoscopic Resection Working Group of the Spanish Society of Digestive Endoscopy (Endoscopic Resection GSEED) has identified the need to develop clinical guidelines for the endoscopic treatment of colorectal lesions.
MethodologyIn June 2014, several members of the Endoscopic Resection Working Group of the SEED were asked to independently develop a draft of these guidelines, including the questions or sections under development. A panel of experts in EMR (Appendix 1) were appointed in order to draft each section of the guidelines as well as the main content within each section. The following corresponding keywords were included: endoscopic mucosal resection, mucosectomy, polypectomy, endoscopic submucosal dissection, colorectal surgery, indications, contraindications, colorectal cancer, colorectal polyps, granular and non-granular laterally-spreading lesions, quality indicators, antiaggregants, anticoagulants, informed consent, patient rights, high-definition colonoscopy, conventional and virtual chromoendoscopy, submucosal invasion, morphologic (Paris, Kudo, Sano, NICE) and histologic (Vienna) classifications, lymph node invasion risk factors, technical aspects of EMR (en-bloc EMR, piecemeal EMR, injection, snare, electrosurgical current and tattoo), bleeding, perforation, post-polypectomy syndrome, complications of sedation, global efficacy, incomplete resection, recurrence, surveillance, surveillance period, learning y competence. In addition, several participants with few or no technical knowledge in EMR (methodologists, resident physicians, nursing staff, patient associations) were also included in order maximize the objectivity of the contents of the guidelines. All participants stated that they had no conflict of interest that could interfere with the development of the guidelines.
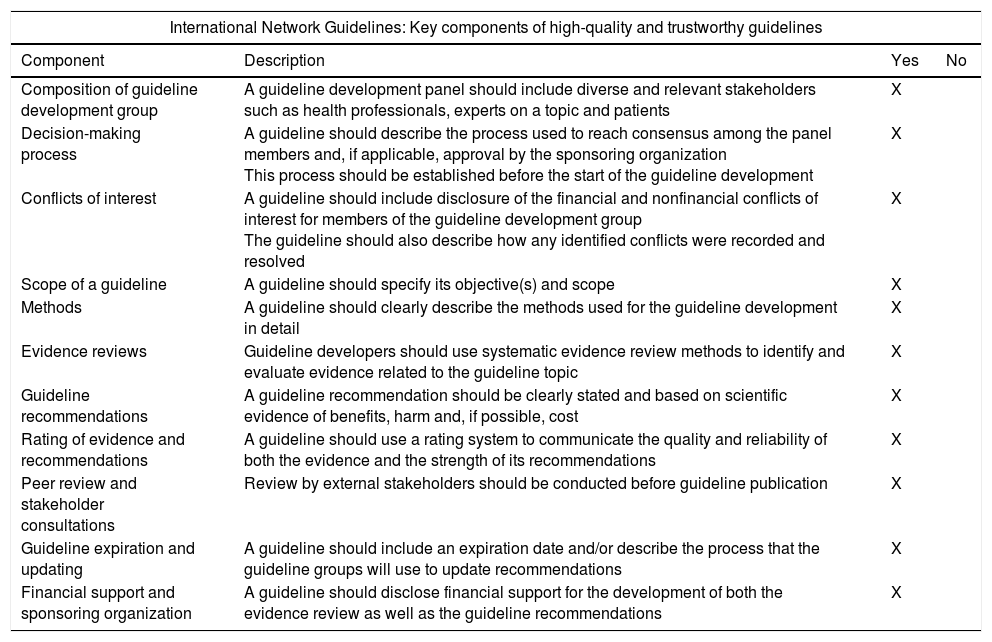
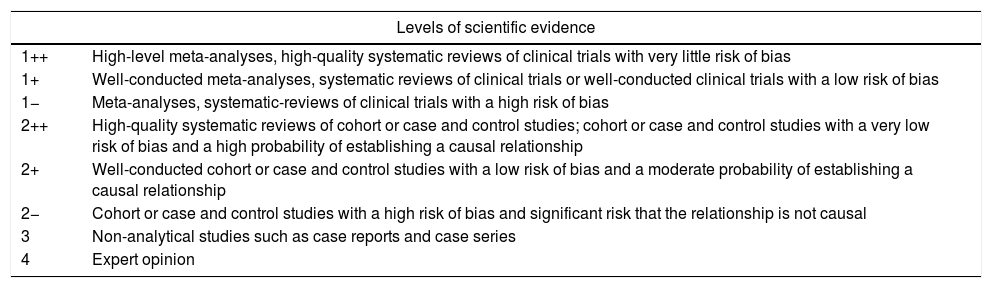
All the general recommendations set out in the International Network Guidelines1 (Appendix 2) were used as well as the levels of evidence of the Scottish Intercollegiate Guidelines2 (Appendix 3). Working groups were created for each section of the guidelines that were comprised of 2–5 individuals led by a coordinator. All groups independently reviewed the available literature in the main scientific databases (Cochrane Library, PubMed, MEDLINE, Pubmed Central [PMD], Embase, Scopus), other secondary publications such as Bandolier, ACP Journal Club, Clinical Evidence and UpToDate, and internet search engines such as the TRIP database and SumSearch. After reviewing the literature, a preliminary draft of the guidelines was drafted, discussed and corrected during a SEED meeting held in Seville in November 2014.
The different chapters were reviewed by coordinators and relevant modifications were made (from January to June 2015). Finally, the guidelines were submitted to a panel of experts for external review (from July to November 2015). The new amendments were reviewed and approved by coordinators of each section. A consensus meeting was held on March 1st, 2016, with the coordinators of the guidelines, the coordinators of each section of the guidelines and the external reviewers. A final draft of the guidelines was developed and sent for editing in printed and digital format. The guidelines can be found in the following link: http://wseed.es/index.php/enlaces/publicaciones/guias-clinicas. This document includes a summary of the guidelines and the recommendations resulting from the consensus meeting.
The purpose of the guidelines is to serve as a reference for Spanish clinicians specializing in digestive endoscopy. The drafting of the guidelines has been a laborious task as it needed to be comprehensive and consensual, and its development has required the collaboration of a large number of professionals. It is likely that some of the content will become obsolete in a short time. For this reason, this document is considered as a starting reference, and an update is planned for 2021. The recommendations are based on the available scientific evidence whenever possible. Due to the nature of the guidelines, scientific evidence is weak in some areas and, therefore, some recommendations are based on a consensus opinion. Obviously, it is imperative that each physician considers the implementation of these recommendations based on their knowledge and available resources.
IndicationsThe cost and complications associated with the treatment of early colorectal neoplasia via open or laparoscopic surgery are significantly higher than those for local treatment. As most colorectal lesions lack invasive potential, the usual treatment of choice is local as they tend to be curative.3–6
The indications for EMR are conditioned by the histological characterization, size (an en bloc EMR can be used if the lesion measures less than 20mm and a fragmented EMR [EMR-f] should be considered when more than 20mm) and location of the lesion. Early colorectal neoplasms without a suspected invasive component (non-invasive intraepithelial neoplasia, categories III-IV in the Vienna Classification System) can be managed with local treatment due to the low possibility of loco-regional or distant metastases. It is not necessary to meet the oncological resection criteria in these cases, therefore an en bloc resection of the lesion is not essential. However, when an early neoplastic lesion does have an invasive component that affects the submucosa or other tissue layers, endoscopic resection alone does not allow for the resection of loco-regional adenopathies. Therefore, this is insufficient from an oncological point of view. Unlike the remainder of the gastrointestinal tract, the colon has the peculiarity that its lymphatic vessels lie in deep submucosal planes. This means that invasive colorectal cancers that affect the most superficial submucosal layer (<1mm) with histological criteria compatible with a good prognosis (high degree of differentiation [G1], lack of lymphatic or vascular invasion and no tumor budding) are associated with insignificant rates of nodal involvement. Therefore, these lesions can be treated in the same way as non-invasive lesions. It is preferable to obtain the lesion in a single piece, and thus perform an en bloc resection which enables an adequate histological study of the mass.5–7
Polypoid lesions (pedunculated [0-Ip]) are usually treated with conventional polypectomy. Lesions with a flat or sessile component and greater than 10mm (0-IIa and 0-Is) are routinely treated by EMR. Other local therapeutic alternatives for the treatment of non-pedunculated lesions (NPCRL) include endoscopic submucosal dissection (ESD) among others and transanal endoscopic surgical techniques (transanal minimally invasive surgery [TAMIS] or transanal endoscopic microsurgery [TEM]) in the rectum. ESD is associated with a higher en bloc resection rate than EMR and therefore the preferred procedure, especially when the lesion must be extracted as a single complete piece. However, colorectal ESD is a technically demanding procedure. Therefore, within Europe, this procedure is only recommended within the context of a clinical trial and only if the prerequisites of the outcome are met in terms of safety and success rates when compared to published reference series. Indications of ESD for the treatment of colon lesions may increase during the next few years. However, the technique is currently limited to a few centers with sufficient experience (see the ESD guidelines of the SEED). In large rectal lesions without an invasive component, ERM and transanal endoscopic surgery have yielded comparable results in terms of efficacy and safety. Hence, the choice of one or other procedure will mainly depend on factors such as the level of experience in either technique. With regard to rectal lesions with a risk of submucosal invasion, ESD and transanal endoscopic surgery provide a similar outcome and the choice of technique will mainly be conditioned by the experience within each center.8,9
The correct description of the lesion is the first point that should be taken into account when performing an EMR as the appearance of the lesion is very useful for predicting the presence of invasive cancer.5 This will be explained in detail in the following section and below are the basic definitions:
- -
NPCRL: A non-pedunculated colorectal lesion is any sessile or flat lesion without a peduncle. According to the Paris classification, this would correspond to type 0-Is and 0-II (see section “Identification and characterization of the lesions”). These lesions account for 13–29% of early neoplasms in the Western hemisphere, with a prevalence similar to that described in Japan.
- -
Laterally spreading tumor (LST) (see section “Identification and characterization of the lesions”): Lesions with a predominantly superficial pattern which can also be exophytic, sessile, flat or slightly depressed (Paris classification 0-II, 0-II-0-Is, 0-Is+0-II), with a size greater than 10mm. When these lesions have a granular surface, they are known as granular LSTs (LST-G). This type of lesion is subdivided into two types: (a) homogeneous LST-Gs, which have a homogeneous granular surface and nodules measuring less than 3mm (Paris 0-IIa); and (b) mixed nodular LST-Gs, which have a granular surface and slightly larger nodules or some sessile areas (Paris 0-IIa+Is/0-Is+0-IIa). When the surface of the LST is even and smooth, it is classified as a non-granular LST (LST-NG). This type of LST is subdivided into elevated flat LSTs (Paris 0-IIa) and pseudo-depressed LSTs (Paris 0-IIc, combined or not with 0IIa or 0IIb).
Over 80% of LSTs are of the mixed and homogeneous LST-G type. Only 15–25% are LST-NG lesions. It is estimated that up to 98% of homogeneous LST-G lesions lack an invasive component; thus they are ideal candidates for en bloc or fragmented EMR. Mixed nodular LST-G lesions are associated with an invasive carcinoma in up to 13% of cases, which almost always lies in the dominant nodule. Therefore, an en bloc EMR of the larger nodules is recommended. LST-NG lesions have an invasive component in up to 12% of cases, which reaches up to 42% when the lesions are of the pseudo-depressed type (Paris 0-IIa and IIc). These characteristics mean that the use of en bloc resection techniques for the treatment of LST-NG lesions should be considered in order to enable an adequate histological study.5,10
The available scientific evidence regarding neoplastic colorectal lesions is based on the fact that adenomas are considered as precursor lesions of colorectal cancer (CRC). Nevertheless, it is estimated that 20–30% of CRCs originate from serrated lesions (SL). Some recent large series have shown that SLs account for up to 16% of all NPCRLs referred for endoscopic treatment. These lesions usually have a smooth and non-granular appearance that could potentially be confused with that of LST-NG lesions. However, their prognosis is much more favorable and they can be treated with EMR-f despite their large size, as the risk of an invasive neoplasia is minimal. This consideration must be taken into account throughout the document.11–13
Rectal carcinoid tumors greater than 15–20mm are suitable candidates for surgical treatment, whereas EMR is considered to be the first-line treatment for lesions of less than 10mm. This includes EMR via the band ligation variant or with a previous circumferential incision. ESD may be considered for rectal carcinoid tumors greater than 10mm and smaller than 15–20mm in size.14
When the position of the lesion makes surgical resection difficult, such as near diverticula, the ileocecal valve, the appendicular orifice or the anal canal, reference centers may be consulted.15
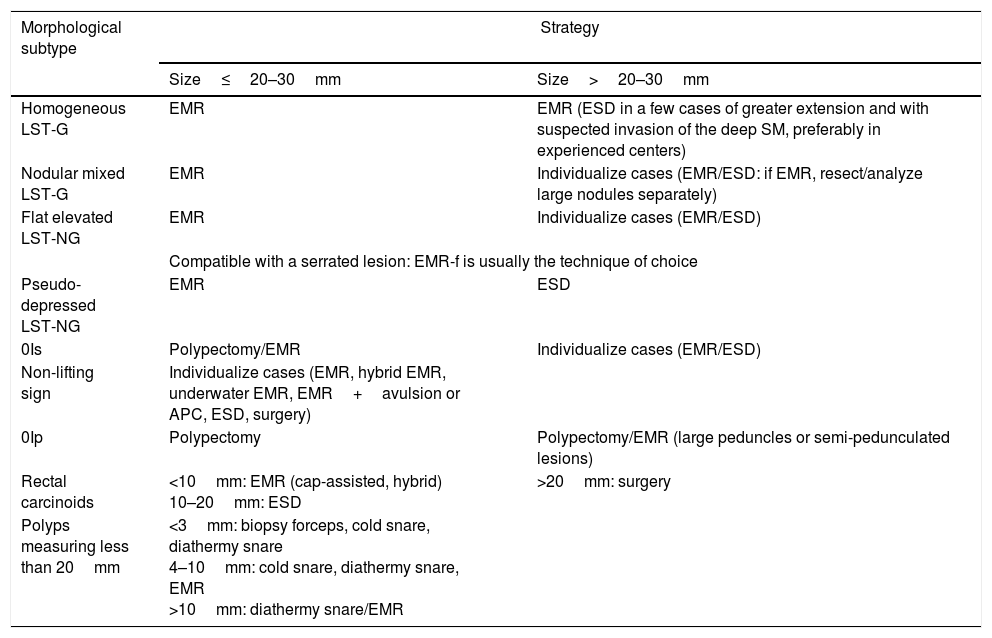
Table 1 indicates the consensus reached by the Endoscopic Resection Working Group of the SEED with regard to the recommended therapeutic strategy for the treatment of early colorectal neoplasia according to the morphological subtype and size of the lesion.
Consensus on the recommended therapeutic strategy for the treatment of early colorectal neoplasia according to the morphological subtype and size of the lesion.
| Morphological subtype | Strategy | |
|---|---|---|
| Size≤20–30mm | Size>20–30mm | |
| Homogeneous LST-G | EMR | EMR (ESD in a few cases of greater extension and with suspected invasion of the deep SM, preferably in experienced centers) |
| Nodular mixed LST-G | EMR | Individualize cases (EMR/ESD: if EMR, resect/analyze large nodules separately) |
| Flat elevated LST-NG | EMR | Individualize cases (EMR/ESD) |
| Compatible with a serrated lesion: EMR-f is usually the technique of choice | ||
| Pseudo-depressed LST-NG | EMR | ESD |
| 0Is | Polypectomy/EMR | Individualize cases (EMR/ESD) |
| Non-lifting sign | Individualize cases (EMR, hybrid EMR, underwater EMR, EMR+avulsion or APC, ESD, surgery) | |
| 0Ip | Polypectomy | Polypectomy/EMR (large peduncles or semi-pedunculated lesions) |
| Rectal carcinoids | <10mm: EMR (cap-assisted, hybrid) 10–20mm: ESD | >20mm: surgery |
| Polyps measuring less than 20mm | <3mm: biopsy forceps, cold snare, diathermy snare 4–10mm: cold snare, diathermy snare, EMR >10mm: diathermy snare/EMR | |
- -
Local treatment is preferred over surgical resection in patients with early colorectal neoplasia due to the lower cost and morbidity and mortality rates (level of evidence 2++; grade of recommendation B).
- -
An adequate assessment of the morphology of the lesion, the superficial mucosal pattern and signs of invasion of the deep submucosa must precede the EMR of non-pedunculated colorectal lesions (NPCRLs) (level of evidence 2++; grade of recommendation B).
- -
EMR is generally indicated for all LCRNPs without suspected deep submucosal invasion and without size limits (level of evidence 1+; grade of recommendation A).
- -
Homogeneous LST-G lesions have an adequate indication for EMR, both en bloc and fragmented (EMR-f), regardless of their size (level of evidence 2+; grade of recommendation C).
- -
In the case of mixed LST-G lesions, an en bloc EMR must be carried out in order to remove the larger nodules as a whole (level of evidence 2+; grade of recommendation C).
- -
Pseudo-depressed LST-NG lesions measuring >20mm are associated with a significant risk of focal submucosal invasion development; therefore alternative techniques must be considered in order to ensure an en bloc resection (ESD, surgery) (level of evidence 2+; grade of recommendation C).
- -
LST-NGs greater than 20mm should be referred to centers specialized in en bloc endoscopic resection, ideally via ESD or an alternative surgical technique (level of evidence 4; grade of recommendation D).
- -
EMR-f is a safe technique for the treatment of serrated lesions, even in the case of lesions greater than 20mm (level of evidence 2+; grade of recommendation C).
- -
EMR should be considered as the first-line treatment option for rectal carcinoid tumors measuring less than 10mm, either using the band ligation variant or with a previous circumferential incision (ESD-hybrid) (level of evidence 2++; grade of recommendation B).
- -
EMR of lesions in difficult locations (diverticula, ileocecal valve, appendicular orifice or anal canal) must be referred to reference centers (level of evidence 4; grade of recommendation D).
Patients should be provided with the necessary information related to their health care in a clear, precise and sufficient manner. A written informed consent must be obtained for invasive diagnostic and therapeutic procedures.
It is advisable to collect diverse data with regard to the patients’ medical history prior to performing an EMR, including the following: information about transmissible diseases, serious comorbidities such as hepatopathies, nephropathies or cardiopathies; anesthesia risk classification (ASA), adverse reactions to drugs, latex or nickel; consumption of toxic substances, likelihood of pregnancy, time elapsed since the last intake of food and the characteristics of the meal and previous surgeries and endoscopies.16
Routine blood tests, electrocardiograms or chest x-rays prior to the EMR is not recommended.17
Electrocautery used in EMR could potentially interfere with pacemakers. With regard to newer devices, no specific care is required prior to the EMR. However, the reprogramming of older devices to their asynchronous mode is recommended in patients who depend on the pacemaker and in situations where prolonged electrocautery is expected. Patients with an implantable cardioverter-defibrillator (ICD) device must be advised to consult with a cardiologist specializing in cardiac arrhythmias prior to undergoing the EMR in order to assess the potential deactivation of the tachyarrhythmia detection function of the ICD device and its subsequent reprogramming. Nowadays, the use of magnets by the cardiologist and/or anesthesiologists can prevent a deactivation.18
The onset of clinically relevant distant infections secondary to endoscopic procedures is anecdotal. Therefore, the administration of prophylactic antibiotics is not recommended for the prevention of an infectious endocarditis or for patients with an orthopedic prostheses.19
Both antiplatelet and anticoagulant therapy increase the risk of immediate and delayed bleeding following an EMR. Therefore, the suspension of anticoagulant treatment prior to the resection of lesions greater than 10mm is recommended. In patients with a high thromboembolic risk, bridge therapy with low molecular weight heparins can be administered. With regard to ASA, the treatment may be discontinued prior to performing the EMR in patients with a low thrombotic risk. Furthermore, it is recommended that thienopyridines (clopidogrel, prasugrel, ticagrelor, ticlopidine) be suspended 5–7 days prior to the EMR. In cases of double anti-aggregation, the discontinuation of thienopyridines and the maintenance of treatment with ASA is recommended. If this is not possible due to the patient's high thromboembolic risk, the EMR should be delayed until the thienopyridine treatment can be suspended.20,21
Specific recommendations- -
The conduct of blood tests, electrocardiograms or chest X-rays prior to an EMR is not recommended in the absence of symptoms or a specific personal history warranting these tests (level of evidence 3; grade of recommendation D).
- -
In the case of old pacemakers, the reprogramming of the device to its asynchronous mode may be beneficial in patients without a proper cardiac rhythm (dependent patient) in which prolonged electrocautery is expected. The devices used nowadays do not require any specific modification (level of evidence 4; grade of recommendation D).
- -
Deactivating the tachyarrhythmia function of the ICD device may be beneficial. It is advisable that the patient consult with a cardiologist specialized in cardiac arrhythmias and trained in the management of ICD devices. These specialists should be responsible for the subsequent deactivation and reprogramming of the device. Nowadays, the use of magnets by cardiologists or anesthesiologists can prevent a deactivation (level of evidence 3; grade of recommendation D).
- -
Antibiotic prophylaxis for the prevention of an infectious endocarditis is not recommended in patients who are scheduled to undergo an EMR, regardless of their cardiac risk factors (level of evidence 2+; grade of recommendation D).
- -
Antibiotic prophylaxis is not recommended either in patients with orthopedic prostheses who are scheduled to undergo an EMR (level of evidence 3; grade of recommendation D).
- -
Anticoagulation therapy significantly increases the risk of bleeding, therefore it is advisable to suspend such treatment prior to performing an EMR (level of evidence 2+; grade of recommendation C).
- -
In patients with a high thromboembolic risk, bridge therapy with low molecular weight heparins is recommended (level of evidence 2+; grade of recommendation C).
- -
An ERM can be carried out in patients receiving acetylsalicylic acid at doses ≤300mg/day. As acetylsalicylic acid seems to increase the risk of bleeding, it could be discontinued prior to the procedure in patients with a low risk of thrombotic events (level of evidence 3; grade of recommendation D).
- -
As the EMR procedure is associated with a high risk of hemorrhage, it is recommended that thienopyridinines (clopidogrel, prasugrel, ticagrelor and ticlopidina) be discontinued 5–7 days prior to performing an EMR (level of evidence 2+; grade of recommendation C).
- -
In patients treated with double antiplatelet therapy, it is advised that thienopyridines be discontinued 5–7 days before the procedure and to continue treatment with acetylsalicylic acid at ≤300mg. If the patient has a high thromboembolic risk, the procedure should be delayed until it is safe to discontinue the thienopyridine therapy (level of evidence 2+; grade of recommendation C).
The patient should be provided with the necessary information for their own health care in a clear, precise and sufficient way. Written informed consent is required when performing invasive diagnostic and therapeutic procedures. The informed consent should include minimum data such as the name, description and objectives of the procedure, general and specific risks, expected benefits and therapeutic alternatives. In addition, the form should also include details of the identification of the patient and should be signed by the patient or legal representative and the physician. The form should also be dated and include information about the right to accept or refuse the proposed procedure and the right to withdraw consent previously granted.22,23
Since intestinal cleansing is essential to accurately identify a lesion and its margins, patients should be instructed how this is performed according to the latest scientific evidence. Testimony from other patients that have undergone the procedure can be disseminated in order to facilitate the understanding of the procedure and mitigate the patient's fears.
Specific recommendations- -
As lesions that are ideal candidates for EMR are often identified during a diagnostic endoscopic procedure, it is recommended that the most important technical considerations of the EMR be included in the general consent for the colonoscopy (level of evidence 4; grade of recommendation D).
- -
With regard to procedures which the endoscopist expects to be highly complex or associated with a risk of complications, the EMR can be delayed and a specific consent form will be required (level of evidence 4; grade of recommendation D).
- -
As bowel cleansing is essential to accurately identify the lesion and its margins, patients should be instructed on how the procedure is performed based on the latest scientific evidence (level of evidence 4; grade of recommendation D).
- -
Testimony from other patients may facilitate the patient's understanding of the procedure and mitigate his/her fears (level of evidence 4; grade of recommendation D).
NPCRLs, especially flat ones (Paris 0-IIa/b), may go unnoticed in diagnostic colonoscopies. Hence, it is important to carry out the colonoscopy in accordance with the quality standards set out by the relevant scientific societies. This involves adequate colonic preparation, a complete colonoscopy, a thorough examination of the entire colon mucosa, an adequate withdrawal time, a correct inspection technique (it is advisable to measure the lesions with open biopsy forceps or snare) and the use of auxiliary chromoendoscopy techniques when necessary. In the case of flat lesions, it is important that the endoscopist is experienced in the detection of subtle lesions which can only be identified by a subtle change in the color of the mucosa, deposits of fecal matter or mucus, fading of the vascular pattern and changes in the roughness of the mucosa. The use of high-definition endoscopy and chromoendoscopy (conventional or electronic) may be of great help in identifying a lesion and delimiting its margins correctly. Therefore, it is recommended that the endoscopists involved in performing an EMR meet the highest standards of quality criteria for colonoscopies and that they are fully familiar with the chromoendoscopy techniques.24 In medium-risk subjects, selective indigo carmine staining is recommended in order to assess the lesions detected with a conventional view. However, panchromoendoscopy is not advised in this situation.25
Furthermore, as mentioned in section “Indications”, a correct characterization of the lesion is crucial in order to plan the most appropriate treatment. The modified Paris26 and LST10,27 classifications are used for morphological characterization of the lesions. The morphological appearance of the lesion is clearly correlated with the likelihood of an invasive component. In addition, the lesion must also be characterized based on its mucosal pattern and other morphological aspects. In general, the presence of indurated areas, converging folds, ulcers, friability, neovascularization, a columnar appearance, areas of retraction, “goose bumps” around the lesion and depressed areas are highly suggestive of an invasive component.28 Together, these characteristics are better at predicting submucosal invasion than the non-lifting sign. Moreover, the mucosal pattern can provide an insight with regard to the existence and depth of the submucosal infiltration and also help differentiate hyperplastic polyps from adenomas. The Kudo's pit pattern is obtained after staining the lesion with a dye (indigo carmine or methylene blue) and using a magnifying or high-resolution endoscope.29 The Sano's capillary pattern is obtained by narrow band imaging (NBI) and magnification.30 The mucosal NICE (NBI International Colorectal Endoscopic Classification) pattern is obtained with NBI and high definition.31
The taking of biopsies and administering submucosal injections is not recommended for candidate EMR lesions. In addition, tattoos close to the lesion in order to avoid submucosal fibrosis and, therefore, facilitate the subsequent resection of the lesion should also be avoided. With regard to endoscopically unresectable lesions, biopsies should be taken from areas with a greater suspicion of malignancy, and only the nearby tissue may be tattooed.
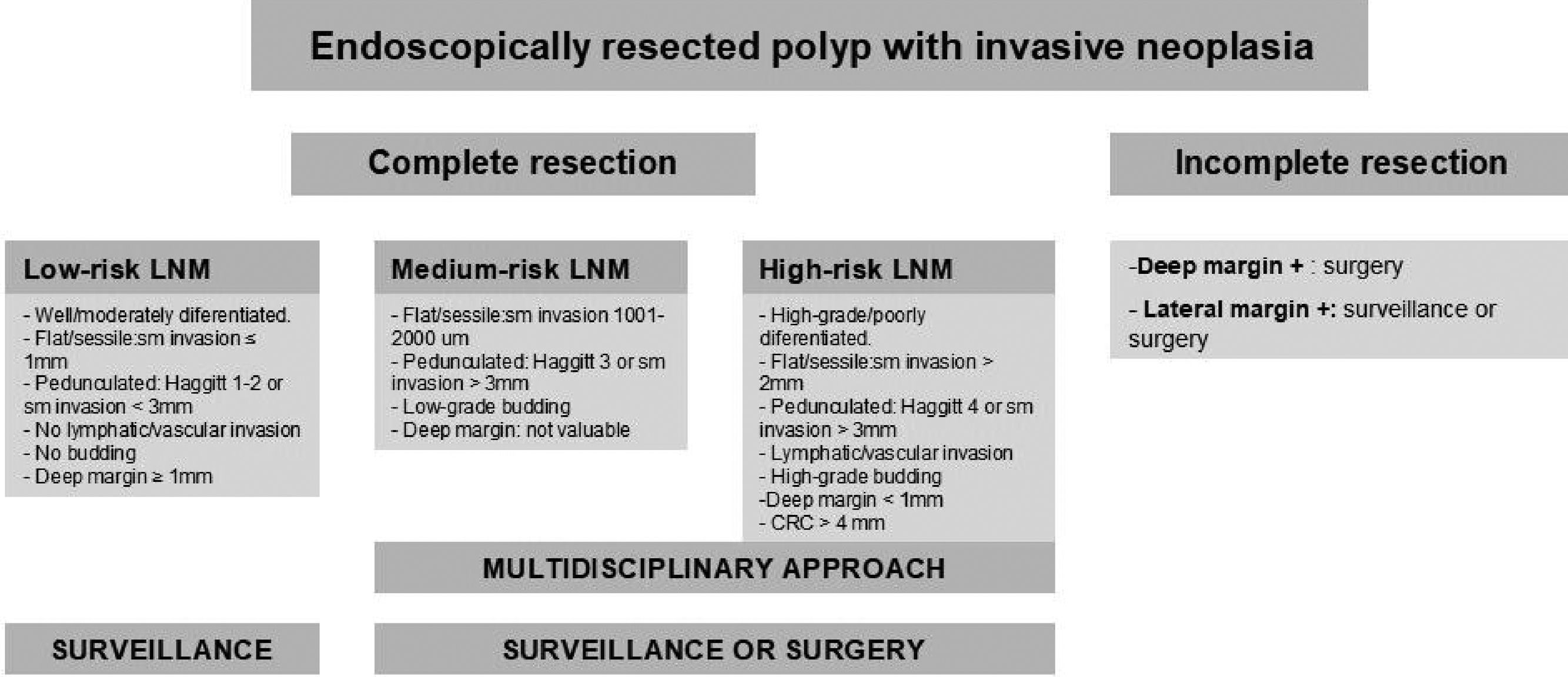
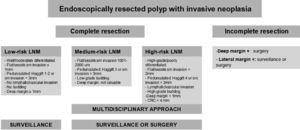
Finally, a correct histological interpretation of the specimen is essential. The pathologist's role is fundamental when analyzing the endoscopic resection specimen, as the histopathological interpretation will determine the subsequent therapeutic plan as well as the post-polypectomy follow-up criteria. The quality of the histological result depends not only on the pathologist but also on the endoscopist and the technique used. An inadequate resection may make the analysis of the specimen difficult for the pathologist, which may consequently impact on patient management. Whenever possible, the resected specimen must be sent to the pathologist stretched and fixed to a firm surface of porous material with pins and the mucosal surface exposed and the entire fragment submerged in formalin. The pathologist should pay particular attention to confirm that a complete resection has been performed (lateral and deep margins greater than 1mm), and to verify the presence or absence of submucosal invasion. The existence of superficial submucosal invasion may be suspected if the degree of invasion measured between the muscle layer and the mucosa is less than 1000μm. Communication between the pathologist and the endoscopist must be maximized in order to optimize the treatment of these patients.32 The use of the modified Vienna classification is recommended for the histological diagnosis of superficial neoplasms of the digestive tract.33Appendix 4 includes the modified Vienna classification. Appendix 5 describes the algorithm used to manage early colorectal cancers. Colorectal adenocarcinoma is defined by the World Health Organization (WHO) as the invasion of neoplastic cells throughout the muscularis mucosae of the submucosa, and staging based on the TNM classification is recommended. With regard to stage-pT1 invasive cancers, the presence or absence of poor prognosis criteria (degree of differentiation, depth of invasion in the submucosa, lymphovascular infiltration and the presence of tumor budding) should be reported. The specimen must be assessed by two pathologists if any of these criteria are met, as additional invasive treatments (surgery) may be required.28 A recommendation for additional surgery of a pT1 adenocarcinoma must be assessed by a multidisciplinary team.
Specific recommendations- -
In medium-risk patients, selective indigo carmine staining is recommended to assess the lesions detected with a conventional view. However, a panchromoendoscopy is not advised (level of evidence 1+; grade of recommendation).
- -
The use of enhancement techniques, such as dye-based chromoendoscopy or optical chromoendoscopy and magnification endoscopy, are recommended for focal interrogation of the mucosa in NPCRL (level of evidence 4; grade of recommendation D).
- -
It is recommended that all NPCRLs be photographed and/or videotaped prior to their resection (level of evidence 4; grade of recommendation D).
- -
An estimation of the size of the NPCRLs with the help of open biopsy forceps or snares is advised (level of evidence 4; grade of recommendation D).
- -
The Paris classification should be used to describe the morphology of the lesion (level of evidence 4; grade or recommendation D).
- -
The description of the characteristics of the surface of the colorectal lesions by means of the NICE classification and/or Sano's capillary pattern and/or Kudo's pit pattern is recommended (level of evidence 4; grade of recommendation D).
- -
The presence of areas of erythema, a firm consistency, an expansive appearance and the convergence of folds and “goose bumps” around the lesion is associated with a greater risk of submucosal invasion (level of evidence 2+; grade of recommendation C).
- -
Endoscopic characterization is better at predicting submucosal invasion than the non-lifting sign (level of evidence 2+; grade of recommendation C).
- -
With regard to lesions that are candidate for endoscopic resection, biopsies, submucosal injections and tattooing near the lesion should be avoided, as these may result in submucosal fibrosis and make the subsequent endoscopic resection of the lesion more difficult (level of evidence 4; grade of recommendation D).
- -
In the case of lesions with signs of endoscopic unresectability due to a suspected deep invasion, biopsies must only be taken of the most suspicious area. These lesions should be tattooed nearby, except in obvious locations (rectum and caecum) (level of evidence 4; grade of recommendation D).
- -
The use of the modified Vienna classification is recommended for the histological diagnosis of superficial neoplasms of the digestive tract (level of evidence 4; grade of recommendation D).
- -
The WHO definition of colorectal adenocarcinoma must be used: “The invasion of neoplastic cells throughout the muscularis mucosae of the submucosa”. The terms intramucosal adenocarcinoma or adenocarcinoma in situ must not be used (level of evidence 4; grade of recommendation D).
- -
The adenocarcinoma must be stratified according to the TNM classification. The version of the TNM classification to be used should be agreed at a national level and formally established by national professional bodies (level of evidence 4; grade of recommendation D).
- -
In order to ensure a correct evaluation of the NPCRLs, it is recommended that the specimen is sent to the pathologist stretched and fixed to a firm surface of porous material with pins, with the mucosal surface exposed and the entire fragment submerged in formalin (level of evidence 4; grade of recommendation D).
- -
The pathologist should pay particular attention to confirm that the resection has been completed (free lateral and deep margins) and to verify the presence or absence of submucosal invasion in the specimens obtained (level of evidence 4; grade of recommendation D).
- -
With regard to stage-pT1 invasive cancers, the presence or absence of criteria of poor prognosis including the degree of differentiation, depth of invasion in the submucosa, lymphovascular infiltration and the presence of tumor budding should be reported (level of evidence 1−; grade of recommendation D).
- -
A margin equal to or less than 1mm, both laterally and in depth, indicates that the margin is affected by the disease (level of evidence 3; grade of recommendation D).
- -
The existence of superficial submucosal invasion may be suspected if the degree of invasion measured between the muscle layer and the mucosa is less than 1000μm (level of evidence 3; grade of recommendation D).
- -
Any histological diagnosis associated with poor prognosis criteria that may warrant potential invasive complementary treatments (surgery) should be assessed by at least two pathologists (level of evidence 4; grade of recommendation D).
- -
Any recommendation for additional surgery of a pT1 adenocarcinoma must be assessed by a multidisciplinary team (level of evidence 4; grade of recommendation D).
EMR is a routine maneuver used in colonoscopies with varying degrees of difficulty. Thus, the expertise of the endoscopist plays a crucial role in the efficacy of the procedure. The lesion factors associated with a greater technical difficulty are: location (ileocecal valve, periapendicular, diverticula and dentate line), a lesion with a difficult access (due to bending or the formation of a loop), involvement of over 50% of the circumference, size >40mm, LST-NG morphology and the absence or deficient lifting after a submucosal injection (non-lifting sign). The presence of fibrosis in the submucosa hinders or even prevents the injection of solutions into this layer. This consequently leads to an increased risk of complications and hinders the endoscopic resection of the lesion, and also increases the risk of incomplete resections and the likelihood of recurrence.34
The EMR procedure is divided into three steps: (a) endoscopic maneuvers enabling the placement of the lesion in a better location for subsequent resection (between the “5 o’clock” and “7 o’clock” position) and the selection of the most appropriate material (the endoscopist must be familiar with all the tools available); (b) resection methods based on the type of lesion; and (c) behavior after the resection.35
Insufflation with CO2 instead of ambient air achieves a greater safety profile and an improvement of the patient's tolerance. With regard to electrocautery, the use of currents controlled by a microprocessor (ENDO CUT®) is recommended due to its efficacy and safety profile. Pure cut currents are associated with a greater risk of immediate bleeding, whereas pure coagulation currents are linked to a greater risk of delayed bleeding.36 For lesions greater than 20mm, the submucosal injection of substances with a low diffusion rate such as glycerin, gelatin succinate, hyaluronic acid or hydroxypropyl methylcellulose are recommended as they are linked to a greater rate of complete resection. Moreover, it is advisable to include sterile stains such as indigo carmine or methylene blue in the dilution of the submucosal injection as these enable a better delimitation of the lesion margins and the cut plane.37,38
In the case of lesions greater than 20–30mm, en bloc EMR is not recommended due to the increased risk of perforation and incomplete resection.39 It is crucial that the EMR be completed in a single session, as the use of more than one session is associated with a greater degree of incomplete resection. The resection should be performed with a diathermy snare, reserving ablative techniques for residual mucosal islets. The post-EMR eschar should be thoroughly reviewed in order to assess the margins and the integrity of the muscle layer.40 The “underwater” EMR technique is a potential alternative that may be of use when residual lesions on the eschars of previous resections are present.41 The injection of local anesthetics improves tolerance in resections located near the dentate line.42 In the case of lesions located behind folds or in the intravalvular part of the ileocecal valve, the use of caps or flexible endoscopes that allow retroflexion is advisable.39 The use of bands in the colon is not recommended due to the high risk of perforation. Intraoperative bleeding can be controlled with APC, coagulation forceps or the tip of the polypectomy snare set to coagulation mode “soft”. If possible, the use of hemostatic clips should be avoided until the resection has been completed.43
The recommended tattooing technique for this area consists in inserting the needle tangentially to the mucosa and injecting physiological saline first in order to create a submucosal chamber into which coal and/or Chinese ink is subsequently injected.44
Although this technique has been performed routinely and frequently for at least a decade, many of the technical aspects have not yet been scientifically assessed and are applied empirically.
Specific recommendations- -
The endoscopist's level of expertise has a very significant impact on the efficacy of the procedure, including the risk of complications, the rate of en bloc resections and the rate of a complete resection (level of evidence 4; grade of recommendation D).
- -
The endoscopist must be familiar with all the available and necessary tools for performing the EMR (level of evidence 4; grade of recommendation D).
- -
It is important to place the polyp between the “5 o’clock” and “7 o’clock” position, as this is where the operating channel of the colonoscopy tends to lie (level of evidence 2+; grade of recommendation D).
- -
Insufflation with C02 instead of ambient air achieves a greater safety profile and an improvement of the patient's tolerance (level of evidence 2+; grade of recommendation D).
- -
The use of currents controlled by a microprocessor (of the ENDO CUT® type) is recommended due to its safety and efficacy profile. On the contrary, the use of pure cut currents should be avoided due to the increased risk of immediate bleeding and pure coagulation currents owing to the increased risk of delayed bleeding (level of evidence 1+; grade of recommendation B).
- -
In the case of NPCRLs greater than 20mm, injection of substances into the submucosa with a low diffusion rate (glycerin, gelatin succinate, hyaluronic acid and hydroxypropyl methylcellulose) is recommended, as they are linked to a greater rate of complete resection (level of evidence 1+; grade of recommendation A).
- -
The inclusion of sterile stains (indigo carmine and methylene blue) in the submucosal injection solution is recommended in order to delimit the margins and cut plane (level of evidence 4; grade of recommendation D).
- -
En bloc EMR is not advised for lesions greater than 20–30mm, due to an increased risk of perforation or incomplete resection (level of evidence 2++; grade of recommendation B).
- -
The resection should be attempted in a single session, as resections carried out over several sessions are associated with a greater risk of failure of the endoscopic therapy (level of evidence 2+; grade of recommendation).
- -
It is recommended that the diathermy snare be used to complete the resection and to reserve ablative techniques for the removal of small islets of residual lesion (level of evidence 2+; grade of recommendation C).
- -
The post-EMR eschar should be thoroughly reviewed in order to assess the margins and the integrity of the muscle layer (level of evidence 3; grade of recommendation D).
- -
The performance of banded EMR in the colon is not considered to be safe, except when it is carried out in the rectum, due to the risk of perforation (level of evidence 1−; grade of recommendation B).
- -
The “underwater” EMR technique is a potential alternative to simple or fragmented EMR that may be of use for the resection of residual lesions present over scars of a previous resection (level of evidence 2++; grade of recommendation C).
- -
The injection of local anesthetics improves tolerance during the resection of lesions that come into contact with the dentate line (level of evidence 3; grade of recommendation D).
- -
The use of APC, hemostatic forceps or the tip of the polypectomy snare set to coagulation mode “soft” is recommended to control intraoperative bleeding (level of evidence 3; grade of recommendation D).
- -
In the case of lesions located behind folds or in the intravalvular part of the ileocecal valve, the use of caps or flexible endoscopes that allow retroflexion is advisable (level of evidence 4; grade of recommendation D).
- -
The recommended tattooing technique with coal and/or Chinese ink for this area consists in inserting the needle tangentially to the mucosa and injecting physiological saline first in order to create a submucosal chamber into which the coal and/or Chinese ink is subsequently injected (level of evidence 4; grade of recommendation D).
The most significant complications related to EMR are bleeding and perforation. These events can be treated and resolved with endoscopic methods in the majority of cases, although surgery may be required in others, especially in the case of a perforation. Furthermore, complications resulting from the colonoscopy, previous intestinal preparation, sedation and suspension or replacement of the antiplatelet or anticoagulant therapy (when applicable) may also arise. The lack of homogeneous criteria to assess these events and classify them a complication has resulted in a wide variability in the incidence of complications reported in the literature. Moreover, there are few studies that prospectively record all events following the EMR, thus minor complications are probably underestimated.
Bleeding is the most frequent complication related to this procedure, and it usually takes place within the first 48h and occasionally up to 14 days later. The incidence of intraoperative bleeding ranges from 3.4% to 24%, 1% to 11% in the case of immediate bleeding and 0% to 9.7% in the case of delayed bleeding. In most cases, the bleeding is self-limited and does not require treatment, with an almost null associated mortality rate. The following characteristics are predisposing factors for a post-EMR bleed: proximal location, large lesion size, intraoperative bleeding, use of electrosurgical units not controlled with a microprocessor, elderly age, high blood pressure and treatment with acetylsalicylic acid or anticoagulants.45–47 The use of adrenaline diluted in the submucosal injection is associated with a decreased incidence of early bleeding but does not affect delayed bleeding.48 Coagulation of visible vessels with argon or coagulation forceps does not reduce the incidence of post-EMR bleeding in the colon or rectum either.49 In addition, there is no scientific evidence to recommend the systematic closure of eschars with hemostatic clips to prevent post-EMR bleeding. However, the closure of mucosal defects with clips after the EMR of a large lesion in the colon is associated with a reduced risk of delayed bleeding.47
The perforation rate is traditionally considered as a quality standard for colonoscopies. It has a rate of 0.03–0.8% during diagnostic procedures and 0.15–3% during therapeutic procedures.50 The European Society of Gastrointestinal Endoscopy (ESGE) considers EMR to have a high risk of perforation, with rates of less than 5% reported for large colon lesions.51,52 Risk factors for post-EMR perforation include a large lesion size and location in the right colon. Other factors, such as advanced age, comorbidities, female sex, inflammatory bowel disease, previous abdominal surgeries, indication for a colonoscopy due to an obstruction, sessile morphology (0-Is), fibrosis or deep invasion of the lesion, poor preparation and inexperience of the endoscopist have also been described as risk factors for perforation during a colonoscopy.53 Early diagnosis is crucial in the management of a perforation and determines the best therapeutic strategy for an optimum patient prognosis. Almost a third of cases are detected immediately and the remaining are detected one to two days after the procedure. Rarely, cases of delayed perforation are detected 3–14 days after the procedure. After the EMR procedure, it is essential to inspect the mucosal defect and rule out the existence of the so-called target sign. This target sign consists of the presence of tissue from the muscle layer at the base of the resected lesion surrounded by submucosa, and is associated with a high likelihood of damage to the muscle layer itself. In these cases, the eschar should be closed with clips in order to avoid delayed perforation and reduce the need for a subsequent surgery and mortality.3 In the case of perforations measuring less than 2cm with an acceptable degree of cleansing of the intestinal lumen that occur in clinically and analytically stable patients, these perforations could be treated endoscopically. Surgery is reserved in the event of failure of the endoscopic treatment, for perforations measuring over 2cm, for cases with a suspicion of sepsis/peritonitis and in patients with a late diagnosis of perforation. In the case of tension pneumoperitoneum, the use of percutaneous decompression maneuvers improves the patient's condition and serves as a bridge therapy until the final treatment to repair the perforation. Broad-spectrum antibiotics must also be added to the endoscopic and surgical treatment regimens.54
Specific recommendations- -
Submucosal injection of adrenaline reduces the incidence of early bleeding but not that of delayed bleeding (level of evidence 1+; grade of recommendation A).
- -
The preventive coagulation of visible vessels with forceps or APC does not prevent the incidence of post-EMR bleeding in the colon or rectum (level of evidence 1++; grade of recommendation A).
- -
There is no available evidence to recommend the systematic closure of eschars after the EMR to prevent delayed bleeding (level of evidence 2−; grade of recommendation D).
- -
It is advisable to explore the eschar and to close the defect with clips if any damage is detected in the muscle layer (level of evidence 3; grade of recommendation D).
- -
Perforations measuring ≤2cm with an adequate degree of cleansing of the intestinal lumen occurring in clinically and analytically stable patients can be treated endoscopically (level of evidence 3; grade of recommendation D).
- -
Surgical treatment is an appropriate option in the event of a failure of the endoscopic treatment, for large perforations, for the suspicion of sepsis/peritonitis and in patients with a late perforation diagnosis (level of evidence 3; grade of recommendation D).
- -
In the case of tension pneumoperitoneum, the use of percutaneous decompression maneuvers improves the patient's condition and serves as a bridge therapy until the final treatment to repair the perforation (level of evidence 3; grade of recommendation D).
- -
The use of broad-spectrum antibiotics in addition to the endoscopic and surgical treatment of the perforation is recommended (level of evidence 3; grade of recommendation D).
- -
Sedation does not seem to increase the risk of complications during an EMR (level of evidence 3; grade of recommendation D).
The estimated global risk of recurrence of a lesion after an EMR is 15% (CI 95% 12–19%), 3% in the case of en bloc resections and 20% in the case of fragmented resections. Approximately 70% of recurrences are identifiable after three months of follow-up and over 90% are detected six months after the procedure. These recurrences can be treated with an endoscopic technique. Therefore, this treatment is definitive in up to 99% of cases after a mean of 1.2 endoscopies for lesions without an invasive component and candidates for endoscopic treatment, and only 1–4% of large NPCRLs require rescue surgery. When considering non-curative resections due to the existence of histological evidence of invasion, synchronous lesions, recurrences that cannot be managed endoscopically and complications, the overall surgery rates reaches 9%. Predictors of therapeutic failure are: lesion size greater than 30mm, incomplete initial resection, fragmented EMR, 0-Is and 0-IIa+Is morphology, Kudo's pit pattern III/IV/V, adenomatous vs serrated histology and high-grade dysplasia and carcinoma with submucosal invasion vs low-grade dysplasia.6,55,56
The treatment of a polyp with EMR is considered as effective when the lesion has been completely resected and no recurrence is detected during the first follow-up evaluation. An early colonoscopy to monitor the post-EMR scar is not necessary in en bloc EMRs involving the complete resection of the lesion. In the case of EMR-f, the first check-up after the complete EMR is considered as part of the therapeutic procedure. This check-up must be aimed at thoroughly examining the post-EMR scar in order to identify signs of recurrence. In the case of lesions greater than 20mm, patients should be monitored every 3–6 months. Chromoendoscopy (electronic and conventional), confocal endomicroscopy and biopsy sampling have proven to be useful for the detection of residual neoplasia in post-EMR scars57; these areas should also be photographed. If a residual lesion is detected and treated, a new endoscopic evaluation 6–12 months later should be performed. If no signs of recurrence are detected, a new evaluation should be performed 12 months later; this indication may vary based on the recommendations of the endoscopist. Due to their high complexity, the treatment of lesions growing on eschars must be carried out by an endoscopist specialized in EMR and very complex cases should be assessed by a multidisciplinary committee in order to make a consensus decision with the patient.47
Patients with adenomas and serrated lesions are at risk of developing metachronous lesions.58 Therefore, follow-up evaluation colonoscopies must be carried out according to the national and European guidelines on post-polypectomy monitoring.59
Specific recommendations- -
An early colonoscopy to monitor the post-EMR scar is not necessary in en bloc EMRs involving a complete resection of the lesion. A follow-up colonoscopy should be carried out in order to rule out the existence of metachronous lesions according to the relevant post-polypectomy monitoring guidelines (level of evidence 1+; grade of recommendation B).
- -
In the case of the fragmented EMR of NPCRLs greater than 20mm, an endoscopic follow-up evaluation 3–6 months after the procedure is recommended in order to rule out the existence of recurrence/residual tissue (level of evidence 1++; grade of recommendation A).
- -
Whenever a recurrence is detected and treated, an early follow-up evaluation should be carried out after 6–12 months (level of evidence 2+; grade of recommendation C).
- -
If no signs of recurrence are found, an endoscopic follow-up evaluation should be carried out 12 months after the previous check-up (level of evidence 2+; grade of recommendation C).
- -
In specific cases, the schedule of follow-up evaluations should be subject to the criteria of the endoscopist (level of evidence 4; grade of recommendation D).
- -
During follow-up colonoscopies, the eschar must be identified, thoroughly examined and photographed. The use of enhancement techniques and biopsies may improve the detection of a residual neoplasia on the eschar (level of evidence 3; grade of recommendation D).
- -
Areas with suspected residual tissue require the diagnostic analysis of the tissue and the administration of a definitive treatment. The treatment can be endoscopic in most cases (level of evidence 3; grade of recommendation D).
- -
Treatment of a recurrence on the eschar must be administered by an endoscopist specialized in EMR (level of evidence 3; grade of recommendation D).
- -
Complex cases of recurrence should be discussed with a multidisciplinary committee and all decisions must be made in consensus with the patient (level of evidence 3; grade of recommendation D).
EMR is a technique used in most endoscopy units within Spain and generally more complex than simple polypectomies carried out with clamps or a snare (cold or diathermy). The learning objectives of the EMR must address all phases of the procedure and include a complete assessment of the patient in order to achieve the following: (a) recognizing the indications of a technique; (b) obtaining an adequate competence in the procedure; (c) establishing an adequate risk/benefit assessment; and c) awareness of patient management after the procedure including any complications. According to the European Guidelines for the assurance of the quality of screening and diagnostic colonoscopies, doctors qualified to perform this screening should be able to treat lesions <25mm by conventional polypectomy and NPCRLs <20mm by EMR (competence level 3). NPCRLs >20mm or those located in hard-to-reach locations must be treated by professionals with the highest level of competence (level 4).60
The efficacy of the EMR technique, defined by the absence of signs of recurrence during follow-up, is greater among expert endoscopists than among inexperienced ones. It is important that an endoscopist treats an adequate volume of cases during their training in order to gain sufficient experience in this field. Fortunately, the prevalence of colonic lesions with an indication for EMR is very high. Therefore, the volume of patients is not an issue in Spain.61
To the present day, no study has assessed the learning curve in colonic and rectal EMRs. According to the British Joint Advisory Group (JAG), the recommended number of procedures in order to obtain temporary accreditation for performing colonoscopies is 200 and the number required to obtain full accreditation is 300 procedures. According to the JAG, level 1 polypectomies are focused on polyps measuring less than 1cm and level 2 polypectomies are used to treat polyps greater than 1cm. The JAG has also developed a special form for the assessment of skills in EMR via direct observation. The form includes the following items: (a) injecting the submucosa correctly using an appropriate injection technique; (b) performing the polypectomy only if the lesion is adequately elevated; (c) selecting the appropriate snare size; (d) directing the snare carefully over the lesion; (e) adequately choosing between an en bloc or fragment resection, depending on the size of the lesion; (f) placing the snare adequately over the lesion when it is closed; (g) ensuring that the correct amount of tissue is taken with the snare; (h) carefully separating the lesion from the rest of the healthy mucosa; (i) choosing between a cold or diathermy snare correctly; and (j) ensuring adequate hemostasis. These forms generate an objective assessment resulting in scores, but they do not provide data on the learning curve of EMRs. After adapting these criteria to the reality of the training in these techniques, the JAG provides a complete certification when an endoscopist has proved sufficient competence in a procedure which allows for an independent practice.62
In Spain, an adapted and translated scoring system for this purpose is lacking.
Specific recommendations- -
Endoscopists who are new to the EMR technique should be proficient in basic diagnostic and therapeutic colonoscopy (simple snare polypectomy and the treatment of complications) (level of evidence 4; grade of recommendation D).
- -
In addition, endoscopists who are new to the EMR technique must be able to identify NPCRLs, identify suspicious endoscopic signs and have experience in chromoendoscopy (level of evidence 4; grade of recommendation D).
The authors declare that they have no conflict of interests.
Coordinators:
- -
Eduardo Albéniz-Arbizu. Complejo Hospitalario de Navarra. Pamplona, Spain
- -
María Pellisé-Urquiza. Hospital Universitari Clínic. Barcelona, Spain
External revision:
- -
Antonio Zebenzuy-Gimeno-García. Hospital Universitario de Canarias. Tenerife, Spain
- -
Alfredo Lucendo-Villarín. Hospital General de Tomelloso. Tomelloso, Ciudad Real. Spain
Introduction:
- -
Leopoldo López-Rosés. Hospital Xeral. Lugo, Spain
Indications:
- -
Coordinator: Alberto Herreros-de-Tejada. Hospital Puerta de Hierro. Majadahonda, Madrid. Spain
- -
Manuel Rodríguez-Téllez. Hospital Universitario Virgen Macarena. Sevilla, Spain
- -
Orlando García-Bosh. Hospital Moisès Broggi de Barcelona. Barcelona, Spain
- -
Alba Zúñiga-Ripa. Resident medical intern. Complejo Hospitalario de Navarra. Pamplona, Spain
- -
Marta Hernández-Conde. Hospital Puerta de Hierro. Majadahonda, Madrid. Spain
Patient requirements and preparation:
- -
Coordinator: David Martínez-Ares. Complejo Hospitalario Universitario de Vigo. Vigo, Spain
- -
Fernando Alberca-de-las-Parras. Hospital Clínico Universitario Virgen de la Arrixaca. Murcia, Spain
- -
Carla Jerusalén Gargallo-Puyuelo. Hospital Clínico Universitario Lozano Blesa. Zaragoza, Spain
- -
Esteban Saperas-Franch. Hospital General de Catalunya. Sant Cugat del Vallés, Barcelona. Spain
Patient and information:
- -
Coordinator: Maite Herráiz-Bayod. Clínica Universidad de Navarra. Pamplona, Spain
- -
Miguel Muñoz-Navas. Clínica Universidad de Navarra. Pamplona, Spain
- -
Javier Gordillo. Nurse at the Endoscopy Unit. Complejo Hospitalario de Navarra. Pamplona, Spain
- -
Felipe Ramos-Zabala. Hospital Universitario Montepríncipe. Madrid, Spain
- -
Jose María Echevarría. Alianza PCCR patient
Identification and characterization of the lesions:
- -
Coordinator: Akiko Ono. Hospital Clínico Universitario Virgen de la Arrixaca. Murcia, Spain
- -
Marco Bustamante. Hospital Universitari i Politècnic La Fe de Valencia. Valencia, Spain
- -
Marco Antonio Álvarez. Hospital del Mar. Barcelona, Spain
- -
Mariano González-Haba. Hospital Puerta de Hierro-Majadahonda. Madrid, Spain
- -
Marta Montes-Díaz. Anatomía Patológica. Complejo Hospitalario de Navarra. Pamplona, Spain
Technical aspects:
- -
Coordinator: Andrés Sánchez-Yagüe. Hospital Costa del Sol. Marbella. Málaga, Spain
- -
Begoña González-Suárez. Hospital Universitari Clínic. Barcelona, Spain
- -
Juan José Vila-Costas. Complejo Hospitalario de Navarra. Pamplona, Spain
- -
Carlos Guarner-Argente. Hospital de la Santa Creu y San Pau. Barcelona, Spain
- -
Ferran González-Huix. Hospital Universitari de Girona Doctor Josep Trueta. Girona, Spain
Complications:
- -
Coordinator: María Fraile-Gónzález. Hospital de San Pedro. Logroño, Spain
- -
Fernando Múgica-Aguinaga. Hospital Universitario Donostia. Donostia-San Sebastián, Spain
- -
Julyssa Cobián. Hospital Universitario Donostia. Donostia-San Sebastián, Spain
- -
Joaquín Rodríguez-Sánchez. Hospital General de Ciudad Real. Ciudad Real, Spain
- -
Bartolome López-Viedma. Hospital General de Ciudad Real. Ciudad Real, Spain
Efficacy:
- -
Coordinator: Pedro Alonso-Aguirre. Complejo Hospitalario Universitario de A Coruña. A Coruña, Spain
- -
Noel Pin-Vieito. Complejo Hospitalario de Ourense. Ourense, Spain
- -
José Carlos Marín-Gabriel. Hospital Universitario 12 de Octubre. Madrid, Spain
- -
Óscar Nogales-Rincón. Hospital Universitario Gregorio Marañón. Madrid, Spain
- -
Eduardo Valdivielso-Cortázar. Complejo Hospitalario Universitario de A Coruña. A Coruña, Spain
EMR, ESD or surgery:
- -
Coordinator: Adolfo Parra-Blanco. Departamento de Gastroenterología. Facultad de Medicina. Pontificia Universidad Católica de Chile. Santiago de Chile, Chile
- -
Joaquín de-la-Peña. Hospital de Valdecilla. Santander, Spain
- -
José Díaz-Tasende. Hospital Universitario 12 de Octubre. Madrid, Spain
- -
Francisco Javier Navajas-León. Complejo Hospitalario de Toledo. Toledo, Spain
- -
Helena León-Brito. Hospital Reina Sofía. Tudela, Navarra. Spain
Surveillance:
- -
Coordinator: Santiago Soto-Iglesias. Complexo Hospitalario de Ourense. Ourense, Spain
- -
David Remedios-Espino. Complexo Hospitalario de Ourense. Ourense, Spain
- -
Jose Miguel Esteban. Hospital Universitario Clínico San Carlos. Madrid, Spain
- -
David Barquero-Declara. Hospital Moisès Broggi de Barcelona. Barcelona, Spain
Learning and competence:
- -
Coordinator: Eduardo Redondo-Cerezo. Hospital Virgen de las Nieves. Granada, Spain
- -
Juan Gabriel Martínez-Cara. Hospital Virgen de las Nieves. Granada, Spain
- -
Felipe Martínez-Alcalá. Centro Andaluz de Gastroenterología Integral. Sevilla, Spain
- -
Iñaki Fernández-Urién-Sainz. Complejo Hospitalario de Navarra. Pamplona, Spain
Conclusions:
- -
Eduardo Albéniz-Arbizu. Complejo Hospitalario de Navarra. Pamplona, Spain
- -
María Pellisé-Urquiza. Hospital Universitari Clínic. Barcelona, Spain
| International Network Guidelines: Key components of high-quality and trustworthy guidelines | |||
|---|---|---|---|
| Component | Description | Yes | No |
| Composition of guideline development group | A guideline development panel should include diverse and relevant stakeholders such as health professionals, experts on a topic and patients | X | |
| Decision-making process | A guideline should describe the process used to reach consensus among the panel members and, if applicable, approval by the sponsoring organization This process should be established before the start of the guideline development | X | |
| Conflicts of interest | A guideline should include disclosure of the financial and nonfinancial conflicts of interest for members of the guideline development group The guideline should also describe how any identified conflicts were recorded and resolved | X | |
| Scope of a guideline | A guideline should specify its objective(s) and scope | X | |
| Methods | A guideline should clearly describe the methods used for the guideline development in detail | X | |
| Evidence reviews | Guideline developers should use systematic evidence review methods to identify and evaluate evidence related to the guideline topic | X | |
| Guideline recommendations | A guideline recommendation should be clearly stated and based on scientific evidence of benefits, harm and, if possible, cost | X | |
| Rating of evidence and recommendations | A guideline should use a rating system to communicate the quality and reliability of both the evidence and the strength of its recommendations | X | |
| Peer review and stakeholder consultations | Review by external stakeholders should be conducted before guideline publication | X | |
| Guideline expiration and updating | A guideline should include an expiration date and/or describe the process that the guideline groups will use to update recommendations | X | |
| Financial support and sponsoring organization | A guideline should disclose financial support for the development of both the evidence review as well as the guideline recommendations | X | |
| Levels of scientific evidence | |
|---|---|
| 1++ | High-level meta-analyses, high-quality systematic reviews of clinical trials with very little risk of bias |
| 1+ | Well-conducted meta-analyses, systematic reviews of clinical trials or well-conducted clinical trials with a low risk of bias |
| 1− | Meta-analyses, systematic-reviews of clinical trials with a high risk of bias |
| 2++ | High-quality systematic reviews of cohort or case and control studies; cohort or case and control studies with a very low risk of bias and a high probability of establishing a causal relationship |
| 2+ | Well-conducted cohort or case and control studies with a low risk of bias and a moderate probability of establishing a causal relationship |
| 2− | Cohort or case and control studies with a high risk of bias and significant risk that the relationship is not causal |
| 3 | Non-analytical studies such as case reports and case series |
| 4 | Expert opinion |
| Grades of recommendation | |
|---|---|
| A | At least one meta-analysis, systematic review or clinical trial classified as 1++ and directly applicable to the target population of the guideline, or a volume of scientific evidence comprising studies classified as 1+, which are highly consistent with each other |
| B | A body of scientific evidence comprising studies classified as 2++, directly applicable to the target population of the guideline and highly consistent with each other, or scientific evidence extrapolated from studies classified as 1++ or 1+ |
| C | A body of scientific evidence comprising studies classified as 2+, directly applicable to the target population of the guideline and highly consistent with each other, or scientific evidence extrapolated from studies classified as 2++ |
| D | Level 3 or 4 scientific evidence, or scientific evidence extrapolated from studies classified as 2+ |
1. Negative for neoplasia/dysplasia
- -
Vienna Category 1
2. Indefinite neoplasia/dysplasia
- -
Vienna Category 2
3. Low grade neoplasia (low grade adenoma/dysplasia)
- -
Vienna Category 3 (non-invasive low grade neoplasia)
4. Intramucosal borderline neoplasia
- -
4.1. High-grade adenoma/dysplasia
- -
4.2. Intramucosal carcinoma well-differentiated
- -
Vienna Category: 4.1–4.4 (non-invasive high grade neoplasia)
5. Definite carcinoma
- -
5.1. Intramucosal carcinoma, moderately or poorly differentiated
- -
5.2. Submucosal carcinoma or beyond
- -
Vienna Category: 5.1–5.2 (invasive neoplasia)
Please cite this article as: Albéniz E, Pellisé M, Gimeno García AZ, Lucendo AJ, Alonso Aguirre PA, Herreros de Tejada A, et al. Guía clínica para la resección mucosa endoscópica de lesiones colorrectales no pediculadas. Gastroenterol Hepatol. 2018;41:175–190.