Many patients with hepatitis C virus (HCV) have associated comorbidities that require complex treatments. We sought to determine the impact of treatment with direct-acting antiviral agents (DAAs) for HCV on adherence to prescribed concomitant medications for associated comorbidities and to identify predictors of non-adherence to comedications.
Patients and methodsHCV-infected patients treated with DAAs in a Spanish hospital between January 2015 and December 2016 and followed-up by the pharmacy unit were included in the study. Adherence to concomitant comedication prescribed before and during HCV therapy with DAAs was compared to adherence during the same number of weeks before DAA initiation. Demographic, clinical and pharmacotherapy variables were analyzed to determine factors associated with non-adherence. A multivariate regression model was created for prediction of non-adherence to concomitant medication.
ResultsData from 214 patients using prescribed concomitant therapies were analyzed. Significant reduction on adherence to comedications was observed after initiation of DAA treatment compared with a similar period before therapy initiation (29.9% vs. 36.9%, p=0.032). The univariate analysis showed that polypharmacy and presence of vascular disease were associated negatively with adherence to concomitant medications (87.8%, p=0.006 and 84.7%, p<0.001, respectively). Multivariate analysis indicated that HIV/HBV coinfection was associated with adherence (OR 0.19; 95% CI 0.09–0.39), while polypharmacy was a predictor for non-adherence (OR 4.54; 95% CI 1.48–13.92).
DiscussionAdherence to concomitant medications decreases in HCV-infected patients when DAA therapy is initiated. Polypharmacy is a predictor for non-adherence, while HIV/HBV coinfection reduce non-adherence rates. Polymedicated patients on DAAs might benefit from close follow-up and educational programmes to improve their adherence.
Muchos pacientes con virus de la hepatitis C (VHC) presentan comorbilidades que requieren tratamientos complejos. Queremos determinar el impacto del tratamiento con antivirales de acción directa (AAD) para el VHC en la adherencia a medicaciones concomitantes e identificar factores predictivos de no adherencia a comedicaciones.
Pacientes y métodosPacientes tratados con AAD entre 2015 y 2016 se incluyeron en el estudio y se comparó su adherencia a medicaciones concomitantes antes y durante la terapia con AAD en un periodo de tiempo similar. Múltiples variables fueron analizadas para identificar factores asociados a la no-adherencia. Se creó un modelo de regresión multivariable para predecir la no adherencia a medicaciones concomitantes.
ResultadosSe analizaron datos de 214 pacientes en tratamientos concomitantes. Tras iniciar la terapia con AAD, la adherencia a las comedicaciones disminuyó respecto a la adherencia en ausencia de AAD (29,9% respecto al 36,9%; p=0,032). El análisis univariante demostró que la polifarmacia y la enfermedad vascular estaban asociadas negativamente con la adherencia a las medicaciones concomitantes (87,8%, p=0,006 y 84,7%, p<0,001, respectivamente). El análisis multivariante indicó que la coinfección con VIH/VHB estaba asociada con la adherencia (OR: 0,19; IC 95%: 0,09-0,39), mientras que la polifarmacia era un predictor de no adherencia (OR: 4,54; IC 95%: 1,48-13,92).
DiscusiónEl inicio del tratamiento con AAD disminuye la adherencia a la comedicación en pacientes con VHC. La polifarmacia es predictor de no adherencia mientras que la coinfección con VIH/VHB la reduce. Aquellos pacientes polimedicados y en tratamiento con AAD podrían beneficiarse de un seguimiento estrecho para aumentar su adherencia.
Hepatitis C virus (HCV) infection is a major public health burden and a leading cause of liver disease, with an estimated prevalence of 1% worldwide.1 Treatment options for chronic HCV infection are evolving rapidly, with direct-acting antivirals (DAAs) becoming the standard of care. DAAs have proven to be highly effective in clinical trials, with sustained virologic response (SVR) rates of over 90%, irrespective of the viral genotype.2,3 The increased virological potency coupled with the excellent tolerability of all currently available oral DAA regimens are prompting their use in many patients previously excluded from interferon-based therapies.4
Another major advance in the available treatments against HCV has been the improvement in the associated pharmacotherapeutic complexity, facilitating treatment adherence, patient satisfaction and achievement of SVR.5 This considerable progress means that DAAs have been incorporated into clinical practice at a rapid rate. Although their effectiveness and safety are yet to be clearly defined in specific populations, such as cirrhotic patients and transplant recipients, recent studies suggest that they are safe and efficacious even those populations.6,7 However, the safety profile of DAAs is restricted by significant drug interactions, and their widespread use is limited by their high cost.8,9
HCV-infected patients often present high levels of comorbidity, and medications are also needed during DAA therapy.10–12 Adherence to these medications might be crucial to avoid serious health complications not related with liver disease. However, most studies have focused only on the adherence to DAAs and have failed to evaluate the effect of antiretroviral therapies on adherence to other medications. The aim of the current study was to determine variations in adherence to other medications during therapy with DAAs against HCV compared to the pre-DAA period, and to describe predictive factors associated with non-adherence to those drugs co-administered with DAAs.
Patients and methodsWe performed a retrospective, single-centre observational study, including all patients ≥ 18 years old starting DAA therapy for HCV infection in our centre from 1 January 2015 until 31 December 2016. Patients who participated in clinical trials were excluded. For this analysis, patients taking prescribed concomitant therapy were selected. Follow-up was performed by the pharmacy department's pharmacy clinic for viral diseases at Hospital de Valme (Seville, Spain).
This study was approved by the hospital ethics committee and met the ethical principles stated in the Declaration of Helsinki. Written informed consent was obtained from all individual participants included in the study.
Definition of study endpointsThe main endpoint was variation in adherence to prescribed concomitant medication. Adherence to comedication over DAA therapy was compared with that over a period of similar length, immediately before DAA initiation. Data relating to DAA use by the patients were collected from the outpatient pharmacy-dispensing programme (Dominion-Farmatools). Information regarding other medication was obtained from the electronic health prescriptions programme of the Andalusian Public Health System.
The other variables collected in order to determine characteristics potentially associated with a modification in adherence were obtained from patients’ medical records and laboratory reports. These included demographic characteristics (sex and age); virological and ultrasound parameters (plasma HCV-RNA [log10IU/mL], HCV genotype, liver stiffness [kPa]); pharmacotherapeutic variables (HIV or HBV coinfection, treatment status [treatment-naïve patient, or treatment-experienced], type of DAA prescribed (a) sofosbuvir plus ribavirin plus interferon [SOF+RBV+INF]; (b) sofosbuvir plus ribavirin [SOF+RBV]; (c) sofosbuvir/ledipasvir [SOF/LDV]; (d) ombitasvir/paritaprevir/ritonavir plus dasabuvir [OBV/PTV/RTV+DSV]; (e) ombitasvir/paritaprevir/ritonavir plus ribavirin [OBV/PTV/RTV+RBV]; (f) sofosbuvir plus simeprevir [SOF+SMV] or (g) sofosbuvir plus daclatasvir [SOF+DCV]), and the generation of DAA prescribed (1st generation [boceprevir, telaprevir], 2nd generation [sofosbuvir, simeprevir, daclatasvir], or 3rd generation [ledipasvir, ombitasvir, paritaprevir, dasabuvir]).13 The type and number of comorbidities (cardiovascular, metabolic, chronic respiratory, central nervous system, dermatologic, digestive, musculoskeletal, hematologic, hormonal and renal disorders, and malignant neoplasms),14 and type and number of concomitant drugs (drugs for cardiovascular diseases, lipid-lowering agents, antidiabetic drugs, psycholeptics or psychoanaleptics, drugs for chronic respiratory disease and for gastric acid-related disorders) were collected. Comedication was included in the analyses if it was prescribed for a minimum of 60 days.
Polypharmacy (defined as taking >5drugs/day),15 adherence to DAAs and the complexity index of the HCV treatment were recorded. The pharmacotherapy complexity index was measured using the Medication Regimen Complexity Index (MRCI). This validated tool includes 65 items grouped into three subgroups: dose forms, dosing frequencies, and additional instructions relevant to drug administration. Scores were calculated using the Colorado University web tool,16 available at: http://www.ucdenver.edu/academics/colleges/pharmacy/Research/researchareas/Pages/MRCTool.aspx.
Patients were stratified by risk level according to the selection and stratification model for pharmaceutical care in HIV patients with or without HCV.17 Adherence to concomitant medication was assessed before DAA initiation and after 12 or 24 weeks of antiviral treatment (depending on the patient) using the Morisky and Green questionnaire18 and the electronic pharmacy refill records, calculated using the formula: [(pills dispensed/pills prescribed per day)/days between refills]×100. This equation has been used in prior studies to evaluate percent adherence to antiviral medications.19–22 Patients were classified as adherent if they responded correctly to the four questions of the Morisky and Green questionnaire and their adherence percentage was above 90% for all the medications. Only chronic and clinically relevant medications were considered for adherence assessment with this method. Drugs modifying the course of the disease were considered of high clinical relevance (drugs of coronary heart disease, myocardial infarction, stroke, diabetes, COPD, osteoporosis, primary prevention and secondary gastrointestinal bleeding, etc.). Drugs for symptomatic treatment of minor conditions (gastroesophageal reflux, constipation, nausea, pain, supplements, topical therapy, etc.) were not considered for adherence estimations.
Statistical analysisQuantitative variables are expressed as median and interquartile range (IQR). Qualitative variables are expressed as percentages (%). To compare mean values of quantitative variables among groups, the Student's t-test was used for independent samples, or the Mann–Whitney non-parametric U-test in cases of non-normal distributions. If any significant differences were observed, 95% confidence intervals (CI) were found for differences in mean (or median, if applicable) values. Contingency tables were prepared, and the chi-square test was used to analyze the relationship between qualitative variables.
Variables that showed an association with the main variable in the univariate analysis with p<0.25 were then included in a multivariate model; a 95% CI was used. Validity of the model was confirmed by the Hosmer and Lemeshow test. The sample size was estimated by the Freeman equation 10×(k+1). The level of statistical significance was set at p<0.05. Data analysis was performed using the statistical package SPSS 22.0 for Windows (IBM Corp., Armonk, NY).
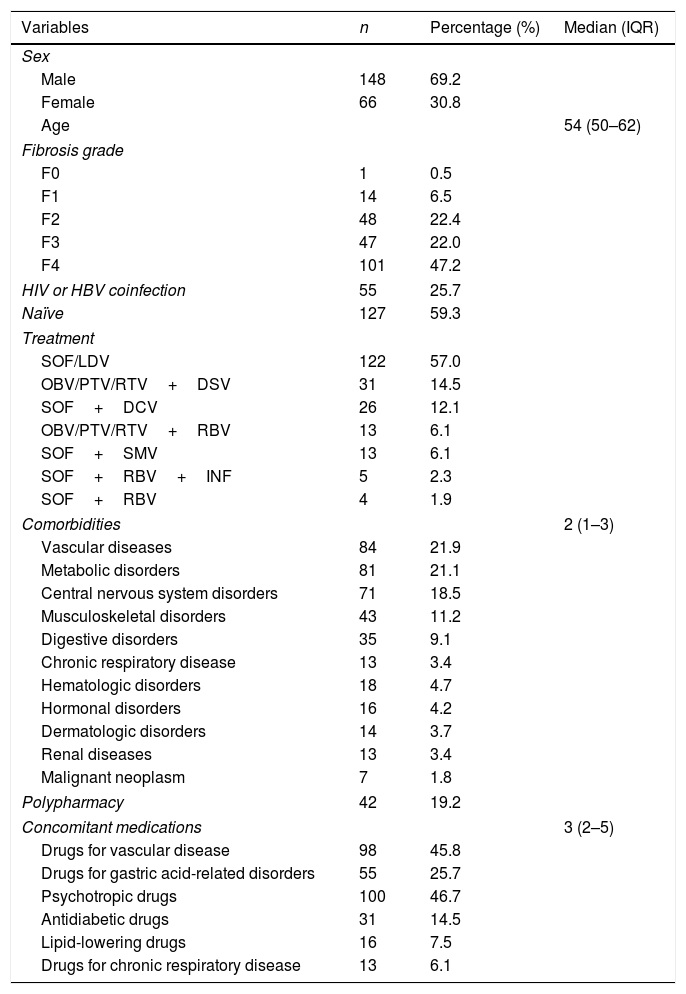
ResultsOf the 384 participants, 214 were taking prescribed comedications for associated comorbidities and were included in the analysis. Demographic, clinical and other patient characteristics are shown in Table 1. The majority of the patients were male (69.2%), with median age 54 years (IQR: 50–62). Considering the degree of fibrosis, the most common stage was F4 (47.2%). Fifty-five patients (25.7%) were also coinfected with HIV or HBV.
Baseline and clinical characteristics of the patients who initiated therapy with direct-acting antivirals and were taking prescribed concomitant medications (N=214).
| Variables | n | Percentage (%) | Median (IQR) |
|---|---|---|---|
| Sex | |||
| Male | 148 | 69.2 | |
| Female | 66 | 30.8 | |
| Age | 54 (50–62) | ||
| Fibrosis grade | |||
| F0 | 1 | 0.5 | |
| F1 | 14 | 6.5 | |
| F2 | 48 | 22.4 | |
| F3 | 47 | 22.0 | |
| F4 | 101 | 47.2 | |
| HIV or HBV coinfection | 55 | 25.7 | |
| Naïve | 127 | 59.3 | |
| Treatment | |||
| SOF/LDV | 122 | 57.0 | |
| OBV/PTV/RTV+DSV | 31 | 14.5 | |
| SOF+DCV | 26 | 12.1 | |
| OBV/PTV/RTV+RBV | 13 | 6.1 | |
| SOF+SMV | 13 | 6.1 | |
| SOF+RBV+INF | 5 | 2.3 | |
| SOF+RBV | 4 | 1.9 | |
| Comorbidities | 2 (1–3) | ||
| Vascular diseases | 84 | 21.9 | |
| Metabolic disorders | 81 | 21.1 | |
| Central nervous system disorders | 71 | 18.5 | |
| Musculoskeletal disorders | 43 | 11.2 | |
| Digestive disorders | 35 | 9.1 | |
| Chronic respiratory disease | 13 | 3.4 | |
| Hematologic disorders | 18 | 4.7 | |
| Hormonal disorders | 16 | 4.2 | |
| Dermatologic disorders | 14 | 3.7 | |
| Renal diseases | 13 | 3.4 | |
| Malignant neoplasm | 7 | 1.8 | |
| Polypharmacy | 42 | 19.2 | |
| Concomitant medications | 3 (2–5) | ||
| Drugs for vascular disease | 98 | 45.8 | |
| Drugs for gastric acid-related disorders | 55 | 25.7 | |
| Psychotropic drugs | 100 | 46.7 | |
| Antidiabetic drugs | 31 | 14.5 | |
| Lipid-lowering drugs | 16 | 7.5 | |
| Drugs for chronic respiratory disease | 13 | 6.1 | |
DCV: daclatasvir; DSV: dasabuvir; HCV: hepatitis C virus; HIV: human immunodeficiency virus; INF: interferon; IQR: interquartile range; LDV: ledipasvir; OBV: ombitasvir; PTV: paritaprevir; RBV: ribavirin; RTV: ritonavir; SMV: simeprevir; SOF: sofosbuvir. Fibrosis grade: three patients were lost to follow-up. Patients may present more than one comorbidity and more than one prescribed medication.
Regarding DAA therapies, the most common regimens were those including SOF/LDV (57%), followed by OBV/PTV/RTV+DSV (14.5%) and SOF+DCV (12.1%).
The median number of comorbidities per patient was 1.0 (IQR: 0.0–2.0). Vascular disease was the most common comorbidity, being present in 21.9% of patients, followed by metabolic disorders in 21.1% of patients and by central nervous system disorders in 18.5%.
The most frequently prescribed therapeutic drugs were psychotropic medications (46.7%) followed by drugs to treat cardiovascular diseases (45.8%) and medication for gastric acid-related disorders (25.7%). The median value of the DAA MRCI was 4 (IQR=2–5).
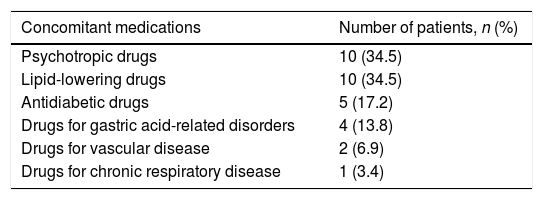
We found a significant variation in adherence to prescribed concomitant medication during DAA therapy as shown in Table 2. Sixty-four patients (29.9%) were adherent while on DAA therapy, compared to 79 (36.9%) (p=0.032) during the period immediately before initiation. Thus, adherence to concomitant medication decreased during DAA treatment by 7%. Specifically, 14 of the 135 patients who were initially not adherent to the concomitant treatments became adherent after initiating DAA. On the other hand, of the 79 patients who were originally adherent to the concomitant medications, 29 became non-adherent after initiating DAA. Regarding medication type, the majority of those patients turned into non-adherent to psycotrophic drugs (34.5%) and lipid-lowering medications (34.5%) followed by antidiabetic drugs (17.2%) (Table 3).
Non-adherent patients by medication class (N=29).
| Concomitant medications | Number of patients, n (%) |
|---|---|
| Psychotropic drugs | 10 (34.5) |
| Lipid-lowering drugs | 10 (34.5) |
| Antidiabetic drugs | 5 (17.2) |
| Drugs for gastric acid-related disorders | 4 (13.8) |
| Drugs for vascular disease | 2 (6.9) |
| Drugs for chronic respiratory disease | 1 (3.4) |
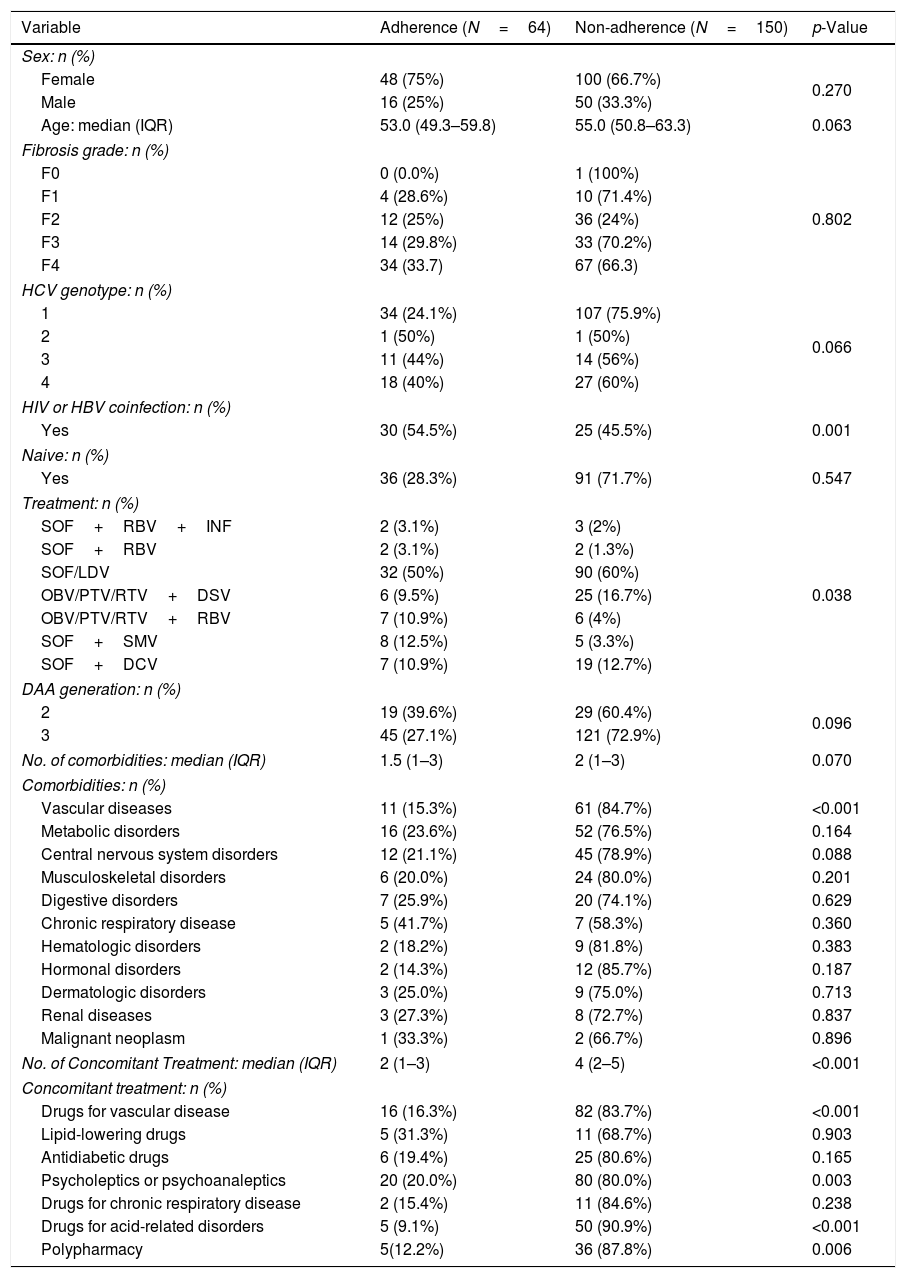
To identify predictors of adherence to concomitant medication, univariate analysis was performed (Table 4). Sex and age did not differ substantially between adherent and non-adherent HCV patients. However, the proportion of patients not coinfected with HBV or HIV was significantly greater in the non-adherent group (p<0.001). Furthermore, polypharmacy was significantly more frequent in non-adherent patients (87.8% vs. 12.2%, respectively; p=0.006).
Univariate analysis of variables associated with adherence to medications for comorbidities in patients receiving DAAs (N=214).
| Variable | Adherence (N=64) | Non-adherence (N=150) | p-Value |
|---|---|---|---|
| Sex: n (%) | |||
| Female | 48 (75%) | 100 (66.7%) | 0.270 |
| Male | 16 (25%) | 50 (33.3%) | |
| Age: median (IQR) | 53.0 (49.3–59.8) | 55.0 (50.8–63.3) | 0.063 |
| Fibrosis grade: n (%) | |||
| F0 | 0 (0.0%) | 1 (100%) | 0.802 |
| F1 | 4 (28.6%) | 10 (71.4%) | |
| F2 | 12 (25%) | 36 (24%) | |
| F3 | 14 (29.8%) | 33 (70.2%) | |
| F4 | 34 (33.7) | 67 (66.3) | |
| HCV genotype: n (%) | |||
| 1 | 34 (24.1%) | 107 (75.9%) | 0.066 |
| 2 | 1 (50%) | 1 (50%) | |
| 3 | 11 (44%) | 14 (56%) | |
| 4 | 18 (40%) | 27 (60%) | |
| HIV or HBV coinfection: n (%) | |||
| Yes | 30 (54.5%) | 25 (45.5%) | 0.001 |
| Naive: n (%) | |||
| Yes | 36 (28.3%) | 91 (71.7%) | 0.547 |
| Treatment: n (%) | |||
| SOF+RBV+INF | 2 (3.1%) | 3 (2%) | 0.038 |
| SOF+RBV | 2 (3.1%) | 2 (1.3%) | |
| SOF/LDV | 32 (50%) | 90 (60%) | |
| OBV/PTV/RTV+DSV | 6 (9.5%) | 25 (16.7%) | |
| OBV/PTV/RTV+RBV | 7 (10.9%) | 6 (4%) | |
| SOF+SMV | 8 (12.5%) | 5 (3.3%) | |
| SOF+DCV | 7 (10.9%) | 19 (12.7%) | |
| DAA generation: n (%) | |||
| 2 | 19 (39.6%) | 29 (60.4%) | 0.096 |
| 3 | 45 (27.1%) | 121 (72.9%) | |
| No. of comorbidities: median (IQR) | 1.5 (1–3) | 2 (1–3) | 0.070 |
| Comorbidities: n (%) | |||
| Vascular diseases | 11 (15.3%) | 61 (84.7%) | <0.001 |
| Metabolic disorders | 16 (23.6%) | 52 (76.5%) | 0.164 |
| Central nervous system disorders | 12 (21.1%) | 45 (78.9%) | 0.088 |
| Musculoskeletal disorders | 6 (20.0%) | 24 (80.0%) | 0.201 |
| Digestive disorders | 7 (25.9%) | 20 (74.1%) | 0.629 |
| Chronic respiratory disease | 5 (41.7%) | 7 (58.3%) | 0.360 |
| Hematologic disorders | 2 (18.2%) | 9 (81.8%) | 0.383 |
| Hormonal disorders | 2 (14.3%) | 12 (85.7%) | 0.187 |
| Dermatologic disorders | 3 (25.0%) | 9 (75.0%) | 0.713 |
| Renal diseases | 3 (27.3%) | 8 (72.7%) | 0.837 |
| Malignant neoplasm | 1 (33.3%) | 2 (66.7%) | 0.896 |
| No. of Concomitant Treatment: median (IQR) | 2 (1–3) | 4 (2–5) | <0.001 |
| Concomitant treatment: n (%) | |||
| Drugs for vascular disease | 16 (16.3%) | 82 (83.7%) | <0.001 |
| Lipid-lowering drugs | 5 (31.3%) | 11 (68.7%) | 0.903 |
| Antidiabetic drugs | 6 (19.4%) | 25 (80.6%) | 0.165 |
| Psycholeptics or psychoanaleptics | 20 (20.0%) | 80 (80.0%) | 0.003 |
| Drugs for chronic respiratory disease | 2 (15.4%) | 11 (84.6%) | 0.238 |
| Drugs for acid-related disorders | 5 (9.1%) | 50 (90.9%) | <0.001 |
| Polypharmacy | 5(12.2%) | 36 (87.8%) | 0.006 |
CNS: central nervous system; DAA; direct-acting antivirals; DCV: daclatasvir; DSV: dasabuvir; HCV: hepatitis C virus; HBV: hepatitis B virus; HIV: human immunodeficiency virus; INF: interferon; IQR: interquartile range; LDV: ledipasvir; OBV: ombitasvir; PTV: paritaprevir; RBV: ribavirin; RTV: ritonavir; SMV: simeprevir; SOF: sofosbuvir. Percentages were calculated by column (sex and treatment) or by row (fibrosis grade, genotype, HIV or HBV coinfection, Naïve, DAA generation, comorbidities and concomitant treatment).
Among the comorbidity variables analyzed, cardiovascular disease was found to be significantly more prevalent in non-adherent patients than in adherent patients (p<0.001). The number of concomitant medications was also higher in non-adherent patients (4.0 vs. 2.0; p<0.001). The percentage of non-adherent patients with concomitant medications to treat cardiovascular diseases, psychiatric conditions, and gastric acid-related disorders was significantly higher than that in adherent patients (83.7% vs. 16.3%, p<0.001; 80% vs. 20%, p=0.003; 90.9% vs. 9.1% p<0.001, respectively).
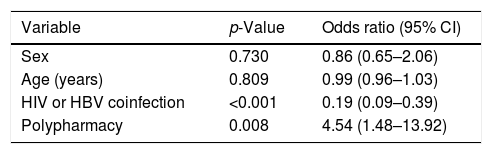
Multivariate analysis subsequently showed that coinfection with HBV or HIV was positively associated with adherence, with an odds ratio of 0.19 (95% CI=0.09–0.39), while polymedicated patients had a four-fold higher probability of being non-adherent to concomitant medication during HCV treatment with DAAs (OR 4.54; 95% CI=1.48–13.92) (Table 5).
Multivariate analysis of variables associated with adherence to concomitant treatment.
| Variable | p-Value | Odds ratio (95% CI) |
|---|---|---|
| Sex | 0.730 | 0.86 (0.65–2.06) |
| Age (years) | 0.809 | 0.99 (0.96–1.03) |
| HIV or HBV coinfection | <0.001 | 0.19 (0.09–0.39) |
| Polypharmacy | 0.008 | 4.54 (1.48–13.92) |
CI: confidence interval; HBV: hepatitis B virus; HIV: human immunodeficiency virus.
Our study found that HCV treatment initiation with DAAs negatively impacted adherence to concomitant medications. Importantly, we also determined that HBV or HIV coinfection and polypharmacy are significantly related to adherence among HCV patients. While coinfected patients on DAAs were more adherent to their comedication, patients receiving several concomitant drugs tended to have less adherence to other prescribed treatments.
The intake of numerous medications for comorbid medical conditions is a frequent event in patients starting HCV treatment.10–12 Since discontinuation of comedication may lead to major health problems,23 it is essential to determine whether initiation of DAA therapy might influence adherence to concomitant medications. Although literature on this topic is scarce, some studies are worth mentioning. Thus, in 2014, a study by Pizzirusso et al.24 showed that initiation of HCV treatment did not adversely impact adherence to concomitant medications. However, it is important to keep in mind that this was a prospective study including HCV naïve-treated patients only. Other reports have shown that prior HCV treatment is associated with lower adherence rates,25 suggesting that patients who have never been on a treatment have higher odds of successfully finishing the therapies. Importantly, non-naïve patient status has also been associated with low adherence rates in HIV treatment in other studies.26
In our study, cardiovascular disease and metabolic and central nervous system disorders were the most prevalent comorbidities. Accordingly, we found that the most commonly prescribed comedications were cardiovascular and psychotropic drugs, and those used in acid-related disorders. This is consistent with previous studies in which an association was found between HCV infection and the appearance of vascular, cognitive and metabolic comorbidities with similar prevalence rates.27–29
Although the complexity of antiretroviral regimens has decreased over recent years, HCV-infected patients still have to deal with a high number of prescribed drugs to treat comorbid diseases. In our study, polypharmacy was significantly more frequent in non-adherent patients. In fact, we found that the number of prescribed concomitant medications was higher in these patients. Specifically, those taking medications to treat cardiovascular, psychiatric and acid-related disorders showed lower adherence than patients taking other types of concomitant medications. Thus, adherence and medication regimen complexity seem closely linked. Previous reports have already demonstrated the effect of HIV treatment complexity on adherence, both in HIV mono-infected and HIV/HCV coinfected patients.30,31 However, to our knowledge, no other study has assessed the effect of treatment complexity on the adherence of HCV-infected patients to prescribed concomitant medications for associated comorbidities.
In our multivariate analysis, coinfection and polypharmacy were found to be associated with adherence. Since non-adherence, in this case related to polypharmacy, may negatively impact therapeutic success, it is important to closely monitor patients at high risk for poor medication adherence, and to choose appropriate interventions to improve compliance. In this sense, the pharmacy clinic staff are uniquely positioned to help patients manage their medications and provide adherence support. This patient-centre care model, based on the ability-motivation-opportunity (AMO) methodology, has already been successfully implemented in our centre.32 We are currently working on applying the results of the current study to improve HCV-patient health outcomes and satisfaction.
Given the association found in this study between polypharmacy and low adherence rates to concomitant medications, the choice of DAA treatment is another variable that should be considered, especially in patients with multiple comorbidities. Physicians should, when possible, prescribe the antiviral treatment that includes the least amount of pills, to increase the likelihood of patient adherence to the concomitant treatment.
This study has several strengths, including a large sample size and the evaluation of multiple variables related to adherence to concomitant medications not previously assessed. However, it also has some limitations. Thus, adherence rates were obtained using pharmacy dispensing records and the MMAS score which, despite being widely used in clinical practice, are known to overestimate rates.33 Another limitation of the study is its observational, retrospective and single-centre nature. Additionally, we did not evaluate over-the-counter and recreational drug use, because these data were not available on all patients.
Despite these limitations, our study has successfully identified the factors that may lead to poor concomitant medication adherence in HCV patients, and has also identified patients who might be particularly adherent to them, stressing the importance of an effective patient care model to closely monitor high risk-individuals.
As HCV-infected patients have associated comorbidities, it is important to monitor their adherence to concomitant medications. Treatments for those comorbidities are usually complex and chronic, and our study has shown that patients tend to focus on DAA therapies that are shorter and simpler and fail to follow concomitant treatments, which may lead to fatal health outcomes. We have identified polypharmacy as a factor associated with non-adherence to concomitant medications. This knowledge will help pharmacy clinic specialists to recognize high-risk patients and to develop personalized follow-up care, thereby ensuring good adherence and response to treatments.
Authors contributionAll named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. RMV conceptualized the study. MMG and MIGR were responsible for data acquisition; JAP and MMG were responsible for the analysis and interpretation of data. All authors (RMV, MMG, JAP, MIGR, MARC) substantially contributed to the writing and editing of the final version. All authors read and approved the final manuscript for this version to be published.
FundingJAP has received a research extension grant from the Programa de Intensificación de la Actividad de Investigación del Servicio Nacional de Salud Carlos III (I3SNS), from Spain and financial support from ISCIII-Subdirección General de Evaluación and the European Regional Development Fund (ERDF) (www.red.es/redes/inicio) (RD16/0025/0040 and RD12/0017/0012).
Medical Writing support was provided by Vanessa Marfil (Medical Science Consulting, Valencia). This service was funded by Gilead.
Conflicts of interestRMV has received lecture fees from Gilead, Janssen-Cilag, Merck Sharp and Abbvie. JAP reports having received consulting fees from Bristol-Myers Squibb, AbbVie, Gilead, Merck Sharp & Dome, and Janssen. He has received research support from Bristol-Myers Squibb, Merck Sharp & Dome, Janssen-Cilag and Gilead and has received lecture fees from AbbVie, Bristol-Myers Squibb, Merck Sharp & Dome, Janssen Cilag, and Gilead.